Summary
Background
Onychomycosis responds to systemic antifungals and sometimes to topical lacquers, but alternative treatments are desirable. Topical application of germicidal ultraviolet (UV) C radiation may be an acceptable and effective therapy for infected nails.
Objectives
To test the ability of UVC to inactivate dermatophyte suspensions in vitro and to sterilize a novel ex vivo model of nail infection.
Methods
Trichophyton rubrum, T. mentagrophytes, Epidermophyton floccosum and Microsporum canis suspensions were irradiated with UVC (254 nm) at a radiant exposure of 120 mJ cm−2 and surviving colony-forming units quantified. T. rubrum infecting porcine hoof slices and human toenail clippings was irradiated with UVC at radiant exposures of 36–864 J cm−2.
Results
In vitro studies showed that 3–5 logs of cell inactivation in dermatophyte suspensions were produced with 120 mJ cm−2 UVC irradiation. Depending on factors such as the thickness and infectious burden of the ex vivo cultures, the radiant exposure of UVC needed for complete sterilization was usually in the order of tens to hundreds of J cm−2. Resistance of T. rubrum to UVC irradiation did not increase after five cycles of subtotal inactivation in vitro.
Conclusions
UVC irradiation may be a less invasive treatment option for onychomycosis, when the appropriate consideration is given to safety.
Keywords: dermatophytes, ex vivo nail infection, onychomycosis, ultraviolet C
Onychomycosis is a chronic fungal nail infection that affects a large percentage of the population. Mycological prevalence in the U.S.A. is 7–10%.1 The prevalence has increased sharply in recent years.2 Onychomycosis can be particularly troublesome to special segments of the population, such as the elderly, patients with diabetes, and immunocompromised individuals. In these patients, onychomycosis increases the risk of recurrent cellulitis and ulceration. Amputation of toes, feet or legs in patients with diabetes is almost always preceded by years of poorly controlled onychomycosis.3 Dermatophytes (including the genera Trichophyton, Epidermophyton and Microsporum) are by far the most common pathogens of onychomycosis,1,4,5 with T. rubrum accounting for 80% of the infections.2 Treatment of onychomycosis is challenging for physicians, and the efficacy of current treatment options, including topical, oral, mechanical and chemical therapies or a combination of these modalities, remains disappointing.6,7
Topical drug treatment for onychomycosis is not usually successful because the topical drugs are typically unable to penetrate the hyperkeratotic nail plate.8,9 As a result, a therapeutically sufficient quantity of drug cannot be delivered to the localized sites of fungal infection. In addition, rapid recurrence of symptoms can occur after discontinuing use.10,11 Oral antifungal agents are more effective although they are also more toxic. There is a significant risk of liver toxicity, prolonged loss of taste, and life-threatening drug interactions.12 When the oral antifungal agents are used on a long-term basis, development of fungal resistance also poses concern. Surgical treatment of onychomycosis is also very difficult.13 Topically applied antifungal drugs may work somewhat better after removing the nail plate by surgery or chemical dissolution.14 However, this is a traumatic procedure that leaves the patient without a nail for months, involves other risks including postoperative infections, and is often ineffective.
Due to the limitations of current treatment options, a simple, nontoxic and effective means to control or cure onychomycosis is clearly warranted.15 Although it has been known since the early 1890s that ultraviolet (UV) radiation (particularly UVC with a wavelength range of 250–280 nm) is highly germicidal, its use to treat actual infections has hardly been developed. The present study was to determine the efficacy of UVC for treatment of onychomycosis and lay the foundation to develop a noninvasive, safe, and highly efficacious treatment for onychomycosis.
