Abstract
Objectives
To evaluate whether BNP changes during sleep are associated with the frequency and severity of apneic/hypopneic episodes, intermittent arousals, and hypoxia.
Background
Sleep apnea is strongly associated with heart failure (HF), and could conceivably worsen HF through increased sympathetic activity, hemodynamic stress, hypoxemia, and oxidative stress. If apneic activity does cause acute stress in HF, it should increase BNP.
Methods
64 HF patients with NYHA class II-III HF and EF <40% underwent a baseline sleep study. Five patients with no sleep apnea and 12 with severe sleep apnea underwent repeat sleep studies, during which blood was collected every 20 minutes for the measurement of BNP. Patients with severe sleep apnea also underwent a third sleep study with frequent BNP measurements while they were administered oxygen. This provided 643 observations with which to relate apnea to BNP. The association of log BNP with each of six markers of apnea severity was evaluated with repeated measures regression models.
Results
There was no relationship between BNP and the number of apneic/hypopneic episodes or the number of arousals. However, the burden of hypoxemia (the time spent with oxygen saturation below 90%) significantly predicted BNP concentrations; each 10% increase in duration of hypoxemia increased BNP by 9.6% [95% CI 1.5-17.7, p=0.02].
Conclusions
Hypoxemia appears to be an important factor that underlies the impact of sleep abnormalities on hemodynamic stress in patients with HF. Prevention of hypoxia might be especially important in these patients.
Keywords: Brain natriuretic peptide, sleep apnea, hypoxia
INTRODUCTION
Sleep apnea has been strongly associated with heart failure (HF) in various cross-sectional studies, with a reported prevalence as high as 40% in HF patients.1,2 Although the extent to which sleep apnea reflects HF severity or causes worsening heart failure is controversial, there are suggestions that sleep apnea can be harmful. HF patients with sleep apnea have a worse prognosis than those without sleep apnea,3,4 and treating sleep apnea can improve hemodynamics, alleviate cardiovascular stress, and potentially prolong survival in HF patients.5,6
The hallmark of sleep apnea is recurrent episodes of hypoxemia and arousals throughout the night. These arousals are accompanied by bursts of sympathetic stimulation which elicit peripheral vasoconstriction and an increase in heart rate and blood pressure.7 Furthermore, the mechanical stresses in obstructive sleep apnea can lead to an increase in left ventricular afterload with further compromise of cardiac output.8 Hypoxia itself can have direct detrimental cardiac effects.9 Thus increased sympathetic activity, hemodynamic stress, and hypoxia could all potentially cause exacerbation of heart failure.
B-type natriuretic peptide (BNP) is a polypeptide with a short half life (approximately 20 minutes) that is secreted by the ventricles in response to cardiomyocycte stretching, and thus is a good marker of hemodynamic stress. If apneic episodes cause acute stress in patients with HF, then BNP levels would be expected to rise and fall contemporaneously. We hypothesized that in patients with HF, the changes in the concentrations of BNP during the night are associated with the frequency of apneic/hypopneic episodes and arousals, as well as the severity of hypoxemia. To address these hypotheses, we designed a study that could evaluate the immediate effects of sleep disturbances on BNP, by measuring BNP levels every 20 minutes during sleep and characterizing their sleep and breathing patterns during these same 20 minute blocks.
METHODS
Study Design
Patient Selection
Subjects were recruited from the patient populations at Johns Hopkins Bayview Medical Center and University of Maryland. All patients had stable symptomatic heart failure and a left ventricular ejection fraction ≤ 40%. They were euvolemic and on a stable heart failure medication regimen for at least one month. Patients were excluded if they had unstable angina, myocardial infarction or hospitalization within the past two months.
Study protocol
Patients underwent three nights of study. On night 1 (baseline test), patients were acclimatized to the sleep laboratory, and underwent a standard sleep study to characterize their sleep and breathing patterns. Baseline recordings were analyzed to categorize patients into one of three groups (No or mild sleep apnea present, overall apnea-hypopnea index (AHI) ≤ 5; sleep apnea of intermediate severity, AHI 6-39.9; severe sleep apnea, AHI ≥ 40).
Patients with no to mild sleep apnea and patients with severe sleep apnea were selected for further study to examine the effects of apnea on BP. They subsequently underwent a second sleep study, now with blood sampling every 20 minutes. (We refer to each sampling interval as an “epoch.”) Patients with intermediate sleep apnea were not further studied because we desired to maximize our sensitivity to detect significant effects of sleep apnea on BNP. Those patients with intermediate sleep apnea were similar to the patients who underwent blood sampling (table 1).
