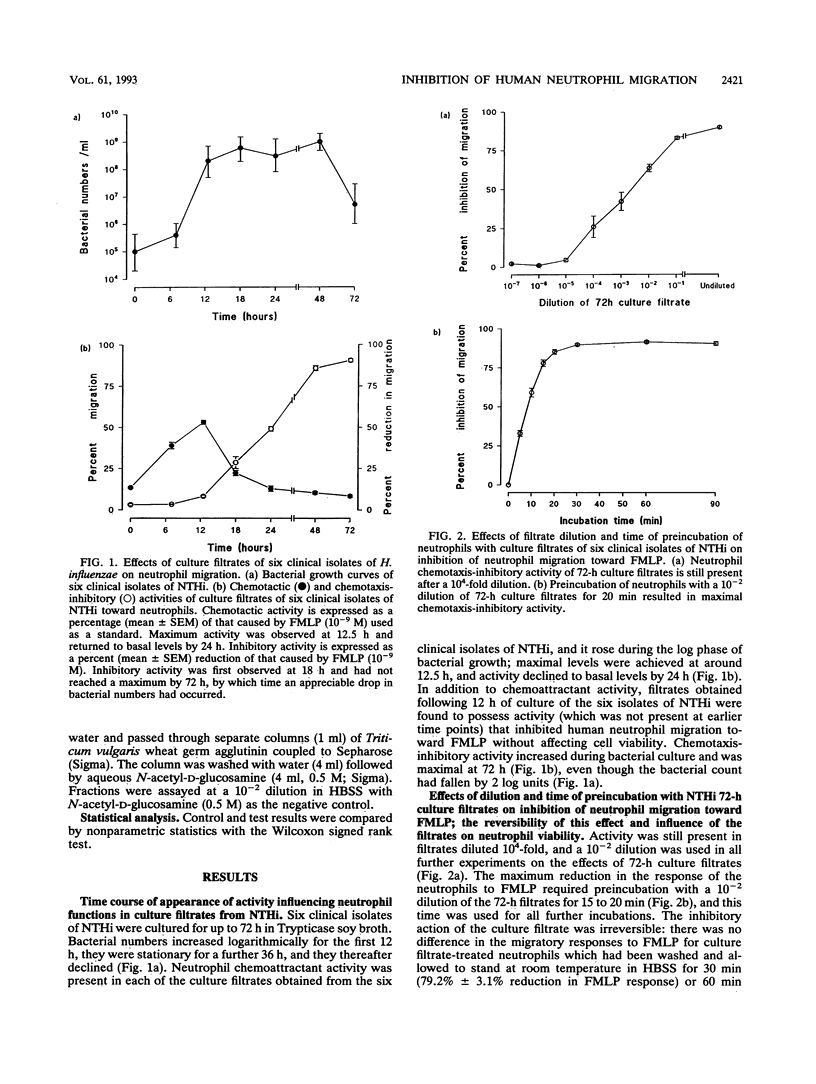
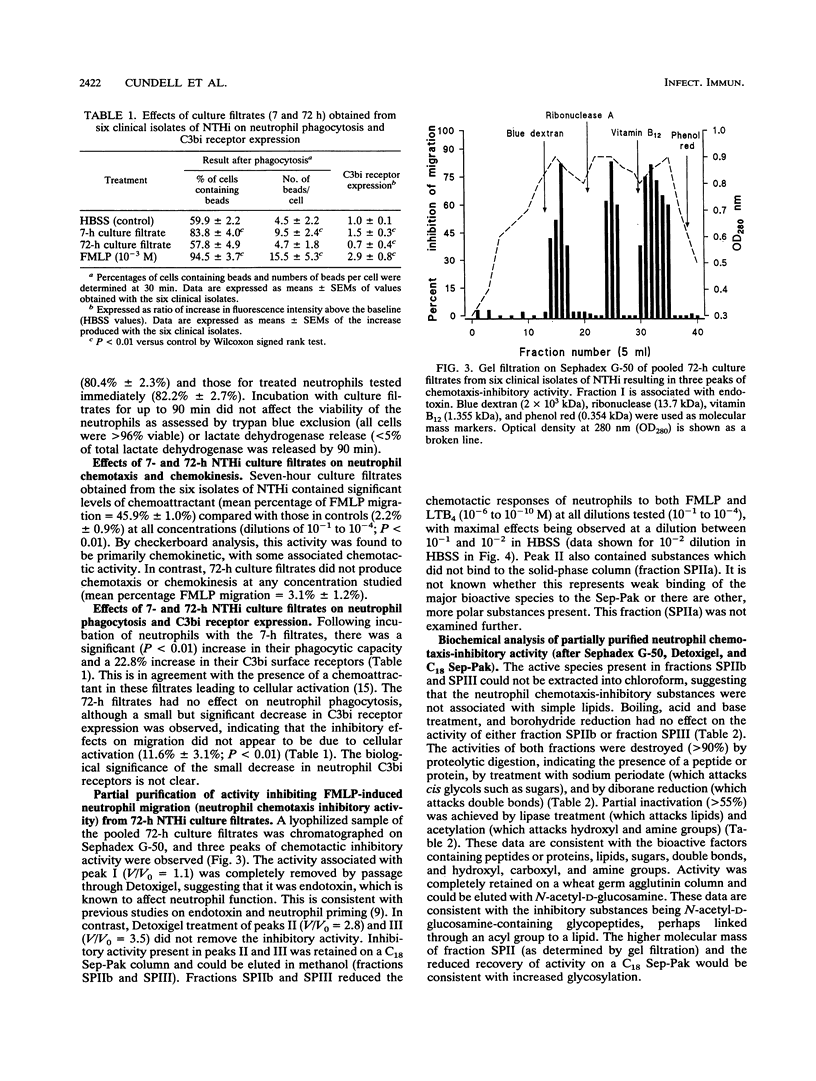
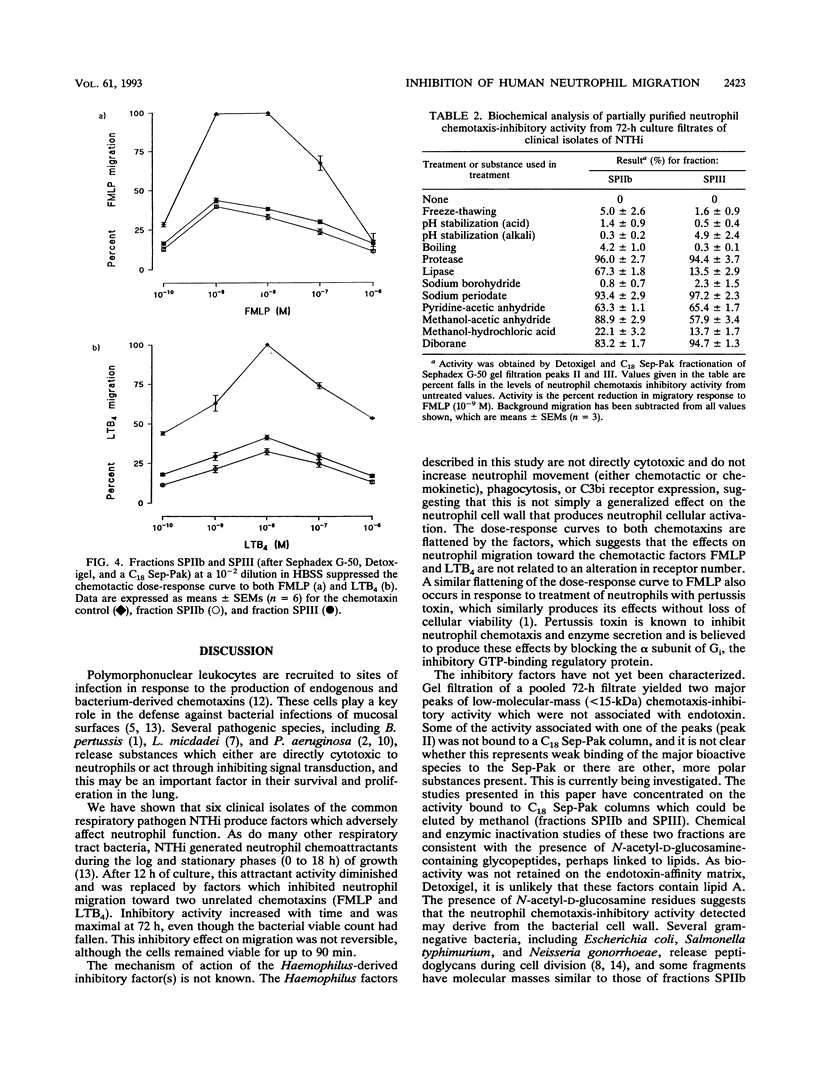
Abstract
Nontypeable Haemophilus influenzae commonly causes infections in the lower and upper respiratory tract, although the mechanisms of its colonization and persistence in the airways are unclear. Culture filtrates from six clinical isolates of this bacterium were assessed for their abilities to influence neutrophil function in vitro. Each culture filtrate was assessed on six separate occasions with neutrophils obtained from six different donors. During the log and early stationary phases of growth (0 to 18 h), culture filtrates contained primarily neutrophil chemokinetic activity but no activity affecting neutrophil migration toward the chemotactic factors N-formyl-L-methionyl-L-leucyl-L-phenylalanine and leukotriene B4. In contrast, filtrates obtained after 24 h of culture contained factors which inhibited neutrophil migration toward both of these chemotactic factors. This chemotaxis-inhibitory activity persisted between 24 and 72 h of bacterial culture, and it was not associated with the presence of either chemotactic or chemokinetic activity as assessed by checkerboard analysis. Gel filtration of pooled 72-h filtrates yielded three major peaks of chemotaxis-inhibitory activity. Endotoxin was present together with two other low-molecular-mass hydrophobic factors of approximately 8 and 2 kDa. These low-molecular-mass factors are chloroform insoluble and heat stable, and they are inactivated by protease, periodate, and diborane reduction. Activity was completely retained on a wheat germ agglutinin column, and it could be eluted with N-acetyl-D-glucosamine. These data suggest that inhibitory activity is associated with N-acetyl-D-glucosamine-containing glycopeptides, possibly derived from the bacterial cell wall. The production of these compounds may contribute to the persistence of this bacterium in vivo by inhibiting neutrophil chemotaxis in the microenvironment of the respiratory mucosa.