Summary
Cogan’s syndrome is defined as a chronic inflammatory disease of unknown origin, an autoimmune disease, characterized by bilateral sensorineural hearing loss, vestibular symptoms, inflammatory ocular manifestations with variable risk of developing into a systemic disease. The onset of disease is variable but is often characterized by isolated ocular symptoms or acute ear and/or vestibular manifestations, variably associated. The diagnosis of Cogan’s syndrome can be a challenge as is evident in the case described here since it is based only on the association between bilateral ocular and vestibuloauditory symptoms with no specific diagnostic tests available.
Keywords: Hearing loss, Interstitial keratitis, Autoimmunity, Cogan’s syndrome
Riassunto
La sindrome di Cogan viene descritta come una patologia infiammatoria cronica di incerta origine, ma più propriamente è una patologia autoimmune, caratterizzata da ipoacusia neurosensoriale bilaterale, sintomatologia vestibolare e manifestazioni infiammatorie oculari con un rischio variabile di sviluppare una malattia sistemica. La malattia può insorgere soltanto con sintomi oculari o con sintomi acuti uditivi e/o vestibolari, variamente associati. Porre diagnosi di sindrome di Cogan può essere una sfida come risulta evidente nel caso descritto perché si basa soltanto sull’associazione di sintomi oculari e audio-vestibolari, in mancanza di tests diagnostici specifici.
Introduction
Cogan’s syndrome is a rare disorder of unknown origin characterized by inflammatory eye disease and vestibulo-auditory symptoms, which primarily affects young white adults, without a hereditary pattern 1. It was first described by an ophthalmologist, Dr David