Table 1.
Baseline characteristics of subjects found to have moderate sleep apnea, mild (or no) sleep apnea, and severe sleep apnea on baseline study. Patients with moderate sleep apnea did not have subsequent study days with blood sampling.
| Moderate Sleep Apnea (n = 47) | Mild Sleep Apnea (n = 5) | Severe Sleep Apnea (n = 12) | |
|---|---|---|---|
| Age (yrs) | 59 ± 12 | 56 ± 12 | 61 ± 13 |
| Sex (M) | 36 (76%) | 3 (60%) | 11 (92%) |
| Race (AA) | 11(23%) | 1 (20%) | 4 (33%) |
| EF (%) | 20 ±7 | 25 ± 6 | 19 ± 4* |
| Cardiomyopathy (Ischemic) | 26 (55%) | 2 (40%) | 6 (50%) |
| HR (min−1) | 68 ± 9 | 76 ± 12 | 64 ± 11* |
| BMI (kg/m2) | 32 ± 7 | 32 ± 7 | 34 ± 8 |
| Prescribed medications | |||
| Beta Blockade | 63(97%) | 5 (100%) | 12 (100%) |
| ACE inhibitors or ARB | 45(95%) | 5 (100%) | 12 (100%) |
| Loop diuretic | 41 (87%) | 4 (80%) | 10 (83%) |
| BNP (pg/ml) prior to sleep | 85 ± 90 | 243 ± 469 |
p < 0.05 patients with severe sleep apnea as compared to those with mild sleep apnea on baseline study
At least 2 weeks patients with severe sleep apnea underwent a third sleep study with frequent blood sampling and also with administration of supplemental oxygen (intervention night). The data from the 2 nights with frequent blood sampling obtained in subjects with severe sleep apnea together with the one night of blood sampling obtained in subjects with no or mild sleep apnea on the baseline test form the basis of the present study. (Note that some of the subjects without sleep apnea on baseline testing had mild or moderate sleep apnea on repeat testing; the group with AHI ≤ 5 on baseline testing is referred to as mild sleep apnea throughout this manuscript).
By comparing the BNP obtained during the normal sleep epochs with the BNP obtained during epochs with sleep abnormalities, the multiple time points provide the power to look at the immediate effects of apneic episodes, arousals and hypoxia on BNP. We evaluated 12 HF patients with severe sleep apnea, both with and without supplemental oxygen, and 5 HF patients with mild to moderate sleep apnea on the baseline test. This provided 643 time-series measurements of BNP levels and apnea counts.
Sleep recording methods
Standardized recording methods were utilized as previously described,10 and included continuous monitoring of left and right electro-oculogram, submental EMG, C3-A2 and C3-O1 electroencephalogram, anterior tibialis electromyogram, oronasal airflow as assessed by both a pressure sensitive nasal cannula and a thermistor, pulse oximetry and thoracic and abdominal movements with piezo-electric gauges, and a modified V5 ECG lead for cardiac rhythm monitoring. Patients were admitted to the unit at 5 p.m. for the sleep study. Thereafter, a standardized meal was provided for dinner at 6 p.m. Sleep study recording sensors were applied, and a forearm intravenous catheter was placed. The sleep study commenced at 10:00 p.m. (lights out), and ended at 6:00 a.m. the following morning.
Frequent venous sampling protocol
Venous blood was drawn every 20 minutes for BNP. An initial baseline sample was drawn at 10 P.M. at “lights out”. The next sample was drawn at 10:40 and every 20 minutes thereafter until 6 AM. The values recorded during the initial 40-minute epoch of all data that are recorded in the form of counts per epoch are deflated by 50% to make the frequencies comparable to those recorded during subsequent 20-minute epochs.
Analytic methods
Sleep study analysis
Standard methods for evaluating sleep structure and respiratory patterns were applied to all sleep study recordings and detailed in the appendix.
Assays
Samples were spun down and stored at −70°. BNP (pg/mL) was assayed by radioimmunoassay (fluorescence immunoassay, Biosite Inc.).
Primary Analysis
We used the data to look for an association between apnea episodes and BNP concentrations over the time the subjects were observed. With 17 subjects, 11 of whom were monitored for two nights, and 23 observations per patient per night (except for one patient who had one less observation) we have 643 observations with which to relate apnea to BNP. (Blood testing was not performed on one of the 12 treatment subjects during the intervention night, so no BNP data are available for this individual for that night) For each observation, we can relate the BNP concentration to sleep disordered breathing exposure over the preceding 20 minute epoch. To account for differences between treatment and non-treatment nights, different nights on which the same subject was observed are treated as independent observations.