Materials and methods
Dermatophytes and preparation of stock inocula
Clinical isolates of typical dermatophytes T. rubrum, T. mentagrophytes, E. floccosum and M. canis were obtained as gifts from Massachusetts General Hospital and Children's Hospital, Boston. All isolates were subcultured on Sabouraud dextrose agar (SDA) (Becton, Dickinson and Co., Sparks, MD, U.S.A.) plates containing 7.5 mg L−1 gentamicin sulphate (Cambrex Bioscience, Walkerville, MD, U.S.A.). The SDA plates were incubated at 30 °C for 10–15 days. Mature colonies were then harvested, suspended in phosphate-buffered saline (PBS), dispersed to single-cell suspensions using a sonic dismembrator (Model 100; Fisher Scientific, Norcross, GA, U.S.A.) and stored at −80 °C in the presence of 20% glycerol as cryoprotectant until used in experiments.
Porcine hoof and human toenail cultures
Porcine hooves obtained from Yorkshire pigs were used as an ex vivo model for human nail. The protocol to use Yorkshire pigs was approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital. The hooves were sliced into square fragments of approximately 0.7 × 0.7 cm. Human toenail clippings were also collected from anonymous volunteers without onychomycosis. The toenail clippings were cut into circular fragments with 4-mm diameter tissue biopsy punches. The thickness of the hoof and toenail fragments ranged from 0·80 to 1·66 mm and from 0·51 to 1·18 mm, respectively, determined by a digital Vernier caliper.
Prior to being autoclaved at 120 °C for 15 min, the hoof or toenail fragments were sterilized by immersion in 70% isopropanol twice for 1 min, and then washed twice (2 min) with sterile water. Initially fragments were inoculated on the ventral surface with 20 μL T. rubrum suspension containing approximately 2·0 × 10 or 2·0 × 10 colony-forming units (c.f.u.) mL−1, and later they were immersed in 100 μL T. rubrum suspension containing 2·0 × 106 c.f.u. mL−1 or 2 h. The fragments were then placed in 35-mm diameter Petri dishes, which were placed inside a 150-mm diameter Petri dish containing sterile water to provide a moist environment. The dishes with hoof or toenail fragments were then incubated at 30 °C for 2–8 weeks before the experiments were performed.
Ultraviolet C irradiation
UVC radiation was delivered by a germicidal lamp (CE-12-2H; American Ultraviolet Company, Lebanon, IN, U.S.A.). Emission spectral measurement of this lamp by a spectroradiometer (SPR-01; Luzchem Research Inc., Ottawa, ON, Canada) showed a peak emission at 254 ± 2 nm wavelength. The irradiance of the UVC irradiation was controlled by adjusting the distance between the lamp and the target and was measured by a UVX radiometer (UVP Inc., Upland, CA, U.S.A.).
In vitro susceptibility testing of different dermatophytes to ultraviolet C irradiation
Prior to UVC exposure, the optical densities (ODs) of all dermatophyte suspensions were adjusted to approximately 6·5 (10-fold dilutions measured) at 570 nm in PBS with a spectrophotometer (UVmini-1240; Shimadzu Corporation, Kyoto, Japan). In addition, the cell densities of the suspensions were counted on a haemocytometer (Hausser Scientific, Horsham, PA, U.S.A.). In each test, a 2-mL aliquot of the dermatophyte suspension was inoculated to a 32-mm diameter Petri dish to give a depth of 2-mm liquid and exposed to UVC irradiation. During the irradiation, the dermatophyte suspension was stirred by a mini-magnetic bar. Aliquots of 40 μL of the suspension were withdrawn at 0, 15, 30, 45, 60 and 75 s, respectively, when 0, 24, 48, 72, 96 and 120 mJ cm−2 UVC had been delivered (UVC irradiance ≈ 1·6 mW cm−2). To disrupt any aggregates, aliquots were treated with a sonic dismembrator. The aliquots were then subjected to five serial 10-fold dilutions in PBS. Six samples of 10 μL, one from each dilution, were spotted at intervals along one side of square SDA plates (Fisher Scientific) in the order of most (1 : 105) to least (1:1) diluted. Each plate was then tipped on to its side (at a 45° angle), and the spots were allowed to migrate in parallel tracks across the agar surface to the opposite side of the plate.16 The plates were then incubated at 30 °C until countable colonies appeared (4–5 days). The experiments were performed in triplicate for each species.
