Abstract
Antagonists for spinal N-methyl-D-aspartate (NMDA) and amino-hydroxy-methtyl-isoxazolepropionate (AMPA) receptors are effective in attenuating acute nociception or injury-induced hyperalgesia. The antinociception of spinal gabapentin is developed in injury-induced hyperalgesia without affecting acute nociception. The authors evaluated the effects of intrathecal gabapentin, NMDA antagonist (MK801) and AMPA antagonist (NBQX) in the formalin test which shows injury-induced hyperalgesia as well as acute pain. We further assessed the interactions between gabapentin and either MK801 or NBQX. Male Sprague-Dawley rats were implanted with intrathecal catheters. To evoke pain, 50 µL of 5% formalin solution was injected into the hindpaw. The interaction was investigated by a fixed dose analysis or an isobolographic analysis. MK801 and NBQX suppressed flinching responses during phase 1 of the formalin test, while gabapentin had little effect on phase 1. All three agents decreased the phase 2 flinching response. A fixed dose analysis in phase 1 showed that gabapentin potentiated the antinociceptive effect of MK801 and NBQX. Isobolographic analysis in phase 2 revealed a synergistic interaction after coadministration of gabapentin-MK801 or gabapentin-NBQX. Correspondingly, spinal gabapentin with NMDA or AMPA antagonist may be useful in managing acute pain and injury-induced hyperalgesia.
Keywords: Antinociception; gabapentin; Drug Interactions; Injections, Spinal; MK801 Dizocilpine Maleate; NBQX; 2,3-dioxo-6-nitro-7-sultamoylbenzo (f) quinoxaline
INTRODUCTION
Gabapentin is a novel antiepileptic agent and a structural analog to γ-aminobutyric acid (GABA) (1). Studies of the activity profile of gabapentin have shown that intrathecal gabapentin counteracts injury-induced hyperalgesia, but it does not reduce acute nociception (2-4). Although the specific mechanism of gabapentin's antinociception is not certain, N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methtyl-4-isoxazolepropionate (AMPA) receptors have been suggested as sites of action in the spinal cord (5).
Glutamate, the excitatory amino acid (EAA), is involved in the transmission of nociceptive information in the spinal cord (6). The excitatory effect of glutamate is considered to be mediated through at least two distinct classes of receptors, NMDA and AMPA receptors (7-9). Intrathecal NMDA antagonists have various effects on acute nociception, but they inhibit injury-induced hyperalgesia (10-15). On the other hand, intrathecal AMPA antagonists alleviate acute nociception with different effects on injury-induced hyperalgesia (2, 7, 16).
These observations suggest that spinal gabapentin, NMDA antagonists and AMPA antagonists may or may not be against nociception according to the nociceptive conditions. However, there has been little data regarding the interaction between gabapentin and antagonists for NMDA or AMPA receptors at the spinal level in the formalin test, which is a preclinical pain model showing acute nociception followed by injury-induced hyperalgesia.
The aim of this study was threefold: 1) to examine the effect of intrathecal gabapentin, NMDA antagonist (MK801) and AMPA antagonist (NBQX) in the rat formalin test, 2) to evaluate the consequence of gabapentin on the effect of MK801 or NBQX in acute nociception, and 3) to determine the spinally mediated antinociceptive interactions between gabapentin and MK801 or NBQX in the injury-induced hyperalgesia.
MATERIALS AND METHODS
This work was carried out with permission from the Animal Care Committee of our Research Institute of Medical Science.
Male Sprague-Dawley rats (250-300 g) were used. Rats were housed in group cages and kept in a vivarium, maintained at 22℃ with a 12 hr alternating night/day cycle, and were given water and food ad libitum. For drug administration, intrathecal catheters were advanced caudally 8.5 cm into the subarachnoid space through an incision in the atlantooccipital membrane during enflurane anesthesia (17).
Rats showing postoperative neurologic sequelae were immediately killed with an overdose of volatile anesthetics. After surgery, the rats were kept in individual cages and allowed to recover for 4-5 days.