G. Cogan, in 1945, who reported on a “syndrome of non-syphilitic interstitial keratitis (IK) and vestibuloauditory symptoms” 2, even though, in 1934, Morgan and Baumgartner had first described a non-syphilitic IK associated with vestibuloauditory dysfunction 3. Since then, more than 100 cases (~150) have been reported in the literature but little is known about the specific origins of the disease even though it is possible to improve some of its sequelae, specifically the hearing impairment, by cochlear implantation 4–10.
Case report
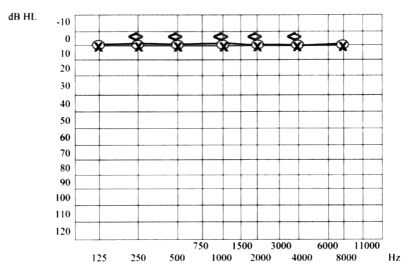
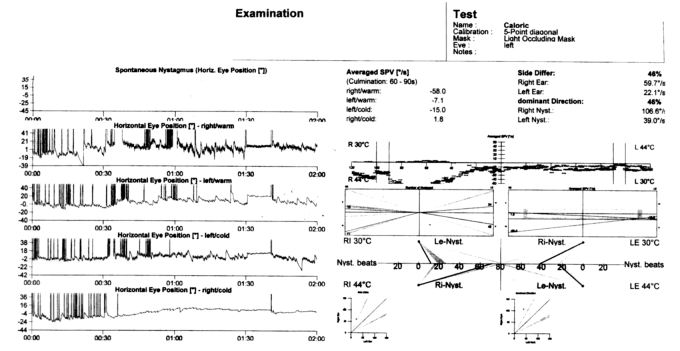
A 31-year-old white female presented with vertigo, hearing loss and tinnitus referred in the left ear, and with a one-week history of bilateral conjunctivitis treated with topical corticosteroids, which appeared after pharyngeal inflammation that occurred 4-5 days previously. On the first otorhinolaryngologic (ORL) evaluation, acute left vestibular paresis was noted without cutaneous herpes signs. Initial treatment consisted of an antiemetic and neuroleptic drug (levosulpiride), vasoactive drugs such as pentoxifylline, 10% glycerol solution and vitamin-B complex. In spite of the symptoms referred by the patient, audiometry revealed normal bilateral and symmetric hearing function (Fig. 1). White blood count was 13640/mm3 and erythrocyte sedimentation rate was elevated (70 mm/h) with all other laboratory tests within normal limits. Ophthalmologic evaluation confirmed a diagnosis of bilateral conjunctivitis. Three days after beginning treatment, worsening of symptoms was noted, with headache, mild left sensorineural hearing loss (Fig. 2) and relevant dizziness. Systemic corticosteroid