In the primary analyses, we modeled the contemporaneous effects of sleep disordered breathing activity on BNP. Our major predictor variables consisted of measures of sleep disordered breathing severity. These metrics included the number of disordered breathing episodes (total, central and obstructive), the number of arousals and the severity of oxyhemoglobin desaturation during each 20 minute epoch. The severity of intermittent desaturation was reflected by the average difference in oxyhemoglobin saturation from the start to the end of each sleep disordered breathing episode (Avg Low SaO2). The overall severity of hypoxemia was represented by the percentage of time spent during each 20 minute epoch at oxygen saturation levels below 90% (t90).
The fundamental challenge in analyzing these relationships is that multiple factors might cause changes in both BNP and apnea characteristics. With repeated measurements of BNP and the independent variables throughout the night, however, we can detect a relationship while accounting separately for variations across individuals and variations over time. We use a variety of standard time-series regression techniques to accomplish this.
Supplementary Analyses
Because BNP remains in the blood after release by the left ventricle (with a half-life of approximately 20 minutes), and also because apneas could theoretically have a cumulative effect on hemodynamic stress, we investigated the possibility that apneas during a previous epoch may affect the level of BNP measured subsequently. We did this by adding five lags of the independent variable in our regressions, as shown in appendix equation (2). This allows us to measure the effect of apneas during the most recent epoch as well as the five previous 20-minute periods on the current level of BNP. We are therefore able to detect the effect of two hours’ worth of apneas on BNP. Further details are in the appendix.
We conducted an additional analysis to distinguish between the effects of hypoxia associated with CSA and hypoxia associated with OSA. We first separated the 643 epochs based on the prevalence of the different types of apneas. We eliminated the 78 epochs with no sleep apneas and separated the remaining 565 epochs into two categories. Predominantly-CSA epochs were those in which at least half of the apneas were central (192 epochs). The remainder were considered Predominantly-OSA epochs.
Any difference between the impact of CSA-associated hypoxia on BNP and the impact of OSA-associated hypoxia on BNP should show up when we distinguish the frequency of hypoxemia during Predominantly-CSA epochs from the frequency during Predominantly-OSA epochs. We therefore ran a multivariate regression of log BNP on two separate hypoxemia variables. The first variable is equal to the percent of time during each Predominantly-CSA epoch that was spent with pO2 < 90%, and it is equal to zero for all Predominantly-OSA epochs. Similarly, the second variable is equal to the percent of time that was spent with pO2 < 90% during Predominantly-OSA epochs. As in Table 3, we ran a fixed-effects regression with clustered standard errors.
Table 3.
Effect of Apneas on B-Type Natriuretic Peptide
| Regression | Independent variable | Coefficient | 95% Confidence Interval |
|---|---|---|---|
| Frequency Parameters | |||
| (1) | Total number of apneas and hypopneas | 0.0020 | [−0.0064, 0.0104] |
| (2) | Number of central apneas | −0.0014 | [−0.0185, 0.0156] |
| (3) | Number of obstructive apneas | 0.0037 | [−0.0094, 0.0168] |
| (4) | Number of arousals | −0.0033 | [−0.0138, 0.0072] |
| Desaturation Parameters | |||
| (5) | Percent with O2 sat. below 90% | 0.0096 | [0.0015, 0.0177]* |
| (6) | Average difference between peak and nadir of O2 saturation | 0.0169 | [−0.0100, 0.0439] |
Each row reports the result of a separate regression of the logarithm of BNP on the independent variable specified. Standard errors are clustered by patient-night, of which there are 28 distinct observations. Every regression also includes individual fixed effects and a linear time trend. The sample size is N=643 for each regression.
p = 0.02
Following the primary and secondary analyses discussed above, we checked the robustness of our general pattern of negative results to various changes in the model specification. The description of these analyses and the results are in the appendix.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
The characteristics of the patients studied are described in table 1. The patients with severe sleep apnea had a lower ejection fraction and a slower heart rate as compared to those with mild sleep apnea.
The sleep study results are shown in table 2. By design, the patients with severe apnea had more severe apneic and hypoxic parameters than those who had mild sleep apnea in the baseline study. These parameters were alleviated by supplementation of oxygen (night 3) as compared to the study without an intervention (night 2). As previously described, oxygen led to a modest reduction in AHI11 and an increased proportion of obstructive, as compared to central and mixed apnea.12
Table 2.