The following drugs were used in this study: gabapentin (1-[aminomethyl] cyclohexanacetic acid; Sigma Chemical Co., St. Louis, MO, U.S.A.), MK801 (NMDA receptor antagonist; Research Biochemical Internationals [RBI], Natick, U.S.A.) and NBQX (AMPA receptor antagonist; Tocris Cookson Ltd., Bristol, U.K.). Gabapentin and MK801 were dissolved in normal saline. NBQX was dissolved in dimethylsulfoxide (DMSO). Intrathecal administration of these agents was performed using a hand-driven, gear-operated syringe pump. All drugs were given in a volume of 10 µL solution, followed by an additional 10 µL of normal saline to flush the catheter.
The formalin test was used as a nociceptive test (4). 50 µL of 5% formalin solution was injected subcutaneously into the plantar surface of the hindpaw. After formalin injection, rats show a characteristic pain behavior, biphasic flinching/shaking of the injected paw. Such pain behavior was therefore quantified. The interval from 0-9 and 10-60 min after the formalin injection was defined as phase 1 and phase 2 of the formalin test, respectively. Upon completion of the 60 min observation, the rats were immediately killed with an overdose of volatile anesthetics.
Animals were behaviorally tested four to five days after intrathecal catheterization. After acclimation for 15-20 min in a restraint cylinder, rats were then placed into one of the experimental groups. The drug vehicles (saline or DMSO) were used as a control for intrathecal drugs. The rats were only used once.
The first series of experiments were performed to examine the time course and dose-dependency of intrathecal gabapentin (10, 30, 100 and 300 µg), MK801 (3, 10 and 30 µg) and NBQX (0.3, 1 and 3 µg) in the formalin test. Intrathecal drugs were given 10 min before the formalin injection. Each ED50 value (effective dose producing a 50% reduction of control formalin response) of three agents was separately determined in two phases.
To determine the characteristics of interaction between gabapentin and the NMDA antagonist, or between gabapentin and the AMPA antagonist, a fixed dose analysis and an isobolographic analysis were used (18).
A fixed dose analysis was used in phase 1 because intrathecal gabapentin did not produce an antinociceptive effect during phase 1. A fixed dose of gabapentin (300 µg) was intrathecally coadministered with various doses of MK801 or NBQX. An isobolographic analysis was used to define the property of interaction during phase 2. This method is based on comparisons of doses that are determined to be equally effective. First, each ED50 value was determined from the dose-response. Then, the ED50 values of the mixture were calculated from the dose-response curves of the combined drugs and used for plotting the isobologram. In order to understand the magnitude of the interaction, a total fraction value was also calculated.
Total fraction value
=ED50 [(drug 1+drug 2)/drug 1+(drug 2+drug 1)/drug 2]
The fraction values indicate what portion of the single ED50 value was accounted for by the corresponding ED50 value for the combination. Values near 1 indicate an additive interaction, values greater than 1 imply an antagonistic interaction and values less than 1 indicate a synergistic interaction. The mixtures were delivered intrathecally 10 min before the formalin test.
To examine the behavioral changes by gabapentin, MK801 and NBQX, the highest dose of each drug was given intrathecally to the additional rats. Motor function was assessed by the placing-stepping reflex and the righting reflex (14). The first was evoked by drawing the dorsum of either hind paw across the edge of the table. Healthy rats generally try to put the paw ahead into a position for walking. The other was evaluated by placing the rat horizontally with its back on the table. Healthy rats give rise to an immediate and coordinated twisting of the body into an upright position.
Data are expressed with a mean±SEM. The time-response data are presented as the number of flinches. The dose-response data are presented as the total sum of flinches in each phase. To calculate the ED50 values of each drug, the number of flinches was converted into percentage of control according to the following formula:
Dose-response data were analyzed by one-way analysis of variance (ANOVA) with Scheffe for post hoc. The dose-response lines were fitted using least-squares linear regression and ED50 and its 95% confidence intervals were calculated according to the method described by Tallarida and Murray (19).
The difference between theoretical ED50 and experimental ED50 was examined by t-test. A p value less than 0.05 was considered significant.
RESULTS
A biphasic flinching response of the injected paw was observed in all rats after subcutaneous injection of formalin into the hindpaw.
