Abstract
This study was done to evaluate changes of microvascular function under cold stimulation by measuring coronary flow velocities (CFVs) in vasospastic angina (VA) patients using transthoracic Doppler echocardiography (TTDE). 14 patients with VA and 15 healthy controls were included. CFVs were measured at the distal left anterior descending coronary artery by TTDE at baseline and under cold stimulation. Hyperemia was induced by intravenous adenosine infusion (140 µg/kg/min). At baseline, CFVs and coronary flow reserve (CFR) were not different between controls and VA patients. Under cold stimulation, the degree of increment of CFV with adenosine was lower in VA patients than in controls. Comparing baseline with cold stimulation, coronary flow reserve (CFR) increased (3.1±0.7 to 3.8±1.0, p=0.06) in controls. In contrast, in VA patients, CFR was decreased (2.8±0.9 to 2.6±0.7, p=0.05) and coronary vascular resistance index markedly increased (0.35 to 0.43, p=0.01). Throughout the study, no patient experienced chest pain or ECG changes. In VA patients, CFR was preserved at baseline, but coronary blood flow increase in response to cold stimulation was blunted and CFR was decreased. These findings suggest that endothelial dependent vasodilation is impaired at the coronary microvascular and the epicardial artery level in VA under cold stimulation.
Keywords: Angina Pectoris, Variant; Coronary Vasospasm; Cold Stimulation; Regional Blood Flow; Microvascular Function; Echocardiography, Doppler
INTRODUCTION
Vasospastic angina (VA) is characterized by chest pain at rest with circadian variation (1-3). It is caused by coronary vasospasm and can be diagnosed by the provocation of epicardial coronary constriction after the infusion of acetylcholine or ergonovine (4-6). VA is relatively common in East Asians and is frequently observed in normal coronary arteries (7, 8). Moreover, it has been reported that the prevalence of Raynaud's phenomena or migraine in VA patients is relatively high (9-11). These findings suggest that VA may be a manifestation of generalized smooth muscle contractile disorder, and that an abnormal contractile response in the coronary microcirculation may also be present. An abnormal endothelial dependent vascular tone is regarded to be a main pathogenetic mechanism in VA (12-14). One of physiologic stimuli used to evaluate endothelial function is the cold pressor test (15). The response of coronary blood flow to cold stimulation in a patient with endothelial dysfunction was reported to be impaired (16). However, changes in coronary blood flow and microvascular function under cold stimulation in VA have not been assessed.
Accordingly, the aims of this study were to evaluate microvascular function in VA at room temperature, and to assess whether microvascular dysfunction can be induced by cold stimulation by measuring coronary flow velocities with transthoracic Doppler echocardiography (TTDE).
MATERIALS AND METHODS
Study population
We studied 14 consecutive patients with chest pain at rest. All of patients had angiographically normal coronary arteries and showed documented coronary spasm associated with chest pain or electrocardiographic (ECG) changes after intracoronary infusion of acetylcholine, as reported previously (17). Fifteen healthy subjects were included as a control group. Six patients in the control group received coronary angiography and did not have significant coronary artery stenosis. There were no symptoms or signs of heart disease in the remaining 9 control subjects. ECG and echocardiography were normal in these patients. No subject had valvular heart disease, idiopathic dilated or hypertropic cardiomyopathy, significant arrhythmia, decreased left ventricular function (ejection fraction <50%) or chronic renal failure.