In vitro dermatophyte resistance to ultraviolet C irradiation
The possibility of the development of dermatophyte resistance to UVC irradiation was investigated by carrying out subtotal UVC inactivation (leaving 100 c.f.u. mL−1 postirradiation) of T. rubrum suspension followed by regrowth and repeated inactivation. Surviving c.f.u. were measured after each cycle and cell killing curves constructed. Five cycles of subtotal UVC inactivation were carried out and the experiments were performed in triplicate for each cycle.
Ultraviolet C transmission measurements
In order to detect any differences in optical properties between human toenail samples and pig hoof slices we measured optical transmission of both sets of samples at 254 nm and also measured the thickness of the samples with a Vernier micrometer. An IL1700 research radiometer with an SED240 detector (International Light Technologies, Newburyport, MA, U.S.A.) was used to determine the transmissivity of 254 nm radiation from the CE-12-2H lamp. The nail or hoof clippings were placed between two metal plates (6·5 mm thick) that had a 0·32-cm hole drilled through each plate. This arrangement permitted only collimated radiation to be measured.
Ultraviolet C inactivation of Trichophyton rubrum ex vivo
During the experiment, the porcine hoof or human toenail fragments were placed into 32-mm Petri dishes with the dorsal surfaces facing the UVC irradiation. To investigate the efficacy of UVC inactivation of T. rubrum in ex vivo porcine hoof culture at different depths, a cryostatic microtome was used. After the UVC irradiation, the porcine hoof fragments were embedded in OCT compound (Sakura Finetek U.S.A. Inc., Torrance, CA, U.S.A.) and frozen at −80 °C for 2 h. The frozen hoof fragments were then carefully sectioned at −25 °C, producing sections of 50-μm thickness. The 50-μm thick hoof sections were inoculated on SDA plates and the time for colony outgrowth determined. This procedure was also carried out with entire infected hoof or toenail fragments after UVC irradiation. This allowed a semiquantitative assay for the inactivation rate based on the start date of colony outgrowth on to SDA plates. A fragment was considered to be sterilized when no colony outgrowth took place from the fragment 14 days after the UVC irradiation. For each irradiated hoof or nail culture, a nonirradiated counterpart from the same hoof or from the same nail donor was used as a control that had the same thickness and postinfection time and was infected with the same fungal inoculum, respectively.
Statistics
Data are presented as mean ± SD, and differences between means were compared for significance by two-tailed t-test assuming unequal variance (single comparisons) or one-way ANOVA (multiple comparisons). Significance of correlation coefficient for polynomial curve was estimated by curvilinear regression analysis.
Results
In vitro susceptibilities of different dermatophytes to ultraviolet C irradiation
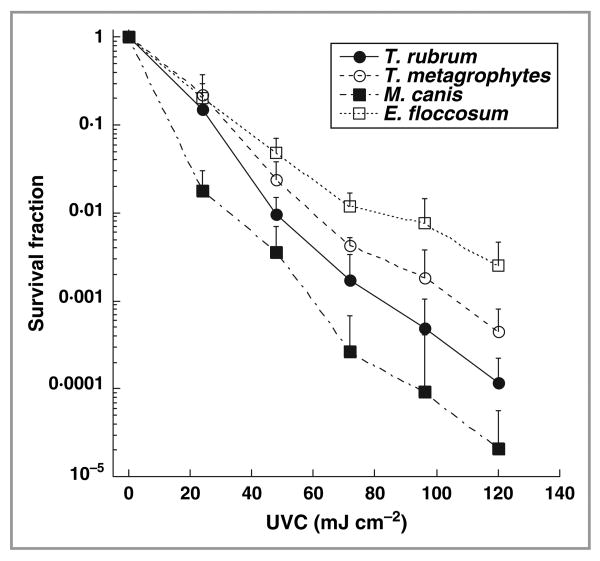
There was the expected semilogarithmic linear dependence of survival fraction with delivered UVC irradiation in suspensions of dermatophytes (Fig. 1). At the same OD (≈ 6·5 at 570 nm), M. canis suspension showed the highest susceptibility to UVC irradiation. Nearly 99·999% or 5 logs of cell inactivation were achieved in M. canis suspensions when 120 mJ cm−2 UVC had been delivered. In contrast, E. floccosum showed the lowest susceptibility to inactivation, and 120 mJ cm−2 of UVC gave < 99·9% or 3 logs of cell killing in E. floccosum suspensions. T. rubrum, the species responsible for most cases of onychomycosis, had a 4 log inactivation at a radiant exposure of 120 mJ cm−2.