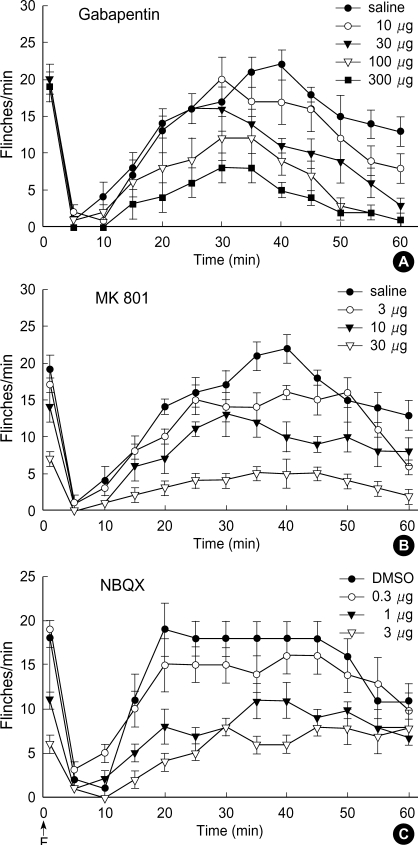
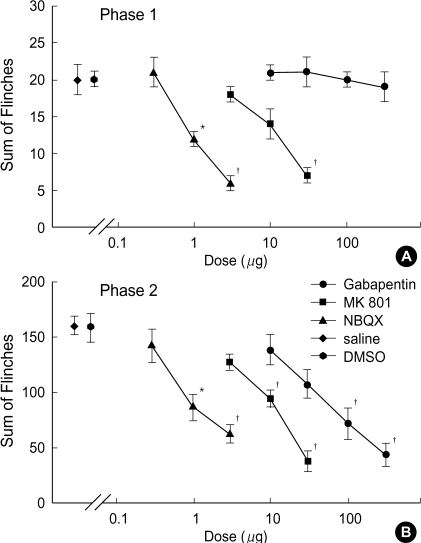
Fig. 1 displays the time course of intrathecal gabapentin, MK801 and NBQX in the formalin test. The sum of the number of flinches in the control group did not differ statistically in either phase (saline vs. DMSO: 20±2 vs. 20±1 in phase 1,160±8 vs. 158±13 in phase 2). Intrathecal MK801 and NBQX, but not gabapentin, produced a dose-dependent reduction of flinching response during phase 1 (Fig. 2A). During phase 2, all three drugs produced a dose-dependent suppression of flinching response (Fig. 2B).
Fig. 1.
Time effect curve of intrathecal gabapentin (A), MK801 (B) and NBQX (C) for flinching response in the formalin test. Each drug was administered 10 min before formalin injection (F). Data are presented as the number of flinches. Each line represents the mean±SEM of 6-8 rats.
Fig. 2.
Dose response curve of intrathecal gabapentin, MK801 and NBQX for flinching response during phase 1 (A) and phase 2 (B) in the formalin test. Data are presented as the total sum of flinches in each phase. Gabapentin reduces flinches in phase 2 but not in phase 1. MK801 and NBQX produce a dose-dependent inhibition of flinches in both phases. Each line represents the mean±SEM of 6-8 rats. Compared with saline or DMSO, *p<0.01, †p<0.001.
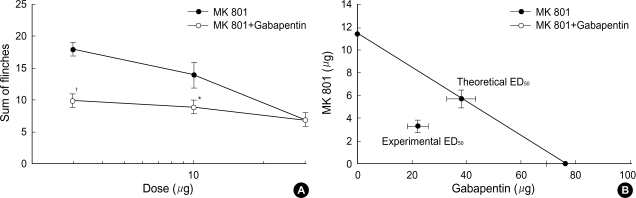
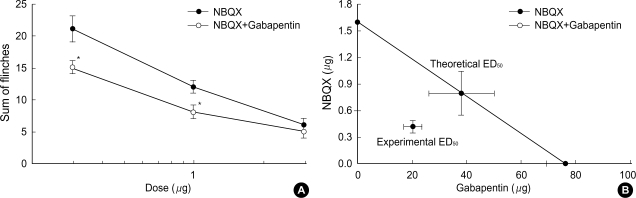
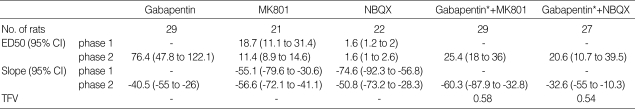
Intrathecal coadministration of MK801 and NBQX with a fixed dose of gabapentin in phase 1 increased the antinociceptive effect of MK801 and NBQX (Fig. 3A, 4A). Isobolographic analysis revealed a synergistic interaction after the concurrent delivery of mixtures of gabapentin-MK801 and gabapentin-NBQX during phase 2 in the formalin test. These experimental ED50 values were significantly lower than those of the theoretical ED50 values (Fig. 3B, 4B) with a total fraction value of less than 1, indicating a synergistic interaction (Table 1).