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Kermode J. C., Naccache P. H., Yassin R., Marsh M. L., Munoz J. J., Sha'afi R. I. The inhibition of neutrophil granule enzyme secretion and chemotaxis by pertussis toxin. J Cell Biol. 1985 May;100(5):1641–1646. doi: 10.1083/jcb.100.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop M. B., Baltch A. L., Hill L. A., Smith R. P., Lutz F., Pollack M. The effect of Pseudomonas aeruginosa cytotoxin and toxin A on human polymorphonuclear leukocytes. J Med Microbiol. 1987 Dec;24(4):315–324. doi: 10.1099/00222615-24-4-315. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Currie D. C., Saverymuttu S. H., Peters A. M., Needham S. G., George P., Dhillon D. P., Lavender J. P., Cole P. J. Indium-111-labelled granulocyte accumulation in respiratory tract of patients with bronchiectasis. Lancet. 1987 Jun 13;1(8546):1335–1339. doi: 10.1016/s0140-6736(87)90647-7. [DOI] [PubMed] [Google Scholar]
- Donabedian H. Human mononuclear cells exposed to staphylococci rapidly produce an inhibitor of neutrophil chemotaxis. J Infect Dis. 1985 Jul;152(1):24–32. doi: 10.1093/infdis/152.1.24. [DOI] [PubMed] [Google Scholar]
- Donowitz G. R., Reardon I., Dowling J., Rubin L., Focht D. Ingestion of Legionella micdadei inhibits human neutrophil function. Infect Immun. 1990 Oct;58(10):3307–3311. doi: 10.1128/iai.58.10.3307-3311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Chaloupka J., Vinter V. Turnover of cell walls in microorganisms. Microbiol Rev. 1988 Dec;52(4):554–567. doi: 10.1128/mr.52.4.554-567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A., Döring G., Høiby N., Valerius N. H. Interaction of Pseudomonas aeruginosa alkaline protease and elastase with human polymorphonuclear leukocytes in vitro. Infect Immun. 