treatment (prednisone 60 mg i.v./day) was started. Caloric testing confirmed the left vestibular paresis (Fig. 3). Auditory Brainstem Responses (ABR) using 120 dB SPL click stimulus demonstrated a delay of I, III, V waves and a normal I-V latency interval on the left side. After 10 days of treatment further worsening of ocular inflammation, headache, left hemifacial pain and periauricular paraesthesia were observed. Neurologic evaluation and laboratory tests (antibodies IgG and IgM against Epstein Barr virus, Cytomegalovirus, Hepatitis virus B and C, HIV, Toxoplasma gondii) were negative. Cerebral high-resolution magnetic resonance imaging (HR-MRI) showed abnormal MRI findings (compatible with inflammatory signals), in the vestibule, semicircular canals, cochlea, vestibular nerve and first part of the facial nerve on the left side of the head (Fig. 4). On the basis of a hypothetic sine herpes vestibuloauditory deficit caused by Herpes Zoster virus, our patient was treated also with valacyclovir. The patient was discharged from hospital with a mild improvement in general conditions but presenting moderate to severe left sensorineural hearing loss on high frequencies and tinnitus (Fig. 5).
Fig. 1.
Normal hearing function.
Fig. 2.
Mild left sensorineural hearing loss.
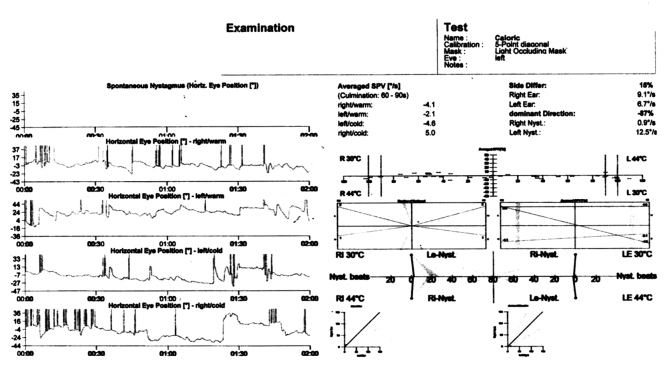
Fig. 3.
Caloric testing showing left vestibular paresis.
Fig. 4.
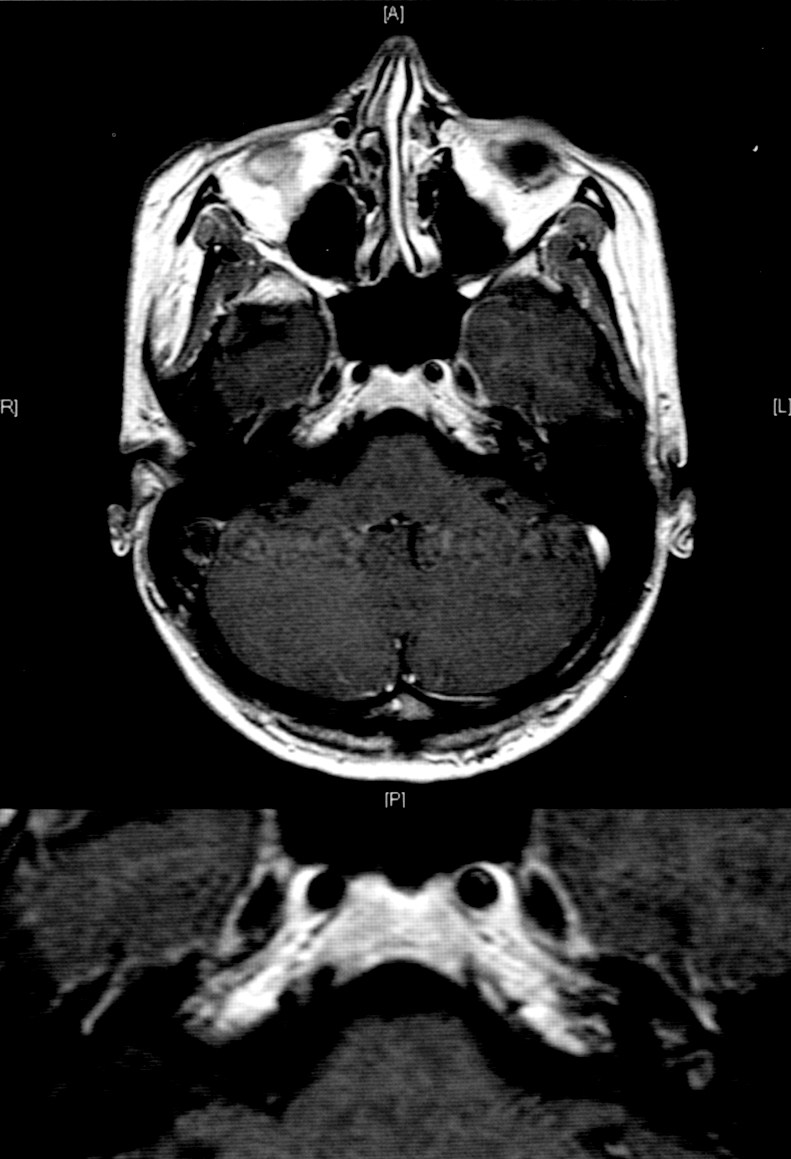
HR-MRI showing abnormal MRI findings in the vestibule, semicircular canals, cochlea, vestibular nerve and first part of facial nerve on left side of head.
Fig. 5.
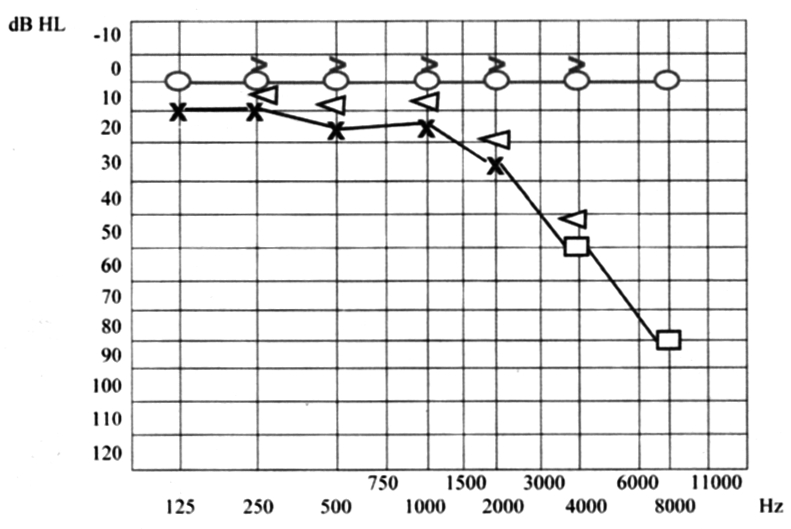
Moderate to severe left sensorineural hearing loss at high frequencies.