Fig. 3.
A fixed dose analysis (A) for the interaction between intrathecal gabapentin (300 µg) and MK 801 during phase 1 and isobologram (B) for the interaction between gabapentin and MK 801 during phase 2 in the formalin test. Data for a fixed dose analysis are presented as the sum of flinches. At a fixed dose analysis, addition of gabapentin to MK 801 was significantly different from that of MK 801 alone, which means that gabapentin increased the antinociception of MK 801. At an isobologram, the ED50 values for each agent are plotted on the x- and y-axes, respectively, and the thick lines represent the S.E.M. of the ED50. The straight line connecting each ED50 value is the theoretical additive line and the point on this line is the theoretical additive ED50. The experimental ED50 point was significantly different from the theoretical ED50 point, indicating a synergistic interaction. Each line represents the mean±SEM of 5-8 rats. Compared with MK 801, *p<0.01, †p<0.001.
Fig. 4.
A fixed dose analysis (A) for the interaction between intrathecal gabapentin (300 µg) and NBQX during phase 1 and isobologram (B) for the interaction between gabapentin and NBQX during phase 2 in the formalin test. Data for a fixed dose analysis are presented as the sum of flinches. At a fixed dose analysis, addition of gabapentin to NBQX was significantly different from that of NBQX alone, which means that gabapentin increased the antinociception of NBQX. At an isobologram, the ED50 values for each agent are plotted on the x- and y-axes, respectively, and the thick lines represent the S.E.M. of the ED50. The straight line connecting each ED50 value is the theoretical additive line and the point on this line is the theoretical additive ED50. The experimental ED50 point was significantly different from the theoretical ED50 point, indicating a synergistic interaction. Each line represents the mean±SEM of 5-7 rats. Compared with NBQX, *p<0.05.
Table 1.
ED50 (µg), slope and total fraction value (TFV) of intrathecal agents in the formalin test
ED50, effective dose producing a 50% reduction of control formalin response; CI, confidence intervals.
*These values imply the ED50 values of gabapentin in the mixtures of gabapentin-MK801 and gabapentin-NBQX.
Intrathecal gabapentin, MK801 and NBQX did not cause any change of motor tone.
DISCUSSION
In the current work, intrathecal gabapentin was active only against the phase 2 flinching response, which implicates that gabapentin may be effective in attenuating the injury-induced hyperalgesia without affecting acute nociception at the spinal level. These results were consistent with those of previous experiments (2-4). Although the antinociceptive mechanisms of gabapentin remains unclear, several targets have been proposed. It has been reported that gabapentin increases the concentration, the rate of synthesis and the release of GABA (1). But intrathecal GABAA or GABAB receptor antagonists failed to reverse the antinociception of gabapentin (5). On the other hand, NMDA and AMPA receptors were reported to be involved in gabapentin-induced antinociception (5). Additionally, gabapentin has been shown to bind specifically to the α2δ subunit of voltage-sensitive calcium channels (20).
In this study, intrathecal MK801 and NBQX resulted in a dose-dependent inhibition of flinching response in both phases. These observations suggest that NMDA receptors and AMPA receptors may be active in the modulation of the injury-induced hyperalgesia as well as acute nociception at the spinal level.
