Abstract
Barrett's esophagus is a premalignant condition of esophageal adenocarcinoma. Inducible nitric oxide synthase (iNOS) is induced by cytokines and can generate locally high concentrations of nitric oxide (NO), whose metabolites can mediate genotoxicity and influence multistage carcinogenesis by causing DNA damage. Therefore, we evaluated the immunolocalization and expression of iNOS in surgically induced rat Barrett's esophagus. Esophagoduodenal anastomosis was performed in rats for inducing reflux of duodenal contents. Rats were killed at postoperative 10, 20, 30 and 40 weeks. We examined histologic changes and iNOS expression in esophagus by immunohistochemistry and reverse transcription-polymerase chain reaction. Eighty six percent of experimental rats showed Barrett's esophagus above esophagoduodenal junction. iNOS immunoreactivity was clearly observed in the epithelial cells of Barrett's esophagus, predominantly at the apical surface of epithelial cells. Cytoplasmic staining was also seen only in atypical Barrett's esophagus. iNOS mRNA was detected only in the lower esophagus of experimental group. In conclusion, this study suggests that iNOS has some roles on Barrett's esophagus formation.
Keywords: Barrett Esophagus, inducible nitric oxide synthase
INTRODUCTION
Nitric oxide (NO) is an important bioregulatory mediator involved in a variety of processes in cardiovascular, nervous, and immune systems (1). It is derived from the amino acid L-arginine in a reaction catalyzed by three different isoforms of nitric oxide synthases (NOS). The constitutive calcium-dependent isoforms, which are known to generate NO at low concentrations, are found in endothelial cells (eNOS) and neuronal tissue (nNOS) (2). An inducible, calcium-independent isoform (iNOS) is expressed in macrophages, neutrophils, endothelial cells, hepatocytes, cardiac myocytes, chondrocytes, and many other cell types (3). It is induced by cytokines and can generate locally high concentrations of NO for prolonged periods of time (4). NO metabolites can mediate genotoxicity and influence multistage carcinogenesis by causing DNA damage through nitrosative deamination, DNA strand breakage, or DNA modification (5, 6).
Several human gastrointestinal neoplasms express iNOS including gastric cancer, colonic adenomas, Barrett's esophagus and associated adenocarcinomas, hepatocellular carcinoma and cholangiocarcinoma (7-10). In addition, iNOS expression was observed in the epithelial cells of rodent colon tumors and stromal cells beneath Barrett's metaplastic epithelium surgically induced by chronic duodenal reflux in rat (11, 12). Reflux of duodenal contents in addition to gastric acid in human seems to contribute to the development of esophagitis and Barrett's esophagus (13, 14). Experimental studies in rat have shown that chronic contact of duodenal contents per se caused squamous cell carcinoma, adenosquamous carcinoma and adenocarcinoma in esophagus (15-17). However, the precise mechanism by which duodenal reflux causes esophageal injury and predisposes to neoplasia is uncertain. We have reported that prostaglandin biosynthetic pathway has an important role in rat esophageal squamous dysplasia and glandular metaplasia induced by chronic duodenal contents reflux (18). Several studies have suggested a cross link between the iNOS and cyclooxygenase pathway (19). Consequently, to study iNOS expression may be useful in understanding the pathogenesis of esophageal lesions related to chronic duodenal contents reflux. In this study, we investigated the immunolocalization and expression of iNOS in Barrett's esophagus induced by esophagoduodenal anastomosis.
MATERIALS AND METHODS
Animals
Thirty seven 7-week old male Sprague-Dawley rats (KIST, Daejun, Korea) were used for control (n=8) and experimental group (n=29). Throughout the experiment, all rats were housed in a controlled environment with 12 hr light/dark cycle and temperature of 22±2℃. After an acclimatization period of 1 week, 29 experimental rats were randomly divided into four groups in a time-course design and underwent an esophagoduodenostomy for inducing duodenal contents reflux for 10 (n=2), 20 (n=12), 30 (n=8) and 40 (n=7) weeks, respectively. Eight control rats underwent sham operation. At the end of the appropriate time period for each group, the control and experimental rats were killed with ether.
