Abstract
This study analyzed the antimicrobial effect of photodynamic therapy (PDT) in association with endodontic treatment. Twenty patients were selected. Microbiological samples were taken after accessing the canal, endodontic therapy, and PDT. At the end of the first session, the root canal was filled with Ca(OH)2, and after 1 week, a second session of the therapies was performed. Endodontic therapy gave a mean reduction of 1.08 log. The combination with PDT significantly enhanced the reduction (1.83 log, p = 0.00002). The second endodontic session gave a similar diminution to the first (1.14 log), and the second PDT was significantly more effective than the first (p = 0.002). The second total reduction was significantly higher than the second endodontic therapy (p = 0.0000005). The total first + second reduction (3.19 log) was significantly different from the first combination (p = 0.00006). Results suggest that the use of PDT added to endodontic treatment leads to an enhanced decrease of bacterial load and may be an appropriate approach for the treatment of oral infections.
Keywords: Endodontics, polyethylenimine and chlorin(e6) conjugate, red laser, root canal
Elimination of the pathogenic microflora from the root canal system during endodontic therapy is one of the main goals of endodontic treatment. Microbial infection plays an important role in the development of necrosis in the dental pulp and the formation of periapical lesions (1). It is well established that the eradication of bacteria from root canals is difficult, and current endodontic techniques are unable to consistently disinfect the canal systems (2). Accepted treatment procedures to eliminate the infection include root canal debridement and mechanical shaping or smoothing (3), irrigation with disinfectant agents such as sodium hypochlorite or hydrogen peroxide, the application of an interappointment dressing containing an antimicrobial agent, and sealing of the root canal (4). In case of infection, the use of antibiotics and antiseptics is an alternative approach, but the long-term use of chemical antimicrobial agents, however, can be rendered ineffective by resistance developing in the target organisms (5–7).
Studies have shown that in cases when a negative microbiological culture has been obtained from the root canal at the time of obturation, there is a 94% success rate. On the other hand, when obturation is performed in a positive culture, the success rate is reduced to 68%. Previous studies have shown that the shoddier healing of periapical lesions is more likely in obturated root canals with positive cultures by the end of the endodontic treatment (8, 9).
Novel approaches to disinfecting root canals have been proposed recently that include the use of high-power lasers (10) as well as photodynamic therapy (PDT) (11, 12). High-power lasers function by dose-dependent heat generation, but, in addition to killing bacteria, they have the potential to cause collateral damage such as char dentine, ankylosis roots, cementum melting, and root reabsorption and periradicular necrosis if incorrect laser parameters are used (13).
PDT is a new antimicrobial strategy that involves the combination of a nontoxic photosensitizer (PS) and a light source (14). The excited photosensitizer reacts with molecular oxygen to produce highly reactive oxygen species, which induce injury and death of microorganisms (15, 16). It has been established that PS, which possess a pronounced cationic charge, can rapidly bind and penetrate bacterial cells, and, therefore, these compounds show a high degree of selectivity for killing microorganisms compared with host mammalian cells (17, 18). PDT has been studied as a promising approach to eradicate oral pathogenic bacteria (19, 20) that cause diseases such as periodontitis (21), peri-implantitis (22), and caries (23). We recently reported on the use of PDT using a polyethyleneimine (PEI) chlorin (e6 [ce6]) conjugate and fiberoptic delivered red light to combat endodontic infection caused by bioluminescent bacteria in an ex vivo model using extracted human teeth (24). When PDT followed conventional endodontic therapy, there was significantly more killing and less bacterial growth than was seen after endodontic therapy alone. Therefore, the aim of the present study was to test this combination of conventional endodontic therapy followed by antimicrobial PDT in a clinical trial in patients requiring endodontic treatment.