Study protocol
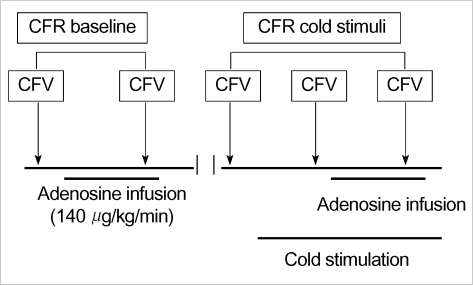
All subjects refrained from caffeine-containing beverages, alcohol and smoking for least 24 hr before the study. All vasoactive medications were also discontinued for 24 hr prior to the study. Coronary flow velocity (CFV) was measured using a high resolution ultrasound system (Sequoir C256 Acuson, Mountain view, CA, U.S.A.), with a 5-MHz transducer, via a transthoracic approach, in the distal left anterior descending coronary artery. Hyperemia was induced by intravenous adenosine infusion (140 µg/kg/min) for 2 min and cold stimulation was performed by immersing both hands into ice water for 2 min. A diagram of the study protocol is shown in Fig. 1. Briefly, CFV was recorded at rest and intravenous adenosine infusion before cold stimulation as baseline. After performing baseline measurements, ten minutes was allowed to allow the hemodynamic condition to fully recover to the resting state, cold stimulation by immersing both hand into ice water was started. CFV before and after adenosine infusion were recorded again under cold stimulation. Throughout the study, ECG was monitored continuously. Blood pressure and heart rate were checked simultaneously with CFV measurements.
Fig. 1.
Study protocol. CFR, coronary flow reserve; CFV, coronary blood flow velocity.
Coronary flow reserve (CFR) was calculated as the ratio of hyperemic to resting mean diastolic coronary flow velocity. The rate-pressure product (RPP) was defined as the product of heart rate and systolic blood pressure.
Coronary vascular resistance index (CVRI) was calculated as follows; [mean arterial pressure at peak blood flow velocity/hyperemic diastolic velocity]÷[mean arterial pressure at basal flow velocity/basal peak diastolic velocity] (18).
Statistical analysis
For descriptive purposes, all data are presented as mean±SD and relative frequencies, as indicated. Absolute changes of CFV induced by adenosine and cold stimulation were analyzed using Wilcoxon sign rank test and Mann-Whitney test. A p-value of <0.05 was considered statistically significant.
RESULTS
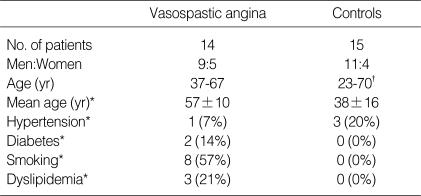
Table 1 summarizes the baseline characteristics of the study population. VA patients were older than the controls (57±10 yr vs. 38±16 yr; p=0.001). The incidences of associated conditions (diabetes mellitus, smoking and dyslipidemia) were higher in VA patients than in controls, except hypertension (7% vs. 20%; p<0.05).
Table 1.
Clinical characteristics of study populations
*p<0.05, Vasospastic angina vs. Controls. †included 9-healthy young volunteers.
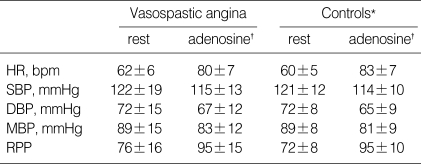
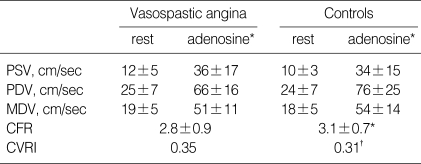
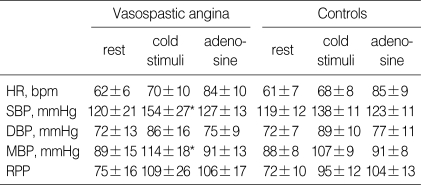
In all subjects, the intravenous infusion of adenosine induced significant increases in heart rate (HR) and RPP and decreases in blood pressure. Hemodynamic responses to adenosine were similar in both groups (Table 2). As shown in Table 3, baseline and hyperemic mean diastolic velocities of coronary flow were similar in VA patients and controls. As a result, CFR and CVRI were also similar in the two groups (CFR, 2.8± 0.9 vs. 3.1±0.7, p=0.21; CVRI, 0.35 vs. 0.31, p=0.08).
Table 2.