Surgical technique and preparation of tissue samples
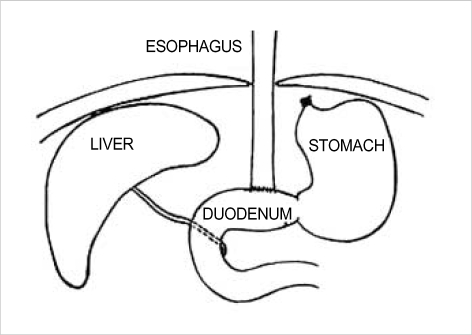
Solid food was withdrawn for 24 hr and water for 12 hr before surgery. Anesthesia was induced and maintained with isoflurane-air mixture. According to the Clark model, esophagoduodenal anastomosis was performed (Fig. 1) (20). In short, a midline laparotomy was performed, and the gastroesophageal junction was identified and mobilized while carefully preserving the vagus nerve. The gastroesophageal junction was ligated, and the distal esophagus was transected 2 mm above the ligature. A total of eight polypropylene 7-0 sutures were placed. Five mm transverse enterostomy was created on the antimesenteric border of duodenum 1 cm distal to pylorus. An end-to-side esophagoduodenstomy was performed. To compensate for blood loss, 1 mL of sterile 0.9% normal saline was instilled in the peritoneal cavity. The abdominal incision was closed in 2 layers and postoperatively the rats were allowed to drink water after 6 hr and were fed on the following day. Immediately after death, the entire esophagus, contiguous anastomotic site and 0.7 cm of duodenal mucosa were removed and the lumen was longitudinally opened (Fig. 2). After snap freezing, a representative sample of duodenum, and upper and lower esophagus was stored at -70℃ for reverse transcription-polymerase chain reaction (RT-PCR). The remaining esophagus including anastomotic site and 0.7 cm of duodenal mucosa was fixed in 10% neutral buffered formalin for 24 hr for histological study and immunohistochemistry.
Fig. 1.
End to side esophagoduodenal anastomosis.
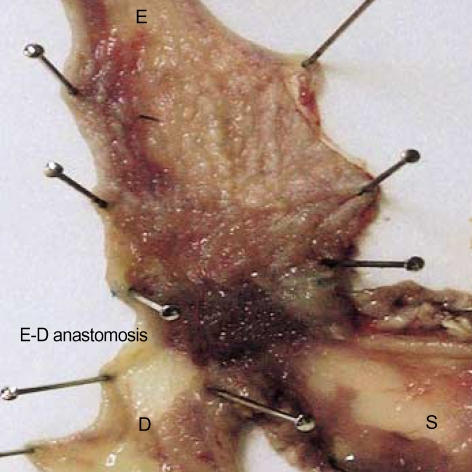
Fig. 2.
Gross finding of esophagoduodenal anastomosis group. Experimental rats show abnormally dilated esophagus, and esophageal inner surface displays whitish nodular patches, which are prominent in lower esophagus. Superficial ulcers are present mainly in lower esophagus. E, esophagus; E-D, anastomosis of esophagoduodenostomy site; S, stomach; D, duodenum.
Pathological analysis
For histologic evaluation, formalin-fixed tissues were embedded in paraffin, cut at 4 µm and stained with H&E. Two pathologists blinded to the experimental groups have assessed all tissues independently. Diagnosis of Barrett's esophagus was made by the presence of columnar metaplasia containing intestinal type goblet cells surrounded by squamous epithelium above the anastomotic site. In addition, Barrett's esophagus was divided into short and long one according to distance from anastomotic site; it was regarded as long one when the specialized intestinal type epithelium was present at a length of >0.5 cm above the anastomotic site. Atypical Barrett's esophagus was diagnosed when glands with atypical features were present both at the superficial and deep portions of the wall.