Materials and Methods
Photosensitizer
The PS used was a conjugate between PEI and ce6, and the synthesis and characterization has been previously described in detail (24, 25). Briefly, high–molecular-weight–branched PEI (MWt ¼10,000–25,000; Aldrich Chemical Catalog #40,872-7, Milwaukee, MI) was reacted with ce6 (Porphyrin Products, Logan, UT) in the presence of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (Sigma, St Louis, MO). The conjugate was purified by size exclusion chromatography and characterized by HPLC on a diol column. The conjugate had an average substitution ratio of 1 ce6 per PEI chain. It was used in a phosphate-buffered saline solution at 60 μmol/L.
Light Source
The illumination was performed with a 300-μm diameter fiber-coupled diode laser (MMOptics, São Carlos, SP, Brazil). The laser delivered 660 nm light at a total power of 40 mW without the fiber. The fiber was initially placed in the apical portion of the root canal at a point in which resistance to the fiber was just felt and spiral movements, from apical to cervical, were manually performed to ensure an equal diffusion of the light inside the canal lumen (26, 27). These movements were repeated approximately 10 times per minute.
Endodontic PDT
The same practitioner performed this study in a private dental office in São Paulo, Brazil. The patients were selected at random; they were in good health and between the ages of 21 and 35. They presented with symptoms of necrotic pulp and periapical periodontitis, all requiring root canal treatment on teeth with closed apices. The protocol was reviewed and approved by the Institutional Review Board of the São Paulo University, and all trial procedures were conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from each subject.
Twenty root canals from anterior teeth (incisors and canines) were treated. A periapical radiograph was taken for each case to determine the presence of apical lesion, the canal morphology, the length, and the number of canals. Only single relatively straight root canal was selected.
The access to the pulp chamber was gained after the installation of a rubber dam, and then the surrounding area was irrigated with 5 mL of chlorhexidine solution at 2% to ensure that the crown of the tooth was with minimal microbial load.
Once the canal was accessed, a K file #10 (Maillefer Instruments SA, Ballaigues, Switzerland) was inserted inside the canal, approximately until the apical portion of the canal. The file was moved in a backwards and forwards motion to remove the necrotic tissue, and then the root canal was irrigated with 1 mL of sterile saline solution and the canal was dried with 3 sterile paper points (Dentsply Latin America, Petrópolis, RJ, Brazil) left inside the root canal for 1 minute. All 3 paper points were combined for initial colony forming unit (CFU) determination. This procedure provided the first microbiological sample representing the initial contamination of the root canal. The paper points were deposited in a sterile bottle with fresh Moller's VMGA (viability medium Göteborg anaerobic) III transport medium.
The canals were prepared with manual instrumentation by K files using a standard crown-down technique working to 1 mm short of the working length. The final geometry was conical; the coronal portion of the canal was prepared with #1, #2, #3 Gates-Glidden drills (Maillefer Instruments SA, Ballaigues, Switzerland); and the apical preparation was made with #40 file resulting in a taper of 0.02. This allowed a good penetration of the irrigating agents and the insertion of the optical fiber until the full working length. The root canals were irrigated with 10 mL of 2.5% sodium hypochlorite solution, followed by 10 mL of 3% hydrogen peroxide solution using an endodontic needle (27G) between each file. At the end of the procedure, the root canals were irrigated with 5 mL of a 17% EDTA (28). The canal was irrigated with 5 mL of sterile saline solution to remove the antimicrobial agent and dried with another 3 paper points (second microbiological sample).
The solution of the photosensitizer was placed inside the root canal (0.5 mL) with an endodontic needle and left inside the root canal for 2 minutes as a preirradiation time. After this time, the root canal was irradiated with the diode laser coupled with the optical fiber for 240 seconds (total energy 9.6 J) as described earlier. A brand new fiber was used for each patient. The root canal was again irrigated with 10 mL of sterile saline solution to remove the photosensitizer and dried with another 3 paper points (third microbiological sample).
A calcium hydroxide paste (Ultradent Products, South Jordan, UT) was placed into the canals. A sterilized cotton ball was placed in the pulp chamber, and the tooth was dressed with temporary restorative material (IRM; Dentsply Latin America, Petrópolis, RJ, Brazil).