Sleep study results of patients with mild sleep apnea at baseline (mild sleep apnea) and those with severe sleep apnea without (night 2) and with (night 3) oxygen supplementation
| Mild Sleep Apnea | Severe Sleep Apnea night 2 | Severe Sleep Apnea night 3 (with O2) | |
|---|---|---|---|
| Total AHI | 16 ± 8 | 66 ± 31 | 48 ± 18* |
| % Obstructive Apneas | 93 ± 8 | 57 ± 37 | 74 ± 34* |
| % Central Apneas | 2.2 ± 1.8 | 18 ± 25 | 15 ± 27 |
| % Mixed Apneas | 4.3 ± 7.4 | 26 ± 22 | 11 ± 14* |
| Total Arousal Index (n/hr) | 13 ± 8 | 47 ± 23 | 39 ± 21 |
| T90 (%) | 0.49 ± 0.60 | 15.2 ± 19.6 | 1.7 ± 3.6* |
| Baseline SaO2 | 96.9 ± 1.4 | 96.0 ± 2.0 | 98.1 ± 0.8* |
| Avg Low SaO2 (%) | 93.6 ± 2.0 | 88.9 ± 4.4 | 94.7 ± 2.4* |
| Baseline log BNP | 3.5 ± 2.1 | 3.6 ± 2.6 | 4.5 ± 1.3 |
| Mean log BNP | 3.9 ± 1.3 | 3.5 ± 2.5 | 4.5 ± 1.4 |
p < 0.05 night 3 v. night 2. Note that patients in the mild sleep apnea group had mild or no sleep apnea on the baseline study, but may have had mild to moderate sleep apnea on the subsequent study. Mean log BNP refers to the mean of all samples obtained through the night.
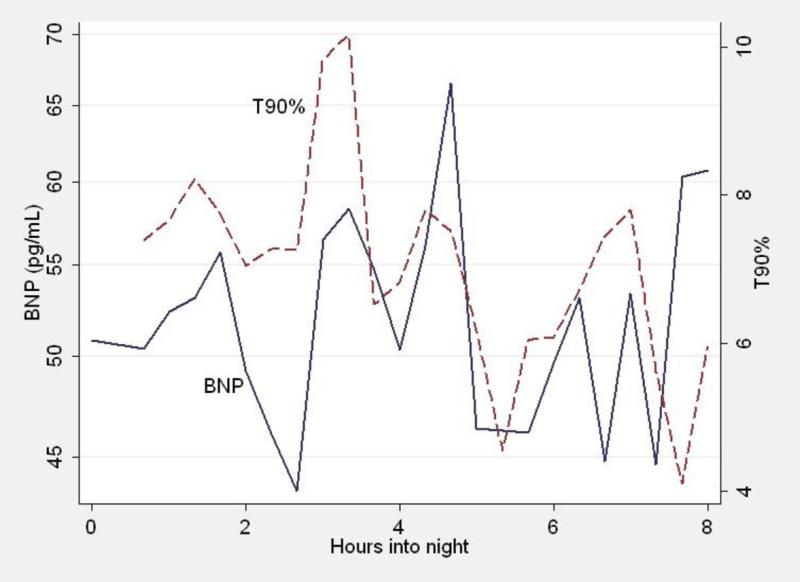
Figure 1 illustrates the time course of mean BNP and hypoxemia in each 20 minute period through the night for all patients. At each time point, the percentage of time for that epoch spent with pO2 < 90% (t90) is shown (dashed line). The values graphed are the means for all patients. Similarly, the mean BNP concentrations for all patients are shown. Overall, no progressive change in BNP is detected over time. However, the individual fluctuations of BNP at various time points appear to coincide with t90.
Figure 1.
For each time point through the night, the mean BNP of all patients (continuous line) and the percentage of time with pO2 < 90% (dashed line) are shown. Note that the BNP appears to fluctuate with the extent of hypoxemia.