Fig 1.
Dose responses to ultraviolet (UV) C irradiation of different dermatophyte suspensions under the same optical density (OD570 nm ≈ 6·5). The data are representative experiments performed in triplicate and are displayed as mean ± SD. T. rubrum, Trichophyton rubrum; T. mentagrophytes, Trichophyton mentagrophytes; M. canis, Microsporum canis; E. floccosum, Epidermophyton floccosum.
Test of dermatophyte resistance to ultraviolet C irradiation
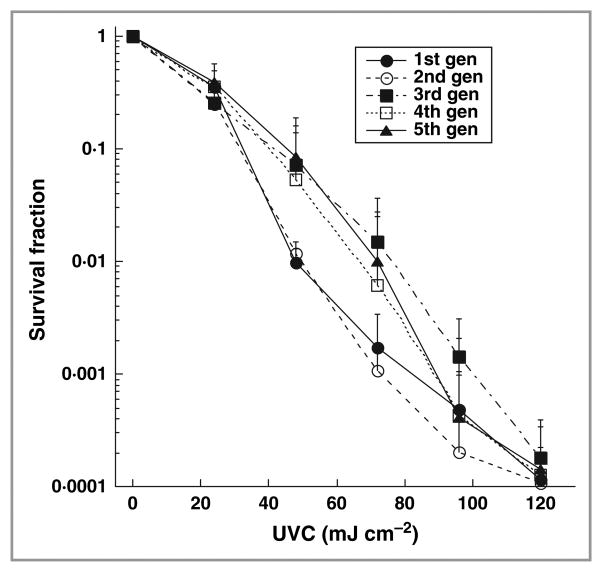
Figure 2 demonstrates the cell inactivation curves of T. rubrum in vitro in response to five consecutive subtotal UVC inactivations. No significant difference was found in cell inactivation rates among the five consecutive cycles when 120 mJ cm−2 UVC had been delivered (ANOVA, P = 0·66). This indicates that resistance is not acquired by T. rubrum cells that are repeatedly exposed to sublethal UVC irradiation.
Fig 2.
Dose responses of Trichophyton rubrum in vitro to five consecutive subtotal ultraviolet (UV) C inactivations. The data are representative experiments performed in triplicate and are displayed as mean ± SD.
Ex vivo models of onychomychosis
Two ex vivo models of onychomycosis were developed in the present study by growing T. rubrum either on the fragments of porcine hoof or on human toenail clippings. Initially we applied drops of fungal suspension with varying cell densities to the ventral surface of porcine hoofs and incubated the fragments for 2–8 weeks. We observed an outgrowth of white mycelia and conidia on the ventral surface in the first 2–4 weeks depending on the initial inoculum, and this growth subsequently regressed over the next 2–4 weeks to give a surface similar to the initial hoof appearance. Figure 3a shows a representative periodic acid–Schiff (PAS)-stained histology of a 4-week porcine hoof culture infected with T. rubrum. Massive T. rubrum invasion was demonstrated by numerous hyphae branching out in every direction and subverting the nail structure. In comparison, Figure 3b shows a PAS-stained histology of an uninfected porcine hoof fragment.
Fig 3.