At a subsequent visit 1 week later, each canal was again sampled to evaluate the recolonization, and then a second endodontic therapy and a second session of PDT was performed following the same procedures as described previously. Each root canal was then sealed by using conventional techniques with Sealer 26 (Dentsply Latin America, Petrópolis, RJ, Brazil), and the tooth was restored with composite resin (Filtek Z350 3M ESPE, St Paul, MN).
Microbiological Analyses
The method of culture was selected to assess the microbial load of common aerobes, facultative anaerobes, and microaerophilic such as Enterococcus sp, Candida sp, Lactobacillus sp, and Porphyromonas sp found in infected root canals. However no attempt was made to identify the specific microbial flora during the process (2).
Once they arrived at the microbiological facility, the paper points were removed from the anaerobic transport medium (VMGA III), placed inside a 1.5-mL microcentrifuge with brain-heart infusion (BHI) broth, and positioned in a vortex for 30 seconds. One hundred–micro-liter aliquots were added to wells of a 96-well plate for serial dilution and streaking on square BHI agar plates for CFU enumeration according to the method of Jett et al. (29). The plates were placed inside a microaerophilic chamber with 5% oxygen, 15% carbon dioxide, and 80% nitrogen and incubated for 72 hours at 37°C (30). At each stage of the treatment (initial, after endodontic treatment, and after PDT), the CFUs were counted. Survival fractions were calculated from each tooth taking into account its initial bacterial load.
Statistical Analysis
Values are given as means, and error bars are standard deviations. Statistical comparisons between means were performed with a paired t test using Microsoft Excel (Redmond, WA); 2-tailed p values are reported.
Results
The radiographic examination confirmed the diagnosis of necrotic pulp and periapical lesions for all the patients selected, and the analysis of the first microbiological sample corroborated the presence of infection in all teeth. The initial infectious burden did vary widely between individual teeth with a mean value of 55,214 CFU per 3 paper points (range, 204,000 to 71). This variation was probably caused by differences in the internal anatomy and geometry of the individual root canal systems and the duration of the infections and the presence or absence of infiltration or caries on the teeth in the beginning of the treatment. The mean values of infectious burden over all 20 teeth for each stage of the study and the log reduction for each step plus the appropriate statistical comparisons are presented in Table 1. The values for individual log reductions are shown in Figure 1. After the initial endodontic therapy, the mean infectious burden was reduced to 7,193 CFU (range 48,000 to 6), a mean log reduction of 1.08 or 91%. The mean infectious burden after subsequent PDT was 2,033 (range, 8,900 to 0), a further mean log reduction of 0.74 or 82%. The overall mean log reduction was 1.83 or 98.5%, and this was significantly greater than that achieved by endodontic therapy alone (p = 0.0005). None of the root canals treated had 100% microbial reduction after endodontic treatment, whereas two teeth showed total absence of microorganisms after the combination of endodontic treatment and PDT.
Table 1.
Qualitative Results, Log Reductions, and Corresponding P Values Obtained in the Study
| Treatment | CFU* | Log Reduction† | Significance‡ |
|---|---|---|---|
| Initial | 55,214 ± 78,595 | ||
| Post 1st endodontic Tx | 7,193 ± 13,549 | 1.08 ± 0.39 | |
| Post 1st PDT | 28,033 ± 3,885 | 0.74 ± 0.6 | |
| Total log redn 1st | 1.83 ± 0.75 | p = 2 × 10−5 vs endo | |
| No. of sterile cultures | Endo = 0 | ||
| PDT = 2 | |||
| Recolonization | 24,280 ± 39,912 | ||
| Post 2nd endodontic Tx | 1,939 ± 4,217 | 1.14 ± 0.31 | |
| Post 2nd PDT | 136 ± 345 | 1.61 ± 0.97 | p = 2 × 10−3 vs 1st PDT |
| Total log redn 2nd | 2.75 ± 1.04 | p = 5 × 10−7 vs 2nd endo | |
| Total log redn 1st + 2nd | 3.19 ± 1.1 | p = 6 × 10−5 vs 1stcombo | |
| No. of sterile cultures | Endo = 0 | ||
| PDT = 5 |
Mean CFU values (± standard deviation) from 3 paper points from each of 20 teeth.