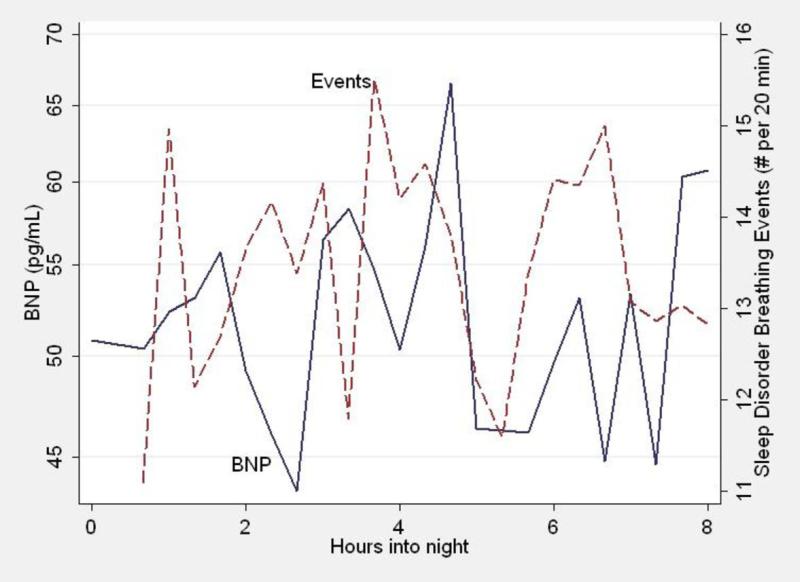
Figure 2 demonstrates the time course of BNP (as described above) and the number of sleep disordered breathing episodes in each 20 minute period through the night for all patients. There is no relationship between the number of sleep disordered breathing episodes at each time point and the change in BNP.
Figure 2.
For each time point through the night, the mean BNP of all patients (continuous line) and the frequency of sleep disordered breathing episodes (dashed line) are shown. Note that the BNP does not appear to fluctuate with the frequency of sleep disordered breathing episodes.
The results of the primary regressions are presented in Table 3. Each row of the table shows the relationship between a different independent variable and the logarithm of BNP, controlling for a linear time trend and individual fixed effects. There was minimal effect of the number of episodes on BNP. However, the impact of hypoxemia is emphasized by the relationship of BNP concentration to both the percent of the epoch that a patient spent with oxygen saturation below 90 percent and the difference in O2 saturation between the start and end of each sleep disordered breathing episode.
The point estimates of BNP responses to sleep disordered breathing frequency parameters are small, not significant, and are neither consistently positive nor negative. (Table 3) For example, the coefficient on the total number of apneas (regression 1) is only 0.002. The standard errors are small enough that we can say with 95 percent certainty that the true coefficient is below 0.0104. Interpreting this clinically, 10 apneas in 20 minutes—or 30 apneas per hour—would increase BNP by less than 10.4%. The coefficients on the other independent apnea variables are also precisely estimated to be close to zero. Even obstructive apneas (regression 3), which would be expected to cause elevations in BNP from hemodynamic stress, have a point estimate of 0.0037, and an upper bound of 0.0168. Thus, 30 extra obstructive apneas per hour would increase BNP at the most by 16.8%.
In contrast to the frequency of sleep disordered events, nocturnal hypoxemia predicts elevations in BNP. The percent of the epoch that a patient spent with oxygen saturation below 90% has a significant positive coefficient of 0.0096, meaning that an extra 10% of the epoch (i.e., 2 minutes) at low oxygenation increases BNP by 9.6%, with an upper bound of 17.7% and a lower bound of 1.5%. A similar effect of intermittent desaturation (regression 6) on BNP was observed. Although not statistically significant, the coefficient of 0.0169 indicates that an extra 10 percentage points of average saturation difference increases BNP by 16.9% (C.I. −10.0% to 43.9%).
We did not detect any significant differences in BNP between nights 2 and 3. The power to compare BNP on night 2 and night 3 is limited by the lack of randomization and the small number of patients. Indeed, baseline log BNP (before oxygen was administered) on night 3 was elevated as compared to night 2. (table 2)
Secondary Analyses
There was no latency to a BNP response. The number of sleep disordered breathing episodes in the preceding 1, 2, 3, 4, or 5 twenty minute epochs did not predict log BNP (table 3 in appendix). Similarly, t90 in preceding epochs generally did not predict log BNP, suggesting that BNP rose contemporaneously with hypoxic exposure. Supplementary table available online.