The hemodynamic changes by adenosine infusion at baseline
*p=not significant, Vasospastic angina vs. Controls, †p<0.05, rest vs. adenosine in each group. HR, heart rate; bpm, beats po2 minute; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; RPP, rate pressure product (mmHg/min/100).
Table 3.
Coronary blood flow by adenosine infusion at baseline
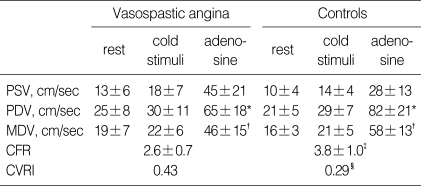
*p=not significant, †p=0.08, Vasospastic angina vs. Controls. PSV, peak systolic velocity; PDV, peak diastolic velocity; MDV, mean diastolic velocity; CFR, coronary flow reserve; CVRI, coronarv vascular resistance index.
Under cold stimulation, heart rate, blood pressure, and RPP increased in both groups. In particular, increases in blood pressure and RPP were prominent in the VA group (Table 4). Coronary flow velocities were increased by cold stimulation in both groups, increases were lower in the VA group (p=0.03, Table 5).
Table 4.
The hemodynamic changes to cold stimulation and adenosine
*p<0.05, Vasospastic angina vs. Controls. HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; RPP, rate pressure product (mmHg/min/100).
Table 5.
Coronary blood flow by adenosine infusion under cold stimulation
*p, †p, ‡p, §p<0.05, Vasospastic angina vs. Controls. PSV, peak systolic velocity; PDV, peak diastolic velocity; MDV, mean diastolic velocity (cm/sec); CFR, coronary flow reserve; CVRI, coronary vascular resistance index.
Hemodynamic responses to adenosine during cold stimulation were similar in the two groups (Table 4). Unlike baseline response, hyperemic response to adenosine was blunted in the VA group under cold stimulation. In VA, the increase in diastolic coronary velocity was lower than in the controls, resulting in a lower CFR and a higher CVRI than for the controls (Table 5).
In summary, at room temperature, coronary flow velocity and CFR were similar in VA and control groups. Under cold stimulation, compared with baseline, CFR was marginally increased in controls (3.1±0.7 to 3.8±1.0, p=0.06). In contrast, in the VA group, CFR decreased (2.8±0.9 to 2.6±0.7, p=0.05) and CVRI increased (0.35 to 0.43, p=0.01).
DISCUSSION
The present study shows the characteristics of coronary blood flow in response to cold stimulation in VA patients by direct measurements of coronary blood flow velocities using TTDE.
Patients with angina caused by epicardial coronary artery spasm are diagnosed as either having "vasospastic angina" or "variant angina" (6). Coronary artery spasm may produce diffuse or focal arterial narrowing and be a manifestation of a generalized smooth muscle contractile disorder (9, 10, 19). However, whether functional abnormality of the coronary microvascular circulation is present in vasosapstic angina is controversial (20, 21). In our study, as the coronary flow reserve was similar to that of controls at baseline, the microvascular function of VA appeared normal. This finding is agreement with a recent study by Sueda et al. (20). They reported no difference in CFR in patients with acetylcholine induced spasm between the spasm positive and spasm negative vessels, and maintained normal in vessel with spasm as control.
There is also some controversy on the status of coronary vasomotor tone. Kasai et al. (22) reported that the basal coronary artery tone of controls and VA patients does not differ significantly. However, Hoshio et al. (23) reported that coronary artery tone is significantly higher in VA patients than in control. In our study, the coronary vascular resistance index of VA patients at room temperature did not differ from that of the controls (0.35 vs. 0.31, p=0.08), but, as the p-value was 0.08, it is possible that CVRI might have been higher VA, if the study population had been larger.