1984 Jan;43(1):161–165. doi: 10.1128/iai.43.1.161-165.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Nagy L., Nagakura T., Walport M. J., Kay A. B. Identification and partial characterization of an exercise-induced neutrophil chemotactic factor in bronchial asthma. J Clin Invest. 1982 Apr;69(4):889–899. doi: 10.1172/JCI110528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malech H. L., Gallin J. I. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- Ras G., Wilson R., Todd H., Taylor G., Cole P. Effect of bacterial products on neutrophil migration in vitro. Thorax. 1990 Apr;45(4):276–280. doi: 10.1136/thx.45.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979 Jun;24(3):869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinman B. D., Devalia J. L., Davies R. J., Crook S. J., Tabaqchali S. Synthesis of histamine by Haemophilus influenzae. Br Med J (Clin Res Ed) 1986 Mar 29;292(6524):857–858. doi: 10.1136/bmj.292.6524.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley R. A., Shaw J., Hill S. L., Burnett D. Neutrophil chemotaxis in bronchiectasis: a study of peripheral cells and lung secretions. Clin Sci (Lond) 1988 Jun;74(6):645–650. doi: 10.1042/cs0740645. [DOI] [PubMed] [Google Scholar]
- Tetteroo P. A., Bos M. J., Visser F. J., von dem Borne A. E. Neutrophil activation detected by monoclonal antibodies. J Immunol. 1986 May 1;136(9):3427–3432. [PubMed] [Google Scholar]
- Tuomanen E., Rich R., Zak O. Induction of pulmonary inflammation by components of the pneumococcal cell surface. Am Rev Respir Dis. 1987 Apr;135(4):869–874. doi: 10.1164/arrd.1987.135.4.869. [DOI] [PubMed] [Google Scholar]
- Turk D. C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984 Aug;18(1):1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