Mean log(10)(CFU before)/(CFU after) (± standard deviation) for each successive treatment.
A two-tailed paired Student t test assuming unequal variance.
Figure 1.
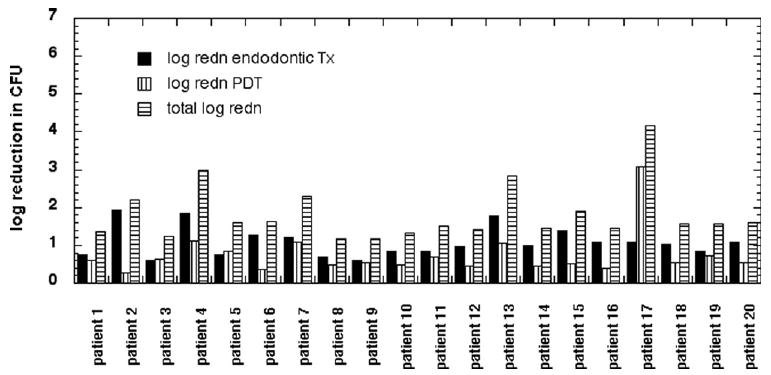
Log(10)(CFU before)/(CFU after) for each patient (3 paper points) after conventional endodontic treatment, after antimicrobial PDT, and total reduction after combination therapy.
All the root canals showed bacterial recolonization after 1 week with the mean CFU value being 24,280 (range, 123,097 to 27), but the recolonization was only about 40% of the original microbial load found. Log reductions in CFU values achieved in the second treatment are shown in Figure 2. The second conventional endodontic therapy achieved a similar reduction in bacterial burden to the first treatment giving a mean CFU value of 1939 (range, 14,273 to 4), a log reduction of 1.14 or 92% (Table 1). However, the second PDT produced a further reduction to a mean CFU value of 136 (range, 1,240 to 0), a log reduction of 1.61 or 97%. This was significantly greater than the reduction achieved by the first PDT (p = 0.002). The additional reduction achieved by the second PDT was highly significant compared with endodontic therapy alone (p = 0.000001). The overall reduction achieved by the two successive combination treatments (a log reduction of 3.19 or more than 99.9%) was significantly greater than that achieved by the first combination treatment (p = 0.00006). Five teeth were completely free of bacteria after the two combination therapies.
Figure 2.
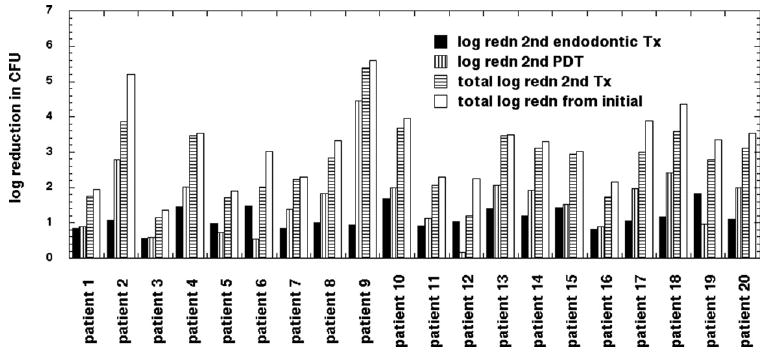
Log(10)(CFU before)/(CFU after) recolonization for each patient (3 paper points) after second conventional endodontic treatment, after second antimicrobial PDT, total log reduction after second combination therapy, and total log reduction after two combination therapies.