The cold pressor test is recognized as a means of evaluating endothelial function and the functional integrity of the vascular wall (24, 25). Normally, cold stimulation induces the sympathetic release of norepinephrine and epinephrine and increases the heart rate, arterial blood pressure, and myocardial oxygen demand. This increase in myocardial metabolic demand has been shown to dilate epicardial arteries and increase coronary blood flow. Moreover, this was found to be mediated through the β-adrenoreceptor by direct nitric oxide synthesis stimulation and through the flow-dependent release of endothelial-derived nitric oxide, despite α-adrenergic coronary artery constriction by the direct stimulation of smooth muscle cells (24, 26, 27). However, it is known that coronary dilatation by cold stimulation is impaired in patients with endothelial dysfunction such as hypertension or diabetes mellitus (15, 28). Infrequently, cold stimulation may cause coronary vasospasm in VA, but the reported sensitivity is about 2 to 10%, which is too low to be clinically significant in the diagnosis of vasosapstic angina (29, 30). Our VA patients did not have chest pain, and did not show ECG changes, left ventricular wall motion abnormality on TTDE, or epicardial coronary artery vasospasm on coronary angiography during cold stimulation.
In this study, coronary blood flow incrementation by cold stimulation was lower in VA patients than in controls, and CFR decreased and CVRI increased in VA under cold stimulation. These findings suggest that sympathetic responses and microvascular integrity are different between VA patients and controls under cold stimulation. Constitutive nitric oxide synthase in the arterial endothelium continuously generates nitric oxide, which has been shown (31) to maintain basal vascular tone in animals and humans. Kugiyama et al. (32) recently showed that there is a deficiency in endothelial nitric oxide bioactivity at the sites of coronary artery spasm, and that this deficiency may play an important role in the pathogenesis of coronary spasm. Our data suggests that flow mediated endothelial dependent vasodilation was impaired at the coronary microvascular level in VA by cold stimulation.
There are several limitations in our study. First, the study population was small to derive definite conclusions. Second, the VA patients were older than the controls. Third, changes in epicardial coronary artery diameters under cold stimulation were measured on coronary angiography only in 8 patients (3 VA patients and 5 controls). However, none of these patients showed coronary artery diameter changes by cold stimulation alone. In remaining study population, no patient experienced chest pain or ECG changes under cold stimuation.
In conclusion, CFR is preserved at room temperature in vasospastic angina, but, compared to controls, increases in coronary blood flow in response to cold stimulation are blunted and CFR is decreased. These findings suggest that endothelial dependent vasodilation is impaired at the coronary microvascular and epicardial artery levels in vasospastic angina under cold stimulation.
Table 6.
Comparison of CFR and CVRI between baseline and cold stimulation
*p<0.05, at baseline vs. under cold stimuli in controls. †p, ‡p<0.05, at baseline vs. under cold stimulation in vasospastic angina; CFR, coronary flow reserve; CVRI, coronary vascular resistance index.
References
- 1.Yasue H, Kugiyama K. Coronary spasm:clinical features and pathogenesis. Intern Med. 1997;36:760–765. doi: 10.2169/internalmedicine.36.760. [DOI] [PubMed] [Google Scholar]
- 2.Yasue H, Touyama M, Shimamoto M, Kato H, Tanaka S. Role of autonomic nervous system in the pathogenesis of Prinzmetal variant form of angina. Circulation. 1974;50:534–539. doi: 10.1161/01.cir.50.3.534. [DOI] [PubMed] [Google Scholar]