EAAs, such as glutamate and aspartate, may play an important role in nociceptive transmission in the dorsal horn of the spinal cord (6). These EAAs have been considered to facilitate spinal sensory transmission and contribute to the enhanced excitability of dorsal horn neurons through NMDA receptors and non-NMDA receptors (8, 9). The NMDA receptors exist in the substantia gelatinosa of the dorsal horn and are postsynaptic to an interneuron mediating an excitation which is responsible for the spinal nociceptive processing, including injury-induced hyperalgesia (21-23). Thus, NMDA antagonists may attenuate the noxious inputs in a tonically active state such as the phase 2 response of the formalin test. On the other hand, AMPA receptors are present in the superficial lamina of the dorsal horn and mediate excitatory transmission involving acute nociceptive inputs (24, 25). Hence, AMPA antagonists may suppress acute excitation induced by high intensity stimuli such as the phase 1 response of the formalin test. Interestingly, in this study, spinal NMDA antagonist and AMPA antagonist reduced not only the phase 1 response but also the phase 2 response. These findings suggest that the NMDA receptor and the AMPA receptor may be involved in the regulation of the injury-induced hyperalgesia as well as acute nociception in the spinal cord. The fact that NMDA antagonist blocked the release of substance-P (26) supports the phase 1 antinociception of MK801 observed in this study. Additionally, the phase 2 response seems to result from a continuous afferent input, which is produced in phase 1. Thus, as the phase 1 component of the formalin stimulus is gradually reduced by AMPA antagonist, the phase 2 response might also be decreased. This effect was observed during phase 2 in this study with AMPA antagonist.
Although NMDA antagonist and AMPA antagonist attenuated both acute nociception and injury-induced hyperalgesia, the relative effectiveness of the drugs was noted. In MK801-treated rats, ED50 in phase 2 was lower than that in phase 1. Moreover, the ED50 value of NBQX in phase 1 was similar to that in phase 2. These findings suggest that the NMDA antagonist appears to be much more effective on injury-induced hyperalgesia than on acute nociception. Additionally, AMPA antagonist shows similar effects for acute nociception and injury-induced hyperalgesia. However, the results observed in the present study are both consistent (11, 13, 15) and inconsistent with previous data (7, 10, 12, 14). This discrepancy may be caused by the different types of tested stimuli, kinds and doses of drugs administered and the relative affinity or selectivity of the drugs.
According to a fixed dose analysis performed in phase 1, intrathecal gabapentin increased the antinociceptive effect of MK801 and NBQX. Isobolographic analysis performed in phase 2 indicated that intrathecal gabapentin showed a synergistic effect with MK801 as well as with NBQX. These results illustrate that spinal gabapentin can augment the antinociceptive action of MK801 and NBQX for injury-induced hyperalgesia as well as acute nociception. Previous studies have shown that intrathecal gabapentin reinforced the effects of clonidine or neostigmine for acute nociception (18). A synergistic interaction has been reported between gabapentin and other analgesics, such as clonidine, naproxen and morphine in numerous nociceptive conditions (16, 27, 28). Several mechanisms would be possible for this synergistic interaction. First, drugs may interact by altering the kinetics of each other. One agent may alter the actions of the other agent at the receptor or channel, thereby leading to synergism. Second, functional interaction may result from distinct drug effects at separate anatomic sites that may act independently and together to inhibit spinal nociceptive processing (29). Thus, a decrease in excitatory neurotransmission with MK801 and NBQX accompanied by inhibiting calcium channels with gabapentin in the spinal cord may lead to synergism. This mechanism was observed in the synergistic interaction of gabapentin and non-NMDA antagonist in a neuropathic pain model of rats (30). Third, synergism may stem from the characteristics of action of gabapentin. Gabapentin would promote the activity of inhibitory neurons following inflammatory injuries (31) and thus the combination of gabapentin with MK801 or NBQX may result in a greater reduction of transmission of nociceptive signals, which may develop into synergism.
Altogether, intrathecal MK801 and NBQX, but not gabapentin, reduced the phase 1 flinching response of the formalin test, and all three drugs reduced the phase 2 flinching response. Intrathecal gabapentin also increased the effects of MK801 and NBQX during phase 1 of the formalin test and interacted with MK801 and NBQX in a synergistic fashion during phase 2.
These results suggest that spinal NMDA and AMPA receptors may be involved in the regulation of the injury-induced hyperalgesia as well as acute nociception. Concurrent delivery of gabapentin with either NMDA antagonist or AMPA antagonist may well have virtue in dealing with pain, as it serves to reduce the required dose of each drug
Footnotes
This work was supported in part by Research Institute of Medical Science, Chonnam National University.