When the results from the individual teeth are inspected, it can be seen that patients 9 to 12, who had a particularly high infectious burden, had relatively modest reductions after the first combination treatment but had some of the highest reductions after the second combination treatment (Fig. 2) largely because of the enhanced efficacy of the second PDT.
Discussion
We have previously shown (24) that a combination of conventional endodontic therapy followed by antimicrobial PDT was highly effective in reducing bacterial load in an ex vivo model of infected human root canals in extracted teeth. In that study, we used bioluminescent bacteria and a conjugate between PEI-ce6 as the PS. The experiments were performed by illuminating inside the root canal for periods of 1, 2, 3, and 4 minutes and measuring the contamination by using bioluminescent images after each minute of illumination (2.4 J/min). That study showed that there was a fluence-dependent reduction in contamination until an energy of 9.6 J (240 seconds) when a plateau was reached and further illumination ceased to have a noticeable effect. Therefore, this fluence was chosen for the clinical PDT trial (24). The positive results of the preclinical study encouraged us to test this novel combination therapy in a clinical trial. The photosensitizer used, PEI-ce6, is a covalent conjugate between a basic synthetic polymer (PEI) and a photosensitizer derived from chlorophyll (ce6) that has a pronounced overall positive charge. It has been designed to bind and penetrate both gram-positive and gram-negative bacterial cell walls that have negative charges while not binding strongly to host mammalian cells. Its binding to bacteria is also rapid, whereas its uptake by mammalian cells is slow because of its large molecular weight (circa 12,000). These two reasons account for its selectivity for bacteria over host cells in antimicrobial PDT. PEI-ce6 has not received regulatory approval for human use, but ce6 itself has been used in PDT clinical trials in Europe and Asia (31).
Some studies (2, 5, 8) have shown that culturing of root canal microflora is complicated, and it demands microbiological facilities in close proximity to the dental office to ensure that microorganisms do not die in transit. However, it is the most effective short-term means of evaluating the disinfection of root canals in vivo (2). To avoid this problem, canal samples were cultured within 1 hour after the sample had been taken. It was decided that a quantitative method to count the total microorganisms assessed inside the root canal would be appropriate because the aim of this study was to verify the number of microorganisms present after endodontic treatment and subsequent antimicrobial PDT.
In conventional endodontic treatment of infected root canals, reducing the bacterial count is accomplished by a combination of mechanical instrumentation, various irrigation solutions, and antimicrobial medication or dressings placed into the canal (27). PDT is a treatment that can be delivered as an addition to conventional endodontic therapy and produces a remarkable additional reduction in bacterial burden. Moreover it appears that a second PDT treatment is even more effective than the first PDT. The reason for this observation is probably that the recolonization of microorganisms occurs in a less complex biofilm compared with the initial infection that is probably in a fully developed biofilm where even the polycationic PS finds difficulty in fully penetrating. Another possible reason could be that the higher pH promoted by the calcium hydroxide paste used between appointments could improve the photoreaction because it has been reported that the probability of reactive oxygen species production, in particular singlet oxygen, is improved in alkaline environment (32). Furthermore, as in the second treatment, the number of viable microorganisms was smaller than in the first treatment; the reactive oxygen species formed during PDT had a bigger chance of producing an irreparable oxidative stress because of the ratio between reactive oxygen species and micro-organisms.
Comparing our results with in vitro studies, Seal et al. (12) and Lee et al. (11) have reported results using PDT in root canal treatments; both authors have used phenothiazine-based PS and low-intensity red lasers against gram-positive bacteria but did not use an optical fiber to access the root canal lumen. Seal et al. (11) found that 3% sodium hypochlorite irrigation killed more Streptococcus intermedius in the endodontic biofilms than PDT with 100 μg/mL toluidine blue and 21 J of 632 nm laser light. Garcez et al. (27) using Enterococcus faecalis, a more relevant endodontic pathogen, and an optical fiber to access the root canal and the same methodology for irradiation achieved better results than the cited authors. These results undoubtedly indicate the use of an optical fiber to improve the irradiation in root canals. The fiber probably distributes homogeneously the light inside the root canal guaranteeing a better photoreaction; also, the technique of irradiation using helicoid movements may have contributed to the results.