Immunohistochemistry
Serial sections of 4 µm thickness were made and spread on poly-L-lysine coated slides. Paraffin sections were immersed in three changes of xylene and hydrated using a graded series of alcohol. Antigen retrieval was performed routinely by immersing the sections in 0.01 M citrate buffer (pH 6.0) in a pressure cooker by autoclaving for 15 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 15 min and then incubated with a primary antibody overnight in a humidified chamber at 4℃. Primary antibodies were polyclonal rabbit anti-iNOS (Transduction laboratories, Lexington, KY, U.S.A.) at a dilution of 1:300. Staining was achieved with a DAKO LSAB+kit (DAKO, Santa Barbara, CA, U.S.A.) and developed with diaminobenzidine tetrahydrochloride. Sections were counterstained for 5 min with Meyer's hematoxylin and then mounted. The hepatic tissue of rat in which lipopolysaccharide (Sigma Chemical Co., St. Louis, MO, U.S.A.) was intraperitoneally administered was used as a positive control. As a negative control, rabbit IgG (DAKO) was used instead of primary antibodies. An estimation of immunohistochemical expression of iNOS was evaluated according to both intensity and area of signal: 0, absent; 1, mild: 2, moderate; 3, severe.
RNA extraction and RT-PCR analysis
Total RNA was extracted and purified from frozen tissues using GeneElute Mammalian Total RNA kit (Sigma Chemical Co.) acccording to manufacturer's instructions. The RNA was quantified by determining absorbance at 260 nm. Two µg of total RNA from each sample was reverse transcribed into cDNA using Superscript II reverse transcriptase (Life Technologies, Inc., Rockville, MD, U.S.A.) and random hexamer primers (Takara, Shiga, Japan). The PCR primers were as follows: glyceraldehydes 3-phosphate dehydrogenase (GAPDH) (311 bp), sense 5'-GAA CGG GAA GCT CAC TGG CAT GGC-3', antisense 5'-TGA GGT CCA CCA CCC TGT TGC TG-3'; iNOS (397 bp), sense 5'-CCA CAA TAG TAC AAT ACT ACT TGG-3', antisense 5'-ACG AGG TGT TCA GCG TGC TCC ACG-3'. After denaturation at 95℃ for 5 min, cDNA amplification was carried out by running 32 cycles of denaturation at 94℃ for 1 min, annealing at 58℃ for 45 sec and extension at 72℃ for 45 sec, followed by further incubation at 72℃ for 7 min. The PCR products were electrophoresed on 1.5% agarose gel containing ethidium bromide and then photographed under UV light.
Statistical analysis
The significance of differences between groups was evaluated by using chi-square test. Differences were considered to be statistically significant at p<0.05.
RESULTS
Experimental rats showed abnormally dilated esophagus, and esophageal inner surface displayed whitish nodular patches, which were prominent in lower esophagus. Superficial ulcers were present mainly in lower esophagus (Fig. 2). All these macroscopic findings were present in all rats except control.
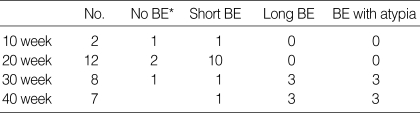
We evaluated the histologic change in the lower esophagus of experimental and control rats. Barrett's esophagus did not occur in control rats. In contrast, 86% of experimental rats showed glandular metaplasia including goblet cells above esophagoduodenal junction. As shown in Table 1, long and atypical Barrett's esophagus were restricted to rats exposed to duodenal contents reflux for 30 and 40 weeks. The glandular metaplasia was thought to have originated from the lower esophagus because all lesions were above the esophageal anastomosis and had an intact esophageal muscularis propria layer on histology.
Table 1.
Incidence of glandular lesions of esophagoduodenal junction in experimental rats
*BE, Barrett's esophagus.