- 3.Yasue H, Omore S, Takizawa A, Nagao M, Miwa K, Tanaka S. Circardian variation of exercise capacity in patients with Prinzmetal's variant angina; role of exercise induced coronary arterial spasm. Circulation. 1979;59:938–948. doi: 10.1161/01.cir.59.5.938. [DOI] [PubMed] [Google Scholar]
- 4.Okumura K, Yasue H, Matsuyama K, Goto K, Miyagi H, Ogawa H, Matsuyama K. Sensitivity and specificity of intracoronary injection of acetylcholine for the induction of coronary artery spasm. J Am Coll Cardiol. 1988;12:883–888. doi: 10.1016/0735-1097(88)90449-4. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki Y, Tokunaga S, Ikeguchi S, Miki S, Iwase T, Tomita T, Murakami T, Kawai C. Induction of coronary artery spasm by intracoronary acetylcholine: comparison with intracoronary ergonovine. Am Heart J. 1992;124:39–47. doi: 10.1016/0002-8703(92)90918-l. [DOI] [PubMed] [Google Scholar]
- 6.Maseri A, Chierchia S. Coronary artery spasm: demonstration, definition, diagnosis, and consequences. Prog Cardiovasc Dis. 1982;25:169–192. doi: 10.1016/0033-0620(82)90015-9. [DOI] [PubMed] [Google Scholar]
- 7.Nobuyoshi M, Abe M, Nosaka H, Kimura T, Yokoi H, Hamasaki N, Shindo T, Kimura K, Nakamura T, Nakagawa Y, Shiode N, Sakamoto A, Kakura H, Iwasaki Y, Kim K, Kitaguchi S. Statistical analysis of clinical risk factors for coronary artery spasm: identification of the most important determinant. Am Heart J. 1992;124:32–38. doi: 10.1016/0002-8703(92)90917-k. [DOI] [PubMed] [Google Scholar]
- 8.Sugiishi M, Takatsu F. Cigarrette smoking is a major risk factor for coronary spasm. Circulation. 1993;87:76–79. doi: 10.1161/01.cir.87.1.76. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Shinozaki N, Hirasawa M, Kato R, Shiraishi K, Kida H, Usuda K, Ishikawa T. Prevalence of migraine and Raynaud's phenomenon in Japanese patients with vasospastic angina. Jpn Circ J. 2000;64:239–242. doi: 10.1253/jcj.64.239. [DOI] [PubMed] [Google Scholar]
- 10.Miller D, Waters DD, Warnica W, Szlachcic J, Kreeft J, Theroux P. Is variant angina the coronary manifestation of a generalized vasospastic disorder? N Engl J Med. 1981;304:763–766. doi: 10.1056/NEJM198103263041306. [DOI] [PubMed] [Google Scholar]
- 11.Cannon RO, 3rd, Cattau EL, Jr, Yakshe PN, Maher K, Schenke WH, Benjamin SB, Epstein SE. Coronary flow reserve, esophageal motility and chest pain in patients with angiographically normal coronary arteries. Am J Med. 1990;88:217–222. doi: 10.1016/0002-9343(90)90145-4. [DOI] [PubMed] [Google Scholar]
- 12.Kiyotaka K, Masamichi O, Takeshi M, Seigo S, Hisao O, Michihiro Y, Yoshito I, Osamu H, Hiriaki K, Hirofumi S, Hirofumi Y. Nitric oxide-mediated flow-dependent dilation is impaired in coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 1997;30:920–926. doi: 10.1016/s0735-1097(97)00236-2. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Tsunoda R, Moriyama Y, Miyao Y, Yoshimura M, Ogawa H, Yasue H. Vitamin E administration improves impairment of endothelium-dependent vasodilation in patients with coronary spastic angina. J Am Coll Cardiol. 1998;32:1672–1679. doi: 10.1016/s0735-1097(98)00447-1. [DOI] [PubMed] [Google Scholar]
- 14.Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77:43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Nitenberg A, Valensi P, Sachs R, Cosson E, Attali JR, Antony I. Prognostic value of epicardial coronary artery constriction to the cold pressor test in type 2 diabetic patients with angiographically normal coronary arteries and no other major coronary risk factors. Diabetes Care. 2004;27:208–215. doi: 10.2337/diacare.27.1.208. [DOI] [PubMed] [Google Scholar]