Literature about antimicrobial PDT shows surprisingly few reports of its use to treat localized infections in vivo. The in vivo studies of Bonsor and coworkers (2, 33) using tolonium chloride as the photosensitizer and a diode laser coupled with an optical fiber as a light source were successful in eliminating all the microorganisms found in the initial root canal infection. The use of a chelating agent after instrumentation, in our case EDTA instead of citric acid used by Bonsor, acts as a cleaner and disrupter of the biofilm expanding the access of the PS to the canal system.
Working in vivo is more complex because the variance of root canal anatomy is higher than in a controlled in vitro experiment. However, the results in vivo for the combined treatments were even better than those obtained in the ex vivo study with extracted teeth. It is possible that in vivo the surrounding tissue could promote light backscattering, thus increasing the number of photons available to the photoreaction.
In conclusion, our results suggest that the use of PDT as an adjuvant to conventional endodontic treatment leads to a significant further reduction of bacterial load, and a second PDT is even more effective than the first. Antimicrobial PDT offers an efficient nontoxic means of destroying microorganisms remaining inside the root canal system after using conventional endodontic chemomechanical therapy.
Acknowledgments
The authors would like to thank Professor Gessé Eduardo Calvo Nogueira for his contribution on the statistical analysis.
MR Hamblin was supported by the U.S. National Institutes of Health (grants R01-CA/AI838801 and R01-AI050875).
References
- 1.Siqueira JF., Jr Endodontic infections: concepts, paradigms, and perspectives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:281–93. doi: 10.1067/moe.2002.126163. [DOI] [PubMed] [Google Scholar]
- 2.Bonsor SJ, Nichol R, Reid TM, Pearson GJ. Microbiological evaluation of photo-activated disinfection in endodontics (an in vivo study) Br Dent J. 2006;200:337–41. doi: 10.1038/sj.bdj.4813371. [DOI] [PubMed] [Google Scholar]
- 3.Bahcall JK, Barss JT. Understanding and evaluating the endodontic file. Gen Dent. 2000;48:690–2. [PubMed] [Google Scholar]
- 4.Sedgley C. Root canal irrigation—A historical perspective. J Hist Dent. 2004;52:61–5. [PubMed] [Google Scholar]
- 5.Reynaud AF, Geijersstam AH, Ellington MJ, Warner M, Woodford N, Haapasalo M. Antimicrobial susceptibility and molecular analysis of Enterococcus faecalis originating from endodontic infections in Finland and Lithuania. Oral Microbiol Immunol. 2006;21:164–8. doi: 10.1111/j.1399-302X.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- 6.Pinheiro ET, Gomes BP, Drucker DB, Zaia AA, Ferraz CC, Souza-Filho FJ. Antimicrobial susceptibility of Enterococcus faecalis isolated from canals of root filled teeth with periapical lesions. Int Endod J. 2004;37:756–63. doi: 10.1111/j.1365-2591.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 7.Figdor D. Microbial aetiology of endodontic treatment failure and pathogenic properties of selected species. Aust Endod J. 2004;30:11–4. doi: 10.1111/j.1747-4477.2004.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 8.Sjogren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297–306. doi: 10.1046/j.1365-2591.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 9.Nair PN, Sjogren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod. 1990;16:580–8. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- 10.Bahcall JK, Miserendino L, Walia H, Belardi DW. Scanning electron microscopic comparison of canal preparation with Nd:YAG laser and hand instrumentation: a preliminary study. Gen Dent. 1993;41:45–7. [PubMed] [Google Scholar]