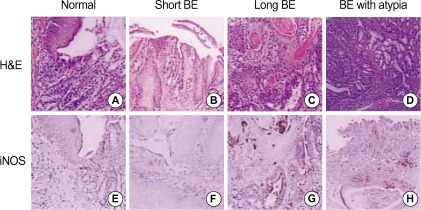
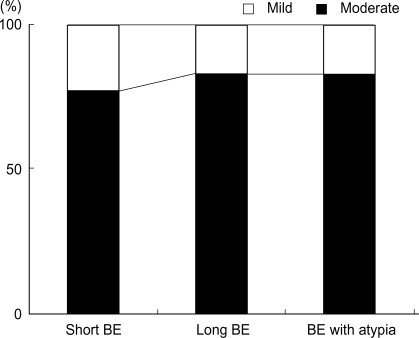
We have performed immunohistochemical staining in order to examine the expression and immunolocalization of iNOS. As shown in Fig. 3, iNOS expression was hardly detectable in either epithelial or stromal cells of control rats. In contrast, iNOS immunoreactivity was clearly observed in the epithelial cells of Barrett's esophagus, predominantly at the apical surface of epithelial cells. Cytoplasmic staining was also seen only in atypical Barrett's esophagus. We compared iNOS expression according to Barrett's esophagus types. As shown in Fig. 4, type did not influence iNOS expression (p>0.05).
Fig. 3.
Histology and immunohistochemical staining of esophagogastric junction and Barrett's esophagus. Histology (A-D) and immunohistochemical staining (E-H, iNOS) of control esophagogastric junction (A, E) and Barrett's esophagus (B-D, F-H). Esophagogastric junction of control rats did not show iNOS expression (E). Short (F) and long (G) Barrett's esophagus revealed iNOS expression in apical surface of epithelial cells. Atypical Barrett's esophagus (H) revealed immunolocalization of iNOS in the cytoplasm of epithelial cells. Magnification: A, E, F ×400; B, C, G ×200; D, H ×100.
Fig. 4.
iNOS expression level according to Barrett's esophagus type by immunohistochemical staining. There is no significant difference (p>0.05).
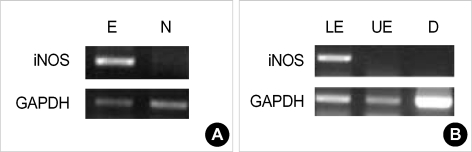
To confirm the immunohistochemical staining, we performed RT-PCR. iNOS mRNA was detected in the lower esophagus of experimental rats and not in that of control rats (Fig. 5A). To determine which sites express iNOS in experimental rats, RT-PCR analysis was performed in duodenum, and upper and lower esophagus. iNOS mRNA was expressed only in lower esophageal portion (Fig. 5B).
Fig. 5.
(A) iNOS mRNA in the lower esophageal tissue of experimental rats (E) and normal one (N). (B) iNOS mRNA in the lower (LE) and upper (UE) esophagus, and duodenum (D) of experimental rats.
DISCUSSION
Eighty six percent (25/29) of rats undergoing esophagoduodenal anastomosis have developed Barrett's esophagus, but definite adenocarcinoma did not occur. This finding may be explained by the experiment of Chen and Yang (21). They have reported that iron supplementation promoted the formation of esophageal adenocarcinoma originated from Barrett's esophagus induced by surgical techniques of esophagoduodenal or esophagogastroduodenal anastomosis (21).
The present study demonstrates that Barrett's esophagus induced by chronic duodenal contents reflux expresses iNOS. Two previous studies reported that iNOS expression was increased in Barrett's esophagus-associated neoplastic progression in human and rat (10, 12). Aberrant iNOS expression may be one of the phenotypical changes in gene expression associated with carcinogenesis of esophageal adenocarcinoma. However, the biological significance of increased iNOS in gastrointestinal carcinogenesis is difficult to specify. It has been reported earlier that iNOS is expressed to an appreciable extent in the epithelium of the normal human colon and that levels are in fact reduced in colonic neoplasms (22, 23). A recent study demonstrated that lack of iNOS promoted intestinal tumorigenesis in the ApcMin/+ mice (24). In contrast, Ahn and Ohshima reported that administration of iNOS inhibitors resulted in significant decrease in adenoma development in the small bowel in the ApcMin/+ mice, and iNOS gene knockout ApcMin/+ mice developed fewer adenomas in both small and large bowels than in wild type mice (25). Takahashi et al. also found that iNOS expression was increased in rat colon tumors induced by azoxymethane (11). Therefore, it may be useful in clarifying the biological role of iNOS expression on Barrett's esophagus-associated adenocarcinoma to study the effect of iNOS inhibitor on animal esophageal adenocarcinoma model.