References
- 1.Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 2.Kaneko M, Mestre C, Sanchez EH, Hammond DL. Intrathecally administered gabapentin inhibits formalin-evoked nociception and the expression of Fos-like immunoreactivity in the spinal cord of the rat. J Pharmocol Exp Ther. 2000;292:743–751. [PubMed] [Google Scholar]
- 3.Shimoyama N, Shimoyama M, Davis AM, Inturrisi CE, Elliott KJ. Spinal gabapentin is antinociceptive in the rat formalin test. Neurosci Lett. 1997;222:65–67. doi: 10.1016/s0304-3940(97)13331-6. [DOI] [PubMed] [Google Scholar]
- 4.Yoon MH, Yaksh TL. The effect of intrathecal gabapentin on pain behavior and hemodynamics on the formalin test in the rat. Anesth Analg. 1999;89:434–439. doi: 10.1097/00000539-199908000-00034. [DOI] [PubMed] [Google Scholar]
- 5.Yoon MH, Choi JI, Jeong SW. Spinal gabapentin and antinociception: mechanisms of action. J Korean Med Sci. 2003;18:255–261. doi: 10.3346/jkms.2003.18.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolle TR, Berthele A, Schadrack J, Zieglgansberger W. Involvement of glutamatergic neurotransmission and protein kinase C in spinal plasticity and the development of chronic pain. Prog Brain Res. 1996;110:193–206. doi: 10.1016/s0079-6123(08)62575-3. [DOI] [PubMed] [Google Scholar]
- 7.Kolhekar R, Meller ST, Gebhart GF. N-methyl-D-aspartate receptor-mediated changes in thermal nociception: allosteric modulation at glycine and polyamine recognition sites. Neuroscience. 1994;63:925–936. doi: 10.1016/0306-4522(94)90560-6. [DOI] [PubMed] [Google Scholar]
- 8.Leem JW, Choi EJ, Park ES, Paik KS. N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor antagonists differentially suppress dorsal horn neuron responses to mechanical stimuli in rats with peripheral nerve injury. Neurosci Lett. 1996;211:37–40. doi: 10.1016/0304-3940(96)12714-2. [DOI] [PubMed] [Google Scholar]
- 9.Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- 10.Hamada F, Noutsuka H, Hamada Y, Goto H. Comparison of the spinal anti-nociceptive effects of ES-242-1 and MK-801, two different NMDA antagonists, in rats. Neurosci Res. 2001;40:61–66. doi: 10.1016/s0168-0102(01)00206-1. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Simpson RK., Jr Intrathecal treatment with MK-801 suppresses thermal nociceptive responses and prevents c-fos immunore-activity induced in rat lumbar spinal cord neurons. Neurol Res. 1999;21:593–598. doi: 10.1080/01616412.1999.11740982. [DOI] [PubMed] [Google Scholar]
- 12.Hunter JC, Singh L. Role of excitatory amino acid receptors in the mediation of the nociceptive response to formalin in the rat. Neurosci Lett. 1994;174:217–221. doi: 10.1016/0304-3940(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 13.Kristensen JD, Karlsten R, Gordh T, Berge OG. The NMDA antagonist 3-(2-carboxypiperazin-4-yl) propyl-1-phosphonic acid (CPP) has antinociceptive effect after intrathecal injection in the rat. Pain. 1994;56:59–67. doi: 10.1016/0304-3959(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama T. Interaction between intrathecal morphine and glutamate receptor antagonists in formalin test. Eur J Pharmacol. 2000;395:203–210. doi: 10.1016/s0014-2999(00)00268-5. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama T, Yaksh TL, Weber E. Effects of intrathecal NMDA and non-NMDA antagonists on acute thermal nociception and their interaction with morphine. Anesthesiology. 1998;89:715–722. doi: 10.1097/00000542-199809000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Matthews EA, Dickenson AH. A combination of gabapentin and morphine mediates enhanced inhibitory effects on dorsal horn neuronal responses in a rat model of neuropathy. Anesthesiology. 2002;96:633–640. doi: 10.1097/00000542-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 18.Yoon MH, Choi JI, Kwak SH. Characteristics of interactions between intrathecal gabapentin and either clonidine or neostigmine in the formalin test. Anesth Analg. 2004;98:1374–1379. doi: 10.1213/01.ane.0000107937.00902.fc. [DOI] [PubMed] [Google Scholar]