- 16.Kang SH, Park HK, Lee CW, Kim JJ, Hong MK, Park SW, Park SJ. Impaired flow-mediated vasodilation of epicardial coronary artery in vasospastic angina. J Korean Med Sci. 1998;13:591–596. doi: 10.3346/jkms.1998.13.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasue H, Horio Y, Nakamura N, Fujii H, Imoto N, Sonoda R, Kugiyama K, Obata K, Morikami Y, Kimura T. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986;74:955–963. doi: 10.1161/01.cir.74.5.955. [DOI] [PubMed] [Google Scholar]
- 18.Antony I, Nitenberg A. Coronary vascular reserve is similarly reduced in hypertensive patients without any other coronary risk factors and in normotensive smokers and hypercholesterolemic patients with angiographically normal coronary arteries. Am J Hypertens. 1997;10:181–188. doi: 10.1016/s0895-7061(96)00330-5. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen K, Ravnsbaek J, Funch-Jensen P, Bagger JP. Oesophageal spasm in patients with coronary artery spasm. Lancet. 1986;1:174–176. doi: 10.1016/s0140-6736(86)90651-3. [DOI] [PubMed] [Google Scholar]
- 20.Sueda S, Kohno H, Fukuda H, Uraoka T. Coronary flow reserve in patients with vasospastic angina: correlation between coronary flow reserve and age or duration of angina. Coron Artery Dis. 2003;14:423–429. doi: 10.1097/00019501-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Anzai H, Saijo T, Nakajima R, Tezuka N, Takagi T, Tsunoda T, Kobayashi N, Nakamura S, Yamaguchi T. Evaluation of coronary flow reserve in patients with vasospastic angina. J Cardiol. 2000;36:17–27. [PubMed] [Google Scholar]
- 22.Kasai JC, Tousoulis D, Gavrielides S, McFadden E, Galassi AR, Crea F, Maseri A. Comparison of epicardial coronary artery tone and reactivity in Prinzmetal's variant angina and chronic stable angina pectoris. J Am Coll Cardiol. 1991;17:1058–1062. doi: 10.1016/0735-1097(91)90830-3. [DOI] [PubMed] [Google Scholar]
- 23.Hoshio A, Kotake H, Mashiba H. Significance of coronary artery tone in patients with vasospastic angina. J Am Coll Cardiol. 1989;14:604–609. doi: 10.1016/0735-1097(89)90100-9. [DOI] [PubMed] [Google Scholar]
- 24.Zeiher AM, Drexler H, Wollschlager H, Saurbier B, Just H. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol. 1989;14:1181–1190. doi: 10.1016/0735-1097(89)90414-2. [DOI] [PubMed] [Google Scholar]
- 25.Zeiher AM, Drexler H, Wollschlager H, Just H. Modulation of coronary vasomotor tone in humans: progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 26.Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by cold pressor test. Circulation. 1988;77:43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- 27.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 28.Antony I, Lerebours G, Nitenberg A. Angiotensin-converting enzyme inhibition restores flow-dependent and cold pressor test-induced dilations in coronary arteries of hypertensive patients. Circulation. 1996;94:3115–3122. doi: 10.1161/01.cir.94.12.3115. [DOI] [PubMed] [Google Scholar]
- 29.Dubois-Rande JL, Dupouy P, Aptecar E, Bhatia A, Teiger E, Hittinger L, Berdeaux A, Castaigne A, Geschwind H. Comparison of the effects of exercise and cold pressor test on the vasomotor response of normal and atherosclerotic coronary arteries and their relation to the flow-mediated mechanism. Am J Cardiol. 1995;76:467–473. doi: 10.1016/s0002-9149(99)80132-5. [DOI] [PubMed] [Google Scholar]
- 30.Waters DD, Szlachcic J, Bonan R, Miller DD, Dauwe F, Theroux P. Comparative sensitivity of exercise, cold pressor and ergonovine testing in provoking attacks of variant angina in patients with active disease. Circulation. 1983;67:310–315. doi: 10.1161/01.cir.67.2.310. [DOI] [PubMed] [Google Scholar]
- 31.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 32.Kugiyama K, Yasue H, Okumura K, Ogawa H, Fujimoto K, Nakao K, Yoshimura M, Motoyama T, Inobe Y, Kawano H. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996;94:266–271. doi: 10.1161/01.cir.94.3.266. [DOI] [PubMed] [Google Scholar]