We have observed over-expression of COX-2, microsomal prostaglandin synthase-1 and EP receptors in this model (18). Recently, PGE2 mediates up-regulation of iNOS in murine breast cancer cell line through EP4 receptors activation (26). NO has been also found to enhance the activity and expression of COX-2 (27, 28). Use of COX-2 inhibitors resulted in a reduction of the development of esophageal adenocarcinoma induced by duodenal reflux (29). These findings suggest that cross talk between NO and COX pathway may play an important role in Barrett's esophagus-associated neoplastic progression. In Barrett's esophagus, iNOS expression was observed at the cytoplasmic area of stromal and epithelial cells (10, 12). In contrast, our study revealed characteristic localization at the apical surface of epithelial cells. This result is in agreement with immunolocalization of iNOS in rat colon tumors induced by azoxymethane (11). Its significance remains to be determined.
ACKNOWLEDGMENT
The present study was supported by research fund from Dongguk University.
References
- 1.Schmidt HH, Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 2.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lala PK, Orucevic A. Role of nitric oxide in tumor progression: Lessons from experimental tumors. Cancer Metastasis Rev. 1998;17:91–106. doi: 10.1023/a:1005960822365. [DOI] [PubMed] [Google Scholar]
- 4.Nathan C, Xie Q. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 5.Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 6.Felley-Bosco E. Role of nitric oxide in genotoxicity: implication for carcinogenesis. Cancer Metastasis Rev. 1998;17:25–37. doi: 10.1023/a:1005948420548. [DOI] [PubMed] [Google Scholar]
- 7.Rachmilewitz D, Karmeli F, Eliakim R, Stalnikowicz R, Ackerman Z, Amir G, Stamler JS. Enhanced gastric nitric oxide synthase activity in duodenal ulcer patients. Gut. 1994;35:1394–1397. doi: 10.1136/gut.35.10.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer II, Kawaka DW, Scott S, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 9.Haswell-Elkins MR, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, Mairiang P, Saitoh M, Yongvanit P, Elkins DB. Liver fluke infection and cholangiocarcinoma: model of endogenous nitric oxide and extragastric nitrosation in human carcinogenesis. Mutat Res. 1994;305:241–252. doi: 10.1016/0027-5107(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 10.Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929–2934. [PubMed] [Google Scholar]
- 11.Takahashi M, Fukuda K, Ohata T, Sugimura T, Wakabayashi K. Increased expression of inducible and endothelial constitutive nitric oxide synthases in rat colon tumors induced by azoxymethane. Cancer Res. 1997;57:1233–1237. [PubMed] [Google Scholar]
- 12.Goldstein SR, Yang GY, Chen X, Curtis SK, Yang CS. Studies of iron deposits, inducible nitric oxide synthase and nitrotyrosine in a rat model for esophageal adenocarcinoma. Carcinogenesis. 1998;19:1445–1449. doi: 10.1093/carcin/19.8.1445. [DOI] [PubMed] [Google Scholar]
- 13.Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, Hagen JA. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus and gastric juice alone. Ann Surg. 1995;222:525–533. doi: 10.1097/00000658-199522240-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillen P, Keeling P, Byrne PJ, Healy M, O'Moore RR, Hennessy TP. Implication of duodenogastric reflux in the pathogenesis of Barrett's esophagus. Br J Surg. 1988;75:540–543. doi: 10.1002/bjs.1800750612. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein SR, Yang GY, Curtis SK, Reuhl KR, Liu BC, Mirvish SS, Newmark HL, Yang CS. Development of esophageal metaplasia and adenocarcinoma in a rat surgical model without the use of a carcinogen. Carcinogenesis. 1997;18:2265–2270. doi: 10.1093/carcin/18.11.2265. [DOI] [PubMed] [Google Scholar]