- 19.Tallarida RJ, Murray R.B. Manual of pharmacologic calculations with computer programs. 2nd ed. New York: Springer-Verlag; 1987. [Google Scholar]
- 20.Bryans JS, Davies N, Gee NS, Dissanayake VU, Ratcliffe GS, Horwell DC, Kneen CO, Morrell AI, Oles RJ, O'Toole JC, Perkins GM, Singh L, Suman-Chauhan N, O'Neill JA. Identification of novel ligands for the gabapentin binding site on the alpha2delta subunit of a calcium channel and their evaluation as anticonvulsant agents. J Med Chem. 1998;41:1838–1845. doi: 10.1021/jm970649n. [DOI] [PubMed] [Google Scholar]
- 21.Benoliel R, Tanaka M, Caudle RM, Iadarola MJ. Co-localization of N-methyl-D-aspartate receptors and substance P (neurokinin-1) receptors in rat spinal cord. Neurosci Lett. 2000;291:61–64. doi: 10.1016/s0304-3940(00)01337-9. [DOI] [PubMed] [Google Scholar]
- 22.Jansen KL, Faull RL, Dragunow M, Waldvogel H. Autoradiographic localisation of NMDA, quisqualate and kainic acid receptors in human spinal cord. Neurosci Lett. 1990;108:53–57. doi: 10.1016/0304-3940(90)90705-e. [DOI] [PubMed] [Google Scholar]
- 23.Yaksh TL. The spinal pharmacology of facilitation of afferent processing evoked by high-threshold afferent input of the postinjury pain state. Curr Opin Neurol Neurosurg. 1993;6:250–256. [PubMed] [Google Scholar]
- 24.Furuyama T, Kiyama H, Sato K, Park HT, Maeno H, Takagi H, Tohyama M. Region-specific expression of subunits of ionotropic glutamate receptors (AMPA-type, KA-type and NMDA receptors) in the rat spinal cord with special reference to nociception. Brain Res Mol Brain Res. 1993;18:141–151. doi: 10.1016/0169-328x(93)90183-p. [DOI] [PubMed] [Google Scholar]
- 25.Headley PM, Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol Sci. 1990;11:205–211. doi: 10.1016/0165-6147(90)90116-p. [DOI] [PubMed] [Google Scholar]
- 26.Cuesta MC, Arcaya JL, Cano G, Sanchez L, Maixner W, Suarez-Roca H. Opposite modulation of capsaicin-evoked substance P release by glutamate receptors. Neurochem Int. 1999;35:471–478. doi: 10.1016/s0197-0186(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 27.Cheng J, Pan H, Eisenach JC. Antiallodynic effect of intrathecal gabapentin and its interaction with clonidine in a rat model of postoperative pain. Anesthesiology. 2000;92:1126–1131. doi: 10.1097/00000542-200004000-00031. [DOI] [PubMed] [Google Scholar]
- 28.Hurley RW, Chatterjea D, Rose Feng M, Taylor CP, Hammond DL. Gabapentin and pregabalin can interact synergistically with naproxen to produce antihyperalgesia. Anesthesiology. 2002;97:1263–1273. doi: 10.1097/00000542-200211000-00033. [DOI] [PubMed] [Google Scholar]
- 29.Roerig SC, Fujimoto JM. Multiplicative interaction between intrathecally and intracerebroventricularly administered mu opioid agonists but limited interactions between delta and kappa agonists for antinociception in mice. J Pharmacol Exp Ther. 1989;249:762–768. [PubMed] [Google Scholar]
- 30.Gu Y, Huang LY. Gabapentin actions on N-methyl-D-aspartate receptor channels are protein kinase C-dependent. Pain. 2001;93:85–92. doi: 10.1016/S0304-3959(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 31.Chen SR, Eisenach JC, McCaslin PP, Pan HL. Synergistic effect between intrathecal non-NMDA antagonist and gabapentin on allodynia induced by spinal nerve ligation in rats. Anesthesiology. 2000;92:500–506. doi: 10.1097/00000542-200002000-00033. [DOI] [PubMed] [Google Scholar]