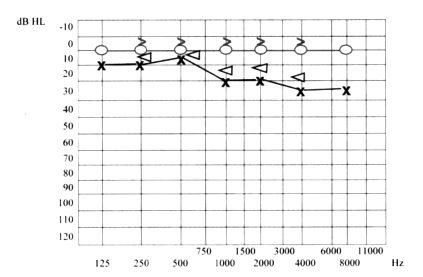
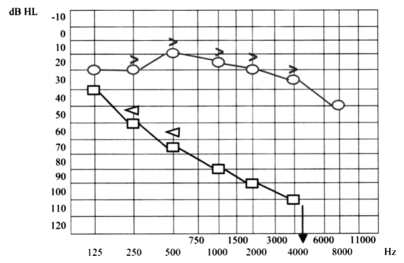
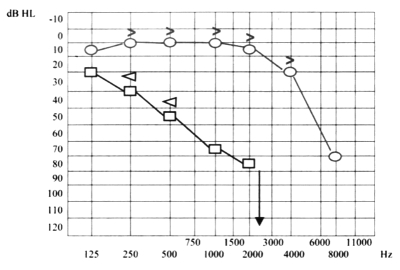
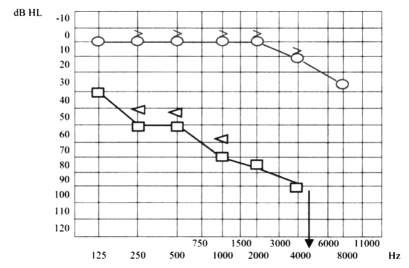
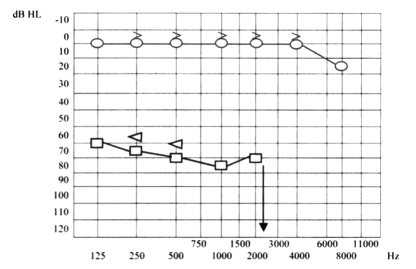
Ten days later, the patient presented even more severe left hearing loss and mild right sensorineural hearing loss (Fig. 6), right paracusia, bilateral aural fullness, dizziness, ataxia, bilateral photophobia, lacrimation, persistent headache and an acute right vestibular paralysis. A few days later caloric testing showed bilateral vestibular paresis (Fig. 7). Vestibular evocated myogenic potentials (VEMPs) were bilaterally absent. Treatment was the same as the previous one. Suspicion of viral meningitis was excluded by a neurological evaluation and normal electroencephalogram (EEG), while, for the first time, Cogan’s syndrome was suspected. Audiometry revealed mild to severe right sensorineural hearing loss at high frequencies (Fig. 8), while cerebral HR-MRI was normal. Finally, the fourth ophthalmologic evaluation revealed the presence of a bilateral IK, an essential element upon which to make a diagnosis of Cogan’s syndrome. Laboratory tests and cerebro-spinal fluid (CSF) analysis were all negative for an acute viral infection (Herpes Simplex 1 and 2, Herpes Zoster), negative for syphilis, Lyme disease, and for auto-antibodies (ANA, AMA, ENA, APCA, ANDNA, ASMA, ACARD, ANCA, LAC). Chest X-rays, echocardiogram, abdomen echography, performed to exclude systemic alterations, were normal. The patient continued systemic corticosteroid treatment and topical corticosteroids for the inflammatory eye disorder. Considering all the disease manifestations, the results of all examinations and the partial response to systemic corticosteroid treatment, a diagnosis of Cogan’s syndrome was finally made. The patient was transferred to a clinic specialized in autoimmune diseases, and began a therapeutic protocol based on methotrexate, cyclophosphamide, cyclosporine, folin, acetylsalicylic acid, and a low dose of corticosteroids on account of iatrogenic Cushing’s syndrome, at least for approximately the first 6 months of treatment, with the aim of achieving improvement or stabilization of the disease. The patient also began an ORL follow-up with audiometric tests after 1 month of treatment and thereafter about every 2 months. After 1 month of this treatment, the patient presented mild right sensorineural hearing loss limited to high frequencies, severe to profound left hearing loss (Fig. 9), oscillopsia and ataxia. After 4 months’ treatment, the patient presented unchanged left hearing loss, improvement of right hearing function (Fig. 10), reduction of oscillopsia and ataxia, persistent bilateral vestibular paresis and disappearance of ophthalmologic symptoms.
Fig. 6.
Severe to profound left hearing loss and mild right sensorineural hearing loss.
Fig. 7.
Caloric testing showing bilateral vestibular paresis.
Fig. 8.
Mild to severe right sensorineural hearing loss at high frequencies, severe to profound left hearing loss.
Fig. 9.
Mild right sensorineural hearing loss limited to high frequencies, severe to profound left hearing loss.
Fig. 10.
Mild right sensorineural hearing loss limited to 8KHz, severe to profound left hearing loss.