- 16.Miwa K, Sahara H, Segawa M, Kinami S, Sato T, Miyazaki I, Hattori T. Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. Int J Cancer. 1996;67:269–274. doi: 10.1002/(SICI)1097-0215(19960717)67:2<269::AID-IJC19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Pera M, Brito MJ, Poulsom R, Riera E, Grande L, Hanby A, Wright NA. Duodenal-content reflux esophagitis induces the development of glandular metaplasia and adenosquamous carcinoma in rats. Carcinogenesis. 2000;21:1587–1591. [PubMed] [Google Scholar]
- 18.Jang TJ, Min SK, Bae JD, Jung KH, Lee JI, Kim JR, Ahn WS. Expression of cyclooxygenase-2, microsomal prostaglandin E synthase-1 and EP receptors is increased in rat esophageal squamous cell dysplasia and Barrett's metaplasia induced by duodenal contents reflux. Gut. 2004;53:27–33. doi: 10.1136/gut.53.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Rosa M, Ialenti A, Ianaro A, Sautebin L. Interaction between nitric oxide and cyclooxygenase (COX) pathways. Prostaglandins Leukot Essent Fatty Acids. 1996;54:229–238. doi: 10.1016/s0952-3278(96)90053-8. [DOI] [PubMed] [Google Scholar]
- 20.Clark GW, Smyrk TC, Mirvish SS, Anselmino M, Yamashita Y, Hinder RA, DeMeester TR, Birt DF. Effect of gastroduodenal juice and dietary fat on the development of Barrett's esophagus and esophageal neoplasia: an experimental rat model. Ann Surg Oncol. 1994;1:252–261. doi: 10.1007/BF02303531. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Yang CS. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis. 2001;22:1119–1129. doi: 10.1093/carcin/22.8.1119. [DOI] [PubMed] [Google Scholar]
- 22.Chhatwal VJ, Nagoi SS, Chan ST, Chia YW, Moochhala SM. Aberrant expression of nitric oxide synthase in human polyps, neoplastic colonic mucosa and surrounding peritumoral normal mucosa. Carcinogenesis. 1994;15:2081–2085. doi: 10.1093/carcin/15.10.2081. [DOI] [PubMed] [Google Scholar]
- 23.Moochhala S, Chhatwal VJ, Chan ST, Ngoi SS, Chia YW, Rauff A. Nitric oxide synthase activity and expression in human colorectal cancer. Carcinogenesis. 1996;17:1171–1174. doi: 10.1093/carcin/17.5.1171. [DOI] [PubMed] [Google Scholar]
- 24.Scott DJ, Hull MA, Cartwright EJ, Lam WK, Tisbury A, Poulsom R, Markham AF, Bonifer C, Coletta PL. Lack of inducible nitric oxide synthase promotes intestinal tumorigenesis in the ApcMin/+ mouse. Gastroenterology. 2001;121:889–899. doi: 10.1053/gast.2001.27994. [DOI] [PubMed] [Google Scholar]
- 25.Ahn B, Ohshima H. Suppression of intestinal polyposis in ApcMin/+ mice by inhibiting nitric oxide production. Cancer Res. 2001;61:8357–8360. [PubMed] [Google Scholar]
- 26.Timoshenko AV, Lala PK, Chakraborty C. PGE2 mediated upregulation of iNOS in murine breast cancer cells through the activation of EP4 receptors. Int J Cancer. 2004;108:384–389. doi: 10.1002/ijc.11575. [DOI] [PubMed] [Google Scholar]
- 27.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvemini D, Seibert K, Masferrer JL, Misko TP, Currie MG, Needleman P. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J Clin Invest. 1994;93:1940–1947. doi: 10.1172/JCI117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, Krishnadath KK, Lutzke LS, Burgart LJ. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology. 2002;122:1101–1112. doi: 10.1053/gast.2002.32371. [DOI] [PubMed] [Google Scholar]