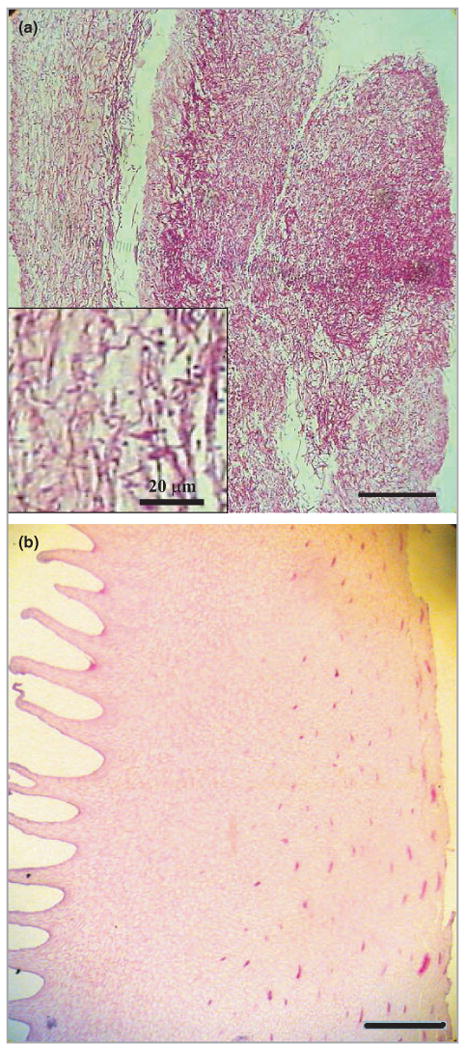
Periodic acid–Schiff-stained histology of (a) a 4-week porcine hoof culture infected with Trichophyton rubrum; (b) a noninfected porcine hoof fragment. Bars = 200 μm.
In later experiments we submerged the hoof or toenail fragments in suspensions of T. rubrum, with the aim of obtaining a more uniform growth of fungus throughout the nail and less discoloration of the nail surface. In this case we observed much less initial white growth of fungus on the surface, and incubation for 4 weeks was sufficient to give full hyphal growth throughout the depth of the nail.
Ultraviolet C inactivation of Trichophyton rubrum ex vivo
Figure 4 shows a representative result (culture 4 in Table 1) illustrating the ability of UVC to penetrate the ex vivo porcine hoof culture and inactivate T. rubrum. Prior to being sectioned by the cryostatic microtome and placed on to SDA plates, the hoof fragment was irradiated under UVC at a power density of approximately 20 mW cm−2 for 8 h, corresponding to a UVC exposure of 576 J cm−2. In this fragment, an inactivation depth of ≈ 850 μm was achieved (Fig. 4a). It was shown from the negative control (a nonirradiated hoof culture; Fig. 4b) that the T. rubrum invasion depth was ≥ 900 μm.
Fig 4.
(a) Consecutive microtome-sliced 50-μm thick sections on Sabouraud dextrose agar (SDA) from an ex vivo porcine hoof culture of Trichophyton rubrum irradiated with an ultraviolet C exposure of 576 J cm−2 (8 h irradiation at an irradiance of 20 mW cm−2); (b) Consecutive 50-μm thick sections from a nonirradiated porcine hoof culture. Numbers indicate the depths (μm) of the sections from the hoof surface. Day 14 culture on SDA.
Table 1.
Summary of ultraviolet (UV) C inactivation of Trichophyton rubrum in ex vivo porcine hoof and human toenail cultures
| Culture | Culture type | Size (cm × cm) | Thickness (mm) | Mode of infection | UVC illumination time (h) | UVC exposure (J cm−2) | Completely sterilized | Delay of colony outgrowth (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | Hoof | 0·35 × 0·7 | 1·50 | A | 11·5 | 828 | No | 0 |
| 2 | Hoof | 0·35 × 0·7 | 1·50 | A | 6 | 432 | No | Not recorded |
| 3 | Hoof | 0·7 × 0·7 | 1·30 | B | 12 | 864 | No | 0 |
| 4 | Hoof | 0·7 × 0·7 | 1·30 | B | 8 | 576 | No | 2 |
| 5 | Hoof | 0·35 × 0·7 | 1·30 | C | 8 | 576 | No | 3 |
| 6 | Hoof | 0·35 × 0·7 | 1·30 | C | 8 | 576 | Yes | |
| 7 | Hoof | 0·7 × 0·7 | 0·80 | C | 6 | 432 | No | 2 |
| 8 | Hoof | 0·4 × 0·4 | 1·14 | D | 8 | 576 | Yes | |
| 9 | Hoof | 0·4 × 0·4 | 1·66 | D | 2 | 144 | No | 4 |
| 10 | Hoof | 0·4 × 0·4 | 1·51 | D | 2 | 144 | Yes | |
| 11 | Hoof | 0·4 × 0·4 | 1·39 | D | 1 | 72 | Yes | |
| 12 | Hoof | 0·4 × 0·4 | 1·34 | D | 1 | 72 | No | 3 |
| 13 | Hoof | 0·4 × 0·4 | 1·27 | D | 1 | 72 | No | 1 |
| 14 | Hoof | 0·4 × 0·4 | 1·19 | D | 1 | 72 | Yes | |
| 15 | Toenail | 0·4 × 0·4 | 0·90 | D | 8 | 576 | Yes | |
| 16 | Toenail | 0·4 × 0·4 | 0·75 | D | 4 | 288 | Yes | |
| 17 | Toenail | 0·4 × 0·4 | 0·51 | D | 4 | 288 | Yes | |
| 18 | Toenail | 0·4 × 0·4 | 1·18 | D | 2 | 144 | Yes | |
| 19 | Toenail | 0·4 × 0·4 | 1·06 | D | 2 | 144 | Yes | |
| 20 | Toenail | 0·4 × 0·4 | 1·14 | D | 1 | 72 | No | 1 |
| 21 | Toenail | 0·4 × 0·4 | 1·10 | D | 1 | 72 | Yes | |
| 22 | Toenail | 0·4 × 0·4 | 0·90 | D | 1 | 72 | Yes | |
| 23 | Toenail | 0·4 × 0·4 | 0·85 | D | 1 | 72 | Yes | |
| 24 | Toenail | 0·4 × 0·4 | 0·70 | D | 1 | 72 | Yes | |
| 25 | Toenail | 0·4 × 0·4 | 0·98 | D | 0·5 | 36 | No | 1 |
| 26 | Toenail | 0·4 × 0·4 | 0·98 | D | 0·5 | 36 | Yes |
A, inoculated with 20 μL T. rubrum suspension containing 2 × 108 colony-forming units (c.f.u.) mL−1, 2-week culture; B, inoculated with 20 μL T. rubrum suspension containing 2 × 106 c.f.u. mL−1, 6-week culture; C, inoculated with 20 μL T. rubrum suspension containing 2 × 106 c.f.u. mL−1, 8-week culture; D, the fragment was immersed in 100 μL T. rubrum suspension containing 2 × 106 c.f.u. mL−1 for 2 h, 4-week culture.
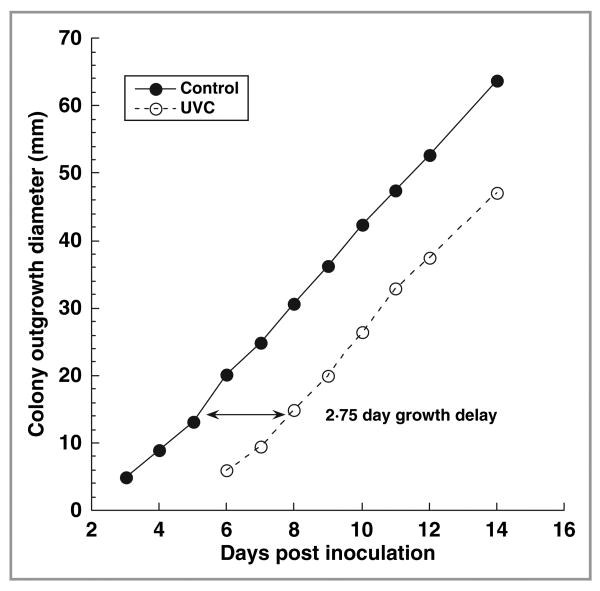
We also assessed the efficacy of UVC inactivation by placing entire hoof or toenail fragments on SDA after UVC irradiation and observing T. rubrum colony outgrowth on SDA. Figure 5 shows a representative result (culture 5 in Table 1) of the time courses of T. rubrum colony outgrowth size (diameter) on SDA from a hoof fragment irradiated at a UVC exposure of 576 J cm−2 (8 h irradiation at an irradiance of 20 mW cm−2), and that from a nonirradiated control. Trichophyton rubrum colony outgrowth was first observed from the nonirradiated control on day 3, and from the UVC-irradiated fragment on day 6. It can be further estimated from the two almost parallel time-course curves that the time delay of the colony outgrowth between the control and irradiated cultures was 2·75 days.
Fig 5.
Time courses of Trichophyton rubrum colony outgrowth size (diameter) on Sabouraud dextrose agar from a porcine hoof culture irradiated with an ultraviolet (UV) C exposure of 576 J cm−2 (8 h irradiation at an irradiance of 20 mW cm−2) and a nonirradiated control, respectively.
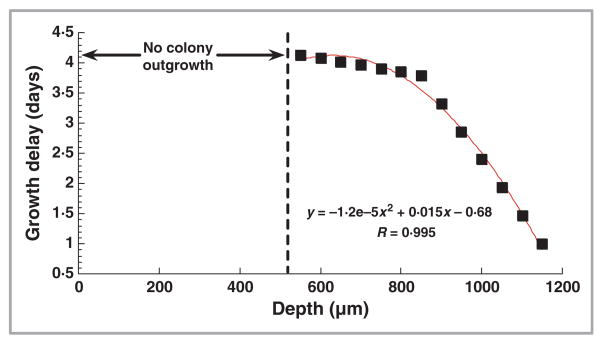
The time delays of colony growth (in comparison with the nonirradiated control) of the microtome-sliced sections at different depths of a UVC-irradiated hoof culture (culture 3 in Table 1) are illustrated in Figure 6. For the sections at the depths of 550–1150 μm, the delays of colony outgrowth could be fairly estimated by a second order polynomial function (R2 = 0·99, P < 0·00001). For the sections at the depths of 500 μm and above, no colony outgrowth was observed until the end of the observation (day 13 after the UVC irradiation).
Fig 6.
Time delays of Trichophyton rubrum colony outgrowth on Sabouraud dextrose agar from microtome-sliced 50-μm thick sections at different depths of an ultraviolet (UV) C-irradiated porcine hoof culture. UVC exposure: 864 J cm−2.
Table 1 summarizes the results of UVC inactivation of T. rubrum-infected ex vivo hoof and toenail cultures. In total, 14 porcine hoof and 12 human toenail cultures were irradiated plus several nonirradiated controls. Depending on factors such as the thickness and degree of discoloration of the ex vivo cultures, the UVC exposure needed for complete sterilization was usually in the order of tens to hundreds of J cm−2. For the human toenail cultures (Table 1, cultures 15–26), when 36 J cm−2 UVC had been delivered, one of two cultures (50%) was sterilized. When the UVC exposure was increased to 72 J cm−2, four of five (80%) cultures were sterilized. When the UVC exposure was further increased to 144 J cm−2 or above, all of five cultures were sterilized. For the porcine hoof cultures, we initially were not able to sterilize cultures that had been infected with drops of T. rubrum suspension (Table 1, cultures 1–7) even when 864 J cm−2 UVC had been delivered, which was probably due to the optical properties (the degree of opacification and discoloration) of the hoof cultures caused by longer incubation time and higher inocula of T. rubrum suspension. Discoloration as well as thickened nail plate clinically represent the symptoms of late-stage onychomycosis.
We checked that the noninfected pig hoof slices did not have significantly different abilities to transmit UVC radiation (transmissivity) compared with the transmissivity of the noninfected human toenail samples as described in Materials and methods. The mean ± SD absorption