- 11.Lee MT, Bird PS, Walsh LJ. Photo-activated disinfection of the root canal: a new role for lasers in endodontics. Aust Endod J. 2004;30:93–8. doi: 10.1111/j.1747-4477.2004.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 12.Seal GJ, Ng YL, Spratt D, Bhatti M, Gulabivala K. An in vitro comparison of the bactericidal efficacy of lethal photosensitization or sodium hypochlorite irrigation on Streptococcus intermedius biofilms in root canals. Int Endod J. 2002;35:268–74. doi: 10.1046/j.1365-2591.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- 13.Bahcall J, Howard P, Miserendino L, Walia H. Preliminary investigation of the histological effects of laser endodontic treatment on the periradicular tissues in dogs. J Endod. 1992;18:47–51. doi: 10.1016/S0099-2399(06)81369-5. [DOI] [PubMed] [Google Scholar]
- 14.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–50. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol. 2004;17:245–54. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42:2595–601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maisch T, Bosl C, Szeimies RM, Lehn N, Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob Agents Chemother. 2005;49:1542–52. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komerik N, Macrobert AJ. Photodynamic therapy as an alternative antimicrobial modality for oral infections. J Environ Pathol Toxicol Oncol. 2006;25:487–504. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.310. [DOI] [PubMed] [Google Scholar]
- 20.Wilson M. Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem Photobiol Sci. 2004;3:412–8. doi: 10.1039/b211266c. [DOI] [PubMed] [Google Scholar]
- 21.Meisel P, Kocher T. Photodynamic therapy for periodontal diseases: state of the art. J Photochem Photobiol B. 2005;79:159–70. doi: 10.1016/j.jphotobiol.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Hayek RR, Araujo NS, Gioso MA, et al. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J Periodontol. 2005;76:1275–81. doi: 10.1902/jop.2005.76.8.1275. [DOI] [PubMed] [Google Scholar]
- 23.Walsh LJ. The current status of laser applications in dentistry. Aust Dent J. 2003;48:146–55. doi: 10.1111/j.1834-7819.2003.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 24.Garcez AS, Ribeiro MS, Tegos GP, Nuñez SC, Jorge AOC, Hamblin MR. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med. 2007;39:59–66. doi: 10.1002/lsm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tegos GP, Anbe M, Yang C, et al. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50:1402–10. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutknecht N, van Gogswaardt D, Conrads G, Apel C, Schubert C, Lampert F. Diode laser radiation and its bactericidal effect in root canal wall dentin. J Clin Laser Med Surg. 2000;18:57–60. doi: 10.1089/clm.2000.18.57. [DOI] [PubMed] [Google Scholar]
- 27.Garcez AS, Nuñez SC, Lage-Marques JL, Jorge AOC, Ribeiro MS. Efficiency of NaOCl and laser-assisted photosensitization on the reduction of Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e93–8. doi: 10.1016/j.tripleo.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66:1375–9. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 29.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–50. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 30.Komiyama EY, Ribeiro PM, Junqueira JC, Koga-Ito CY, Jorge AOC. Prevalence of yeasts in the oral cavity of children treated with inhaled corticosteroids. Pesqui Odontol Bras. 2004;18:197–201. doi: 10.1590/s1806-83242004000300004. [DOI] [PubMed] [Google Scholar]
- 31.Sheleg SV, Zhavrid EA, Khodina TV, et al. Photodynamic therapy with chlorin e(6) for skin metastases of melanoma. Photodermatol Photoimmunol Photomed. 2004;20:21–6. doi: 10.1111/j.1600-0781.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 32.DeRosa MC, Crutchley RJ. Photosensitized singlet oxygen and its applications. Coord Chem Rev. 2002:233–234. 351–71. [Google Scholar]
- 33.Bonsor SJ, Nichol R, Reid TM, Pearson GJ. An alternative regimen for root canal disinfection. Br Dent J. 2006;22:101–5. doi: 10.1038/sj.bdj.4813819. [DOI] [PubMed] [Google Scholar]