The results of the analysis comparing the effects of central and obstructive sleep apnea are as follows:
The brackets following each coefficient show 95% confidence intervals. A t-test for equality of the 2 coefficients is unable to reject equality (P=0.85). Thus it appears that hypoxemia associated with CSA has the same impact on BNP as hypoxemia associated with OSA
DISCUSSION
The present study found that in patients with stable HF, changes in BNP during the night were related to the severity of nocturnal hypoxemia, but not to the frequency or type of sleep disordered breathing episodes or associated arousals. Mechanical and neurohormonal effects of sleep disordered breathing might be expected to produce hemodynamic stress. Nevertheless, intensive sampling of BNP showed no relationship between BNP and the frequency of sleep disordered breathing events or arousals. In contrast, the severity of hypoxemia (defined as percentage of time with an oxygen saturation < 90%) did appear to correlate with increased BNP. Few previous studies have separated out hypoxia from non-hypoxic arousals and obstruction, and our findings show divergent effects of hypoxic and non-hypoxic episodes. This suggests that sleep disordered breathing may be detrimental in HF because of hypoxia exerting adverse effects.
Hemodynamic Effects of Sleep Apnea
The interaction between heart failure and sleep apnea is bi-directional. Hemodynamic disturbances associated with left ventricular dysfunction predispose to periodic breathing and a worsening of sleep apnea,13,14 but there is also evidence that sleep apnea exacerbates heart failure and treatment can improve outcomes.5,6 Although apnea has hemodynamic consequences, it is unclear whether these adverse cardiac sequellae are due to the hypoxia that is associated with the apnea or to the direct hemodynamic effects of the apneas.
Upper airway obstruction does increase cardiac preload and afterload when dogs inspire repeatedly against an occluded upper airway, but these effects are transient and dissipate during expiration. Perhaps more important are the findings that arousals can exacerbate left ventricular dysfunction by their effects on sympathetic drive, arterial vasoconstriction, and blood pressure.15 Similarly, sleep apnea is associated with recurrent nocturnal surges in blood pressure following each apnea cycle.16 These findings could be secondary to either direct hemodynamic effects or hypoxia. While sleep apnea is clearly associated with hemodynamic changes, the present study suggests that the acute hemodynamic effects of obstructive apnea do not cause elevations in BNP.
Cardiovascular effects of hypoxia
In contrast to the lack of impact of apnea or arousal frequency on BNP, the relationship with hypoxia suggests that mechanisms caused by hypoxia do impact cardiovascular function and stress. There is growing evidence that hypoxia is the cause of the cardiovascular effects of sleep apnea. In anesthetized dogs and sedated pigs, investigators have found that the hemodynamic sequellae of sleep apnea are related to the severity of oxyhemoglobin desaturations during apneic episodes17. The mechanisms might include the negative inotropic effect of hypoxia18 or the sympathetic drive caused by hypoxia. Hypoxia impairs contractility by altering the action potential which, in turn, interferes with excitation-contraction coupling.19 Hypoxia also leads to a rise in intracellular sodium and calcium, which can be deleterious to the myocytes. Interestingly, in single adult rat myocytes, moderate hypoxia may cause earlier calcium loading and more progressive cell destruction than complete anoxia.20 Hypoxia leads to increased sympathetic activity and arousals at the termination of apneic episodes.21 Moreover, central autonomic responses to intermittent hypoxemia and arousal overwhelm local vasodilatory responses and trigger pronounced sympathetic neural discharge, peripheral vasoconstriction and increases in blood pressure and cardiac after-load.19
The association of sleep apnea and hypertension may also be related to hypoxia, rather than arousals. While recurrent arousals increase blood pressure acutely following apneic episodes, blood pressure remains elevated during wakefulness only when nocturnal intermittent hypoxemia is superimposed.22 Indeed, intermittent hypoxemia in combination with arousals may be responsible for the finding in humans that sympathetic nerve activity is elevated in apneic patients compared to weight, age and gender matched controls.23
Repeated cycles of hypoxemia followed by re-oxygenation triggers the generation of reactive oxygen species and increases oxidative stress,24 ,25 which, in turn, induces the release of inflammatory cytokines (e.g., TNF-α).26 These alterations may, in the long term, exacerbate left ventricular dysfunction.
Biomarkers of Cardiovascular Stress in Heart Failure and Sleep Apnea
B-type natriuretic peptide (BNP), a non-invasive marker of cardiovascular stress, reflects the hemodynamic status and prognosis of heart failure patients. Numerous studies have demonstrated the relationship between BNP and heart failure severity.27 Importantly, BNP can acutely reflect changes in hemodynamics. For example, it closely tracked improvement in the pulmonary arterial wedge pressure when HF patients were treated for pulmonary edema.28
Previous studies in sleep apnea patients suggest that natriuretic peptide concentrations might be affected by sleep apnea and may be impacted by treatment.29, 30, 31 In a very small study, OSA appeared to increase ANP overnight, but not BNP.32 In contrast, in 22 children, BNP increases overnight did correlate with AHI.33 Supporting the findings of the present study, hypoxemia increased the levels of NT-pro-BNP in healthy young men34 and oxygen therapy decreased BNP concentrations in heart failure patients with central sleep apnea.35 These studies, however, did not look at the cause of decreases in BNP.
CONCLUSION
Our findings suggest that hypoxia is an important factor in the impact of sleep abnormalities in patients with heart failure. The present study does not identify whether hypoxia mediates BNP release through direct effects on cardiac contractility, sympathetic nervous system activation, oxidative stress, or other unknown mechanisms. However, the finding that acute changes in BNP are associated with hypoxia rather than apneic episodes suggests a mechanism for the association of sleep abnormalities with heart failure. Prevention of hypoxia might be especially important in patients with sleep disordered breathing and heart failure. A larger sample size and randomized study design will be required to test this hypothesis.
Supplementary Material
Acknowledgments
Funding Sources This study was supported by Grant Number R01 HL071506 from the National Heart Lung and Blood Institute (NHLBI), NIH, and by Grant Number M01-RR02719 from the National Center for Research Resources (NCRR), a component of NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. This study was supported, in part, by the Intramural Research Program of the National Institute on Aging, NIH, a portion of which was through a Research and Development Contract with MedStar Research Institute. BNP assays were provided by Biosite, Inc., San Diego, CA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 2.Lanfranchi PA, Somers VK, Braghiroli A, Corra U, Eleuteri E, Giannuzzi P. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation. 2003;107(5):727–32. doi: 10.1161/01.cir.0000049641.11675.ee. [DOI] [PubMed] [Google Scholar]
- 3.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153(1):272–6. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 4.Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, Giannuzzi P. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–40. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 5.Bradley TD, Holloway RM, McLaughlin PR, et al. Cardiac output response to continuous positive airway pressure in congestive heart failure. Am Rev Respir Dis. 1992;145(2 Pt 1):377–382. doi: 10.1164/ajrccm/145.2_Pt_1.377. [DOI] [PubMed] [Google Scholar]
- 6.Sin DD, Logan AG, Fitzgerald FS, et al. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000;102(1):61–66. doi: 10.1161/01.cir.102.1.61. [DOI] [PubMed] [Google Scholar]
- 7.O'Donnell CP, Schwartz AR, Smith PL, et al. Reflex stimulation of renal sympathetic nerve activity and blood pressure in response to apnea. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1763–1770. doi: 10.1164/ajrccm.154.6.8970368. [DOI] [PubMed] [Google Scholar]
- 8.Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation. 2003;107(12):1671–8. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 9.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–8. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayappa I, Norman RG, Krieger AC, et al. Non-Invasive detection of respiratory effort-related arousals (REras) by a nasal cannula/pressure transducer system. Sleep. 2000;23(6):763–771. doi: 10.1093/sleep/23.6.763. [DOI] [PubMed] [Google Scholar]
- 11.Gold AR, Schwartz AR, Bleecker ER, Smith PL. The effect of chronic nocturnal oxygen administration upon sleep apnea. Am Rev Respir Dis. 1986;134:925–929. doi: 10.1164/arrd.1986.134.5.925. [DOI] [PubMed] [Google Scholar]
- 12.Gold AR, Bleecker ER, Smith PL. A shift from central and mixed sleep apnea to obstructive sleep apnea resulting from low-flow oxygen. Am Rev Respir Dis. 1985;132:220–223. doi: 10.1164/arrd.1985.132.2.220. [DOI] [PubMed] [Google Scholar]
- 13.Cherniack NS, Longobardo GS. Cheyne-Stokes breathing. An instability in physiologic control. N Engl J Med. 1973;288:952–7. doi: 10.1056/NEJM197305032881810. [DOI] [PubMed] [Google Scholar]
- 14.Longobardo GS, Gothe B, Goldman MD, et al. Sleep apnea considered as a control system instability. Respir Physiol. 1982;50(3):311–333. doi: 10.1016/0034-5687(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 15.Schneider H, Schaub CD, Chen CA, et al. Neural and local effects of hypoxia on cardiovascular responses to obstructive apnea. J Appl Physiol. 2000;88(3):1093–1102. doi: 10.1152/jappl.2000.88.3.1093. [DOI] [PubMed] [Google Scholar]
- 16.Ringler J, Garpestad E, Basner RC, et al. Systemic blood pressure elevation after airway occlusion during NREM sleep. Am J Respir Crit Care Med. 1994;150:1062–1066. doi: 10.1164/ajrccm.150.4.7921437. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Sica AL, Scharf SM. Mechanisms of acute cardiovascular response to periodic apneas in sedated pigs. J Appl Physiol. 1999;86(4):1236–1246. doi: 10.1152/jappl.1999.86.4.1236. [DOI] [PubMed] [Google Scholar]
- 18.Kusuoka H, Weisfeldt ML, Zweier JL, Jacobus WE, Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circ Res. 1986;59:270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- 19.Stern MD, Silverman HS, Houser SR, Josephson RA, Capogrossi MC, Nichols CG, Lederer WJ, Lakatta EG. Anoxic contractile failure in rat heart myocytes is caused by failure of intracellular calcium release due to alteration of the action potential. Proc Natl Acad Sci U S A. 1988;85:6954–8. doi: 10.1073/pnas.85.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverman HS, Stern MD, Lakatta EG. Contrasting effects of anoxia and graded hypoxia on single cardiac myocyte function. Am J Cardiovasc Pathol. 1992;4:256–64. [PubMed] [Google Scholar]
- 21.O'Donnell CP, Ayuse T, King ED, et al. Airway obstruction during sleep increases blood pressure without arousal. J Appl Physiol. 1996;80(3):773–781. doi: 10.1152/jappl.1996.80.3.773. [DOI] [PubMed] [Google Scholar]
- 22.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106–9. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips BG, Kato M, Narkiewicz K, et al. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279(1):H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 24.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162(2 Pt 1):566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 25.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162(6):2166–2171. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 26.Ertel W, Morrison MH, Ayala A, et al. Hypoxemia in the absence of blood loss or significant hypotension causes inflammatory cytokine release. Am J Physiol. 1995;269(1 Pt 2):R160–R166. doi: 10.1152/ajpregu.1995.269.1.R160. [DOI] [PubMed] [Google Scholar]
- 27.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–68. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Maisel A. B-type natriuretic peptide in the diagnosis and management of congestive heart failure. Cardiol Clin. 2001;19(4):557–571. doi: 10.1016/s0733-8651(05)70243-5. [DOI] [PubMed] [Google Scholar]
- 29.Krieger J, Laks L, Wilcox I, et al. Atrial natriuretic peptide release during sleep in patients with obstructive sleep apnoea before and during treatment with nasal continuous positive airway pressure. Clin Sci (Lond) 1989;77(4):407–411. doi: 10.1042/cs0770407. [DOI] [PubMed] [Google Scholar]
- 30.Kita H, Ohi M, Chin K, et al. The nocturnal secretion of cardiac natriuretic peptides during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res. 1998;7(3):199–207. doi: 10.1046/j.1365-2869.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- 31.Tasci S, Manka R, Scholtyssek S, Lentini S, Troatz C, Stoffel-Wagner B, Löderitz B. NT-pro-BNP in obstructive sleep apnea syndrome is decreased by nasal continuous positive airway pressure. Clin Res Cardiol. 2006 Jan;95(1):23–30. doi: 10.1007/s00392-006-0315-9. [DOI] [PubMed] [Google Scholar]
- 32.Svatikova A, Shamsuzzaman AS, Robert Wolk R, Phillips BG, Olson LJ, Somers VK. Plasma Brain Natriuretic Peptide in Obstructive Sleep Apnea. Am J Cardiol. 2004;94:529–532. doi: 10.1016/j.amjcard.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Kaditis AG, Alexopoulos EI, Hatzi F, Kostadima E, Kiaffas M, Zakynthinos E, Gourgoulianis K. Overnight change in brain natriuretic peptide levels in children with sleep-disordered breathing. Chest. 2006;130:1377–84. doi: 10.1378/chest.130.5.1377. [DOI] [PubMed] [Google Scholar]
- 34.Due-Andersen R, Pedersen-Bjergaard U, Høi-Hansen T, Olsen NV, Kistorp C, Faber J, Boomsma F, Thorsteinsson B. NT-pro-BNP during hypoglycemia and hypoxemia in normal subjects: impact of renin-angiotensin system activity. J Appl Physiol. 2008;104(4):1080–5. doi: 10.1152/japplphysiol.01082.2007. [DOI] [PubMed] [Google Scholar]
- 35.Shigemitsu M, Nishio K, Kusuyama T, Itoh S, Konno N, Katagiri T. Nocturnal oxygen therapy prevents progress of congestive heart failure with central sleep apnea. Int J Cardiol. 2007;115:354–60. doi: 10.1016/j.ijcard.2006.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.