Abstract
The T-cell-stimulating activity of staphylococcal enterotoxin B (SEB) is an important factor in the pathogenesis of certain staphylococcal diseases. To investigate the immunologically active domains of the SEB molecule, we have produced truncated fragments of recombinant SEB by C-terminal and N-terminal deletions. The fragments were expressed as fusion proteins with protein A, including a cleavage site to remove the protein A part. Mutant proteins were tested for the ability to stimulate human resting T cells and SEB-reactive T-cell clones. Deletion of only 9 amino acids from the C terminus leads to complete loss of T-cell-stimulating activity. Removing further amino acids from the SEB molecule did not lead to a reexpression of T-cell-mitogenic activity. A mutant protein, however, in which the 9 C-terminal amino acids were replaced with a tail of 68 amino acids encoded by the vector was fully active. Two mutant proteins with N-terminal deletions of 60 and 81 amino acids were inactive as well. A neutralizing monoclonal antibody against a conformational epitope lost binding with all the inactive mutant proteins only, whereas a monoclonal antibody recognizing an epitope involved in emetic activity reacted with all mutant proteins. These results suggest that even small deletions at the C terminus affect the three-dimensional conformation of the SEB molecule.
Full text
PDF
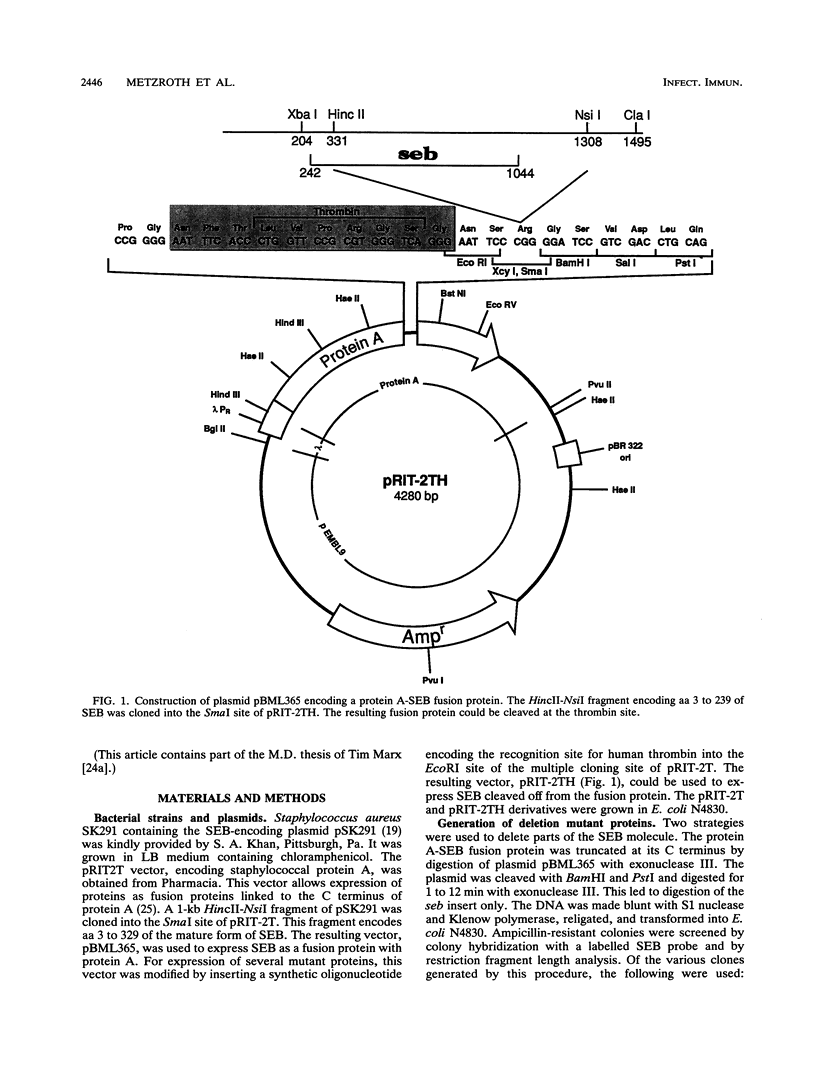
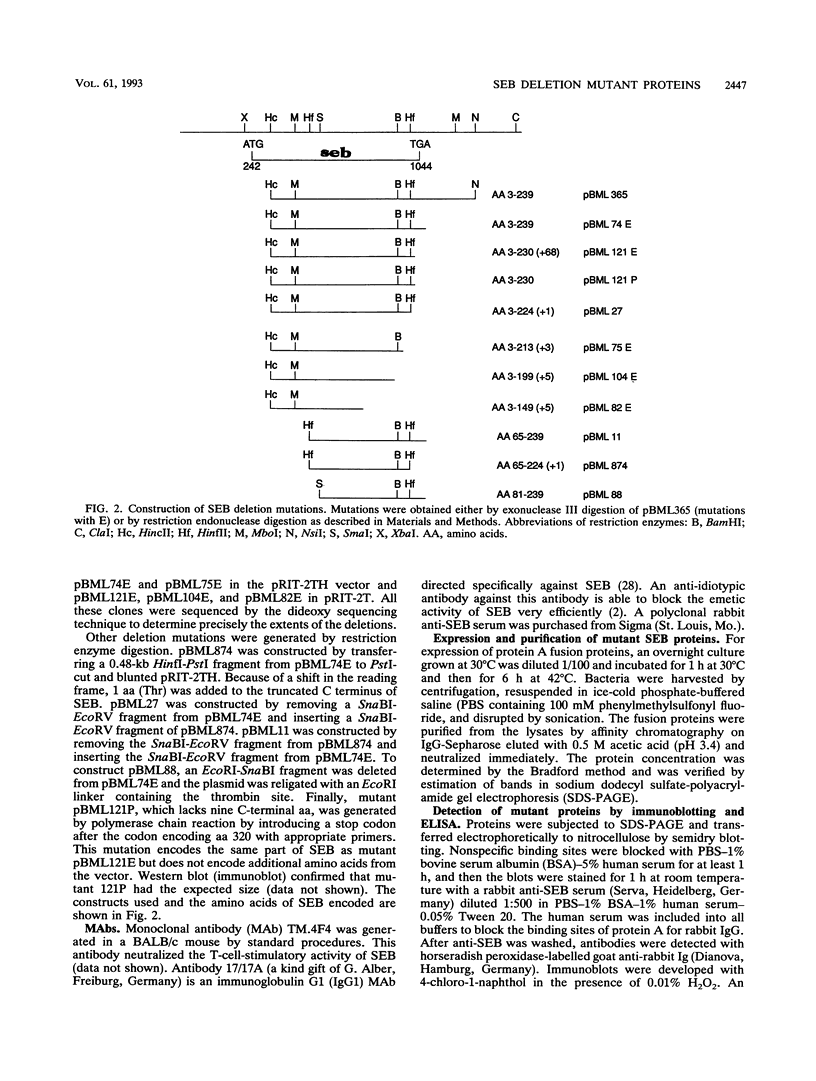

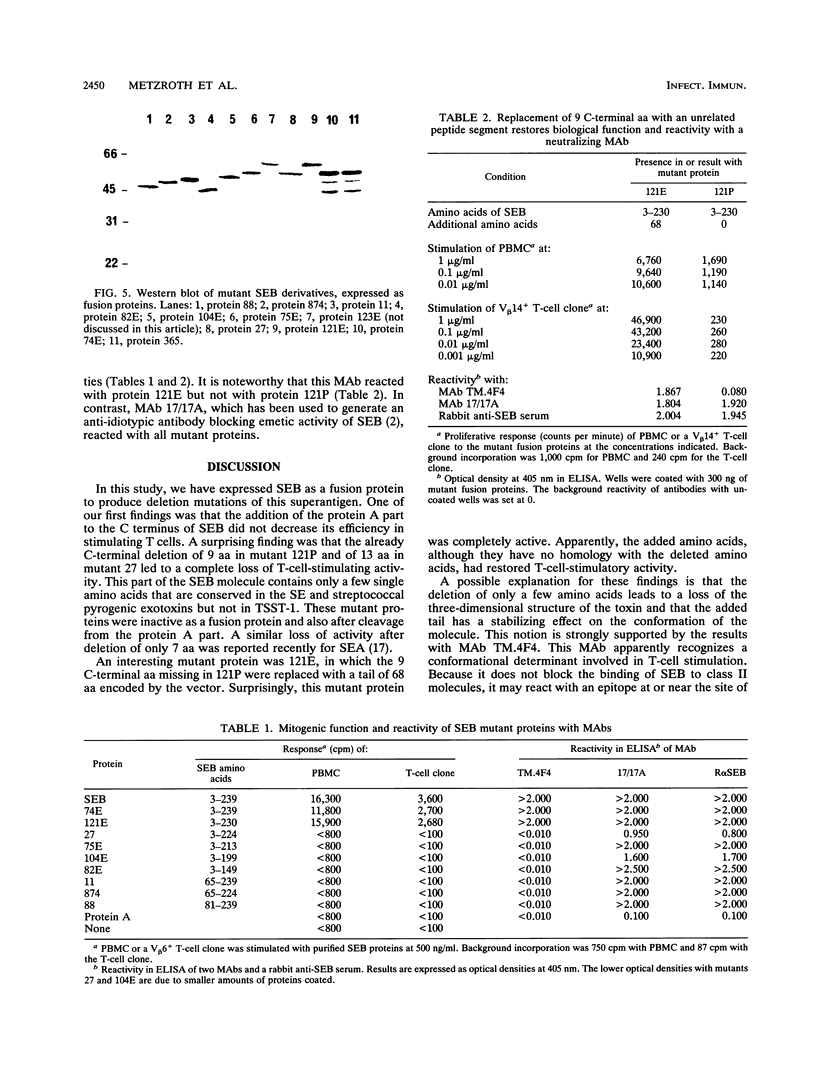


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber G., Hammer D. K., Fleischer B. Relationship between enterotoxic- and T lymphocyte-stimulating activity of staphylococcal enterotoxin B. J Immunol. 1990 Jun 15;144(12):4501–4506. [PubMed] [Google Scholar]
- Bamberger U., Scheuber P. H., Sailer-Kramer B., Bartsch K., Hartmann A., Beck G., Hammer D. K. Anti-idiotypic antibodies that inhibit immediate-type skin reactions in unsensitized monkeys on challenge with staphylococcal enterotoxin. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7054–7058. doi: 10.1073/pnas.83.18.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomster-Hautamaa D. A., Novick R. P., Schlievert P. M. Localization of biologic functions of toxic shock syndrome toxin-1 by use of monoclonal antibodies and cyanogen bromide-generated toxin fragments. J Immunol. 1986 Dec 1;137(11):3572–3576. [PubMed] [Google Scholar]
- Bohach G. A., Handley J. P., Schlievert P. M. Biological and immunological properties of the carboxyl terminus of staphylococcal enterotoxin C1. Infect Immun. 1989 Jan;57(1):23–28. doi: 10.1128/iai.57.1.23-28.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröker M. Improved "ATG vector" series for bacterial synthesis of proteins and protein fragments. Biotechniques. 1988 Sep;6(8):734–734. [PubMed] [Google Scholar]
- Buelow R., O'Hehir R. E., Schreifels R., Kummerehl T. J., Riley G., Lamb J. R. Localization of the immunologic activity in the superantigen Staphylococcal enterotoxin B using truncated recombinant fusion proteins. J Immunol. 1992 Jan 1;148(1):1–6. [PubMed] [Google Scholar]
- Chintagumpala M. M., Mollick J. A., Rich R. R. Staphylococcal toxins bind to different sites on HLA-DR. J Immunol. 1991 Dec 1;147(11):3876–3881. [PubMed] [Google Scholar]
- Couch J. L., Soltis M. T., Betley M. J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988 Jul;170(7):2954–2960. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin C., Kass E. H. Identification of functional antigenic segments of toxic shock syndrome toxin 1 by differential immunoreactivity and by differential mitogenic responses of human peripheral blood mononuclear cells, using active toxin fragments. Infect Immun. 1989 Jul;57(7):2230–2236. doi: 10.1128/iai.57.7.2230-2236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Bailey C. J. Recombinant epidermolytic (exfoliative) toxin A of Staphylococcus aureus is not a superantigen. Med Microbiol Immunol. 1992;180(6):273–278. doi: 10.1007/BF00191548. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Gerardy-Schahn R., Metzroth B., Carrel S., Gerlach D., Köhler W. An evolutionary conserved mechanism of T cell activation by microbial toxins. Evidence for different affinities of T cell receptor-toxin interaction. J Immunol. 1991 Jan 1;146(1):11–17. [PubMed] [Google Scholar]
- Fleischer B., Schmidt K. H., Gerlach D., Köhler W. Separation of T-cell-stimulating activity from streptococcal M protein. Infect Immun. 1992 May;60(5):1767–1770. doi: 10.1128/iai.60.5.1767-1770.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989 May 18;339(6221):221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- Grossman D., Cook R. G., Sparrow J. T., Mollick J. A., Rich R. R. Dissociation of the stimulatory activities of staphylococcal enterotoxins for T cells and monocytes. J Exp Med. 1990 Dec 1;172(6):1831–1841. doi: 10.1084/jem.172.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagle W. O., Tremaine M. T., Betley M. J. The carboxyl-terminal region of staphylococcal enterotoxin type A is required for a fully active molecule. Infect Immun. 1991 Jun;59(6):2126–2134. doi: 10.1128/iai.59.6.2126-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M. J., Hudson K. R., Fraser J. D., Gascoigne N. R. Enterotoxin residues determining T-cell receptor V beta binding specificity. Nature. 1992 Oct 29;359(6398):841–843. doi: 10.1038/359841a0. [DOI] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kotzin B., Herron L., Gelfand E. W., Bigler R. D., Boylston A., Carrel S., Posnett D. N., Choi Y., Marrack P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989 May 19;244(4906):811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Baschieri S., Lees R. K. Clonal expansion precedes anergy and death of V beta 8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol. 1991 Aug;21(8):1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- Marrack P., Blackman M., Kushnir E., Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990 Feb 1;171(2):455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Abrahmsén L., Uhlén M. Immobilization and purification of enzymes with staphylococcal protein A gene fusion vectors. EMBO J. 1985 Apr;4(4):1075–1080. doi: 10.1002/j.1460-2075.1985.tb03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noskova V. P., Ezepchuk YuV, Noskov A. N. Topology of the functions in molecule of staphylococcal enterotoxin Type A. Int J Biochem. 1984;16(2):201–206. doi: 10.1016/0020-711x(84)90073-9. [DOI] [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Johnson H. M. Structural basis for differential binding of staphylococcal enterotoxin A and toxic shock syndrome toxin 1 to class II major histocompatibility molecules. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):125–128. doi: 10.1073/pnas.88.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck B., Scheuber P. H., Londong W., Sailer-Kramer B., Bartsch K., Hammer D. K. Protection against the staphylococcal enterotoxin-induced intestinal disorder in the monkey by anti-idiotypic antibodies. Proc Natl Acad Sci U S A. 1988 May;85(9):3170–3174. doi: 10.1073/pnas.85.9.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust C. J., Verreck F., Vietor H., Koning F. Specific recognition of staphylococcal enterotoxin A by human T cells bearing receptors with the V gamma 9 region. Nature. 1990 Aug 9;346(6284):572–574. doi: 10.1038/346572a0. [DOI] [PubMed] [Google Scholar]
- Spero L., Morlock B. A. Biological activities of the peptides of staphylococcal enterotoxin C formed by limited tryptic hydrolysis. J Biol Chem. 1978 Dec 25;253(24):8787–8791. [PubMed] [Google Scholar]
- Swaminathan S., Furey W., Pletcher J., Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature. 1992 Oct 29;359(6398):801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]