Discussion
The diagnosis of classic Cogan’s syndrome is made on the basis of non-syphilitic IK, acute-onset sensorineural hearing loss and vestibular symptoms such as Ménière’s disease, and progressive hearing loss up to deafness within 2 years. Diagnosis of atypical Cogan’s syndrome can also be made when the classic pattern of autoimmune-type vestibuloauditory symptoms is associated with inflammatory eye disease other than interstitial keratitis or when the interval between the onset of ophthalmologic, and the onset of vestibuloauditory, symptoms is more than 2 years. Cogan’s syndrome usually affects young white adults, in general; about 30% of patients develop systemic manifestations (systemic vasculitis, aortitis, musculoskeletal complaints, neurological symptoms, etc.) 9 11 12. It is not easy to recognize the type of Cogan’s syndrome (typical or atypical), because several patients do not present interstitial keratitis at the onset and develop this condition in the following phase, as occurred in the patient described here. Timing and association of manifestations of Cogan’s syndrome are extremely variable but, as reported in the literature, a viral prodrome, within the few weeks preceding disease onset, is constant, and both ophthalmologic and vestibuloauditory symptoms are present within a few days to weeks after disease onset 0. From the few cases published, it is difficult to determine the presence of systemic disorders, which can include: headache, fever, musculoskeletal pain, splenomegaly, lymphadenopathy and vasculitis affecting the aortic valve, coronary arteries and small kidney vasculature 9 10. In our case, as in others reported in the literature, the patient presented few abnormal laboratory tests: only leukocytosis and elevated erythrocyte sedimentation rate; sometimes C-reactive protein is elevated, but often no alteration in inflammation parameters is observed, therefore, no specific diagnostic test is performed 13–15. Radiographic studies, such as cranial computed tomography (CT) and magnetic resonance imaging (MRI), are often normal and the result is useful to exclude other causes of vestibuloauditory symptoms such as stroke or neoplasm 10, even though some Authors have reported the presence of labyrinthine aspecific radiological abnormalities, as in our case. Since it can be difficult to evaluate the activity of the disease, Helmchen et al. 16 used high resolution magnetic resonance imaging (HR-MRI) to differentiate between active and inactive stages and found that abnormal MRI signals of the inner ear were related to the activity of the disease. In fact, the patients studied during an acute exacerbation showed abnormal MRI signals in the vestibule, semicircular canals, vestibular nerve and cochlea, which disappeared after relapse of the disease.
Considering the variable onset and development of symptoms as well as the lack of specific laboratory tests, the diagnosis of Cogan’s syndrome is a challenge and is often based upon a good response to corticosteroid treatment 17 18. Nevertheless, as in our case, corticosteroids have proved to be of short-term benefit, associated with relevant side-effects. Some authors have noted that, with corticosteroid treatment, hearing could be stabilized but total bilateral vestibuloauditory dysfunction may occur and deafness could not be prevented 19. Immunosuppressive drugs are used to cure vestibuloauditory disease, reduce adverse effects of corticosteroids, avoid ocular recurrence, but are, in general, restricted to patients willing to endure the attendant risks, with controversial benefits 20, while long-term ophthalmologic outcomes are rare. The administration of toxic medications to preserve hearing, at all costs, is a less desirable option with the opportunity of cochlear implantation 7 9 10 21 22.
Cogan’s syndrome is a cause of progressive deafness, it is generally assumed that it is an “autoimmune disease” 19 23. Autoimmune inner ear diseases (AIEDs) are unusual causes of progressive bilateral sensorineural hearing loss and vestibular defects: three kinds of syndromes have been described so far:
progressive bilateral sensorineural hearing loss (which could be the first symptom) associated with systemic manifestations characteristic of a specific autoimmune disease such as: polyarthritis, rheumatoid arthritis (AR), etc.;
progressive bilateral sensorineural hearing loss associated with inflammatory involvement of eyes and specifically with interstitial keratitis (Cogan’s syndrome);
progressive bilateral sensorineural hearing loss exclusively.
Patients could develop all kind of syndromes, in general, including all the above-mentioned 11.
AIED is a rare disease, accounting for < 1% of all cases of hearing impairment or dizziness. The diagnosis of AIED might be overlooked due to the lack of a specific diagnostic test, as for Cogan’s syndrome. Presentation of audiovestibular symptoms is similar to that in Cogan’s syndrome: the progression of hearing loss is too rapid to be diagnostic for presbyacusis and too slow to conclude a diagnosis of sudden sensorineural hearing loss (SNHL). Occasionally, only one ear is initially affected, but bilateral hearing loss occurs, in most patients, with symmetric or asymmetric audiometric threshold. Almost 25-50% of patients also have tinnitus and aural fullness which can fluctuate. Vestibular symptoms may be present in almost 50%. Systemic autoimmune diseases coexist in 15-30% of patients 14 18. It is difficult to distinguish AIED and Cogan’s syndrome since AIED can be associated with vestibular symptoms and also because AIED can “become or overlap” develop into Cogan’s syndrome. Moreover, Cogan’s syndrome could start without ocular inflammation which is essential to make diagnosis of typical or atypical Cogan’s syndrome 22.
Many patients initially present only vestibuloauditory symptoms which can be considered classic Ménière syndrome episodes, therefore, it is not easy to make the correct diagnosis which is, indeed, important to establish the correct treatment as soon as possible 24. In order to emphasize the differences between Cogan’s syndrome and Ménière’s disease, it is important to consider the fact that vestibular symptoms associated with Cogan’s syndrome are generally more pronounced and long-standing, with the possibility of persisting incessantly for several days, or indefinitely, also fluctuating without a period of complete remission, as in the case described here. Moreover, Cogan’s syndrome results in a bilateral vestibular injury that may lead to symptoms of complete peripheral vestibular organ dysfunction such as ataxia and/or oscillopsia 10.
Considering the limited efficacy and the risks associated with the treatment options described, and also the risk of systemic disease, the importance of being aware of Cogan’s syndrome becomes clear 25. Follow-up of patients presenting sudden or rapidly progressive hearing loss, with or without vestibular symptoms, is also important, because these symptoms could represent the initial manifestation of Cogan’s syndrome. Since about two thirds of patients meet the classic diagnostic criteria for Cogan’s syndrome 10, the follow-up of patients who present only vestibuloauditory symptoms should be guaranteed for at least two years. Therefore, we propose a follow-up schedule with a monthly vestibuloauditory assessment for the first 3 months, then every 3 months for the first and second year, every 6 months in the third year and then once a year, considering that the duration between the onset of ophthalmologic and vestibuloauditory symptoms could be significantly variable and unpredictable 10.
Only a few cases of Cogan’s syndrome have been published in the literature 7, but this disease needs to be accurately documented and described in order to offer a better understanding of the pathogenesis and in order to contribute towards developing efficacious treatment strategies.
References
- 1.Cundiff J, Kansal S, Kumar A, Goldstein DA, Tessler HH. Cogan’s syndrome: a cause of progressive hearing deafness. Am J Otolaryngol 2006;27:68-70. [DOI] [PubMed] [Google Scholar]
- 2.Cogan DG. Syndrome of nonsyphilitic interstitial keratitis and vestibuloauditory symptoms. Arch Ophthalmol 1945;33:144-9. [DOI] [PubMed] [Google Scholar]
- 3.Morgan RF, Baumgartner CJ. Ménière’s disease complicated by recurrent interstitial keratitis: excellent result following cervical ganglionectomy. West J Surg 1934;42:628-31. [Google Scholar]
- 4.Haynes BF, Kaiser-Kupfer MI, Mason P, Fauci AS. Cogan syndrome: studies in thirteen patients, long-term follow-up, and a review of the literature. Medicine (Baltimore) 1980;59:426-41. [PubMed] [Google Scholar]
- 5.McDonald TJ, Vollertsen RS, Younge BR. Cogan’s syndrome: audiovestibular involvement and prognosis in 18 patients. Laryngoscope 1985;95:650-4. [DOI] [PubMed] [Google Scholar]
- 6.Vollertsen RS, McDonald TJ, Younge BR, Banks PM, Stanson AW, Ilstrup DM. Cogan’s syndrome: 18 cases and a review of the literature. Mayo Clin Proc 1986;61:344-61. [DOI] [PubMed] [Google Scholar]
- 7.Minet M, Deggouj N, Gersdorff M. Cochlear implantation in patients with Cogan’s syndrome: a review of four cases. Eur Arch Otorhinolaryngol 1997;254:459-62. [DOI] [PubMed] [Google Scholar]
- 8.St Clair EW, McCallum RM. Cogan’s syndrome. Curr Opin Rheumatol 1999;11:47-52. [DOI] [PubMed] [Google Scholar]
- 9.Gaubitz M, Lübben B, Seidel M, Schotte H, Gramley F, Domschke W. Cogan’s syndrome: organ-specific autoimmune disease or systemic vasculitis? A report of two cases and review of the literature. Clin Exp Rheumatol 2001;19:463-9. [PubMed] [Google Scholar]
- 10.Gluth MB, Baratz KH, Matteson EL, Driscoll CL. Cogan syndrome: a retrospective review of 60 patients throughout a half century. Mayo Clin Proc 2006;81:483-8. [DOI] [PubMed] [Google Scholar]
- 11.Vinceneux P, Couloigner V, Pouchot J, Bouccara D, Sterkers O. Autoimmune deafness. Presse Med 1999;28:1904-10. [PubMed] [Google Scholar]
- 12.Grasland A, Pouchot J, Hachulla E, Blétry O, Papo T, Vinceneux P, Study Group for Cogan’s Syndrome. Typical and atypical Cogan’s syndrome: 32 cases and review of the literature. Rheumatology (Oxford) 2004;43:1007-5. [DOI] [PubMed] [Google Scholar]
- 13.Berrettini S, Ravecca F, Bruschini L, Ursino F, Sellari-Franceschini S. Progressive sensorineural hearing loss: immunologic etiology. Acta Otorhinolaryngol Ital 1998;18:33-41. [PubMed] [Google Scholar]
- 14.Bovo R, Aimoni C, Martini A. Immune-mediated inner ear disease. Acta Otolaryngol 2006;126:1012-21. [DOI] [PubMed] [Google Scholar]
- 15.Bonaguri C, Orsoni JG, Zavota L, Monica C, Russo A, Pellistri I, et al. Anti-68 kDa antibodies in autoimmune sensorineural hearing loss. Autoimmunity 2007;40:73-8. [DOI] [PubMed] [Google Scholar]
- 16.Helmchen C, Jäger L, Büttner U, Reiser M, Brandt T. Cogan’s Syndrome. High resolution MRI indicators of activity. J Vestib Res 1998;8:155-67. [PubMed] [Google Scholar]
- 17.Casselman JW, Majoor MH, Albers FW. MR of the inner ear in patients with Cogan syndrome. AJNR Am J Neuroradiol 1994;15:131-8. [PMC free article] [PubMed] [Google Scholar]
- 18.Broughton SS, Meyerhoff WE, Cohen SB. Immune-mediated inner ear disease: 10-year experience. Semin Arthritis Rheum 2004;34:544-8. [DOI] [PubMed] [Google Scholar]
- 19.Pleyer U, Baykal HE, Rohrbach JM, Bohndorf M, Rieck P, Reimann J, et al. Cogan I syndrome: too often detected too late? A contribution to early diagnosis of Cogan I syndrome. Klin Monatsbl Augenheilkd 1995;207:3-10. [DOI] [PubMed] [Google Scholar]
- 20.Ruckenstein MJ. Autoimmune inner ear disease. Curr Opin Otolaryngol Head Neck Surg 2004;12:426-30. [DOI] [PubMed] [Google Scholar]
- 21.Pasanisi E, Vincenti V, Bacciu A, Guida M, Berghenti T, Barbot A, et al. Cochlear mplants. Otol Neurotol 2003;24:601-4. [DOI] [PubMed] [Google Scholar]
- 22.Selivanova O, Haxel BR, Mann WJ. Cogan’s syndrome: a diagnostic challenge. HNO 2006;54:619-23. [DOI] [PubMed] [Google Scholar]
- 23.Lunardi C, Bason C, Leandri M, Navone R, Lestani M, Millo E, et al. Autoantibodies to inner ear and endothelial antigens in Cogan’s syndrome. Lancet 2002;360:915-21. [DOI] [PubMed] [Google Scholar]
- 24.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière’s disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg 1995;113:181-5. [DOI] [PubMed] [Google Scholar]
- 25.Orsoni JG, Zavota L, Pellistri I, Piazza F, Cimino L. Cogan syndrome. Cornea 2002;21:356-9. [DOI] [PubMed] [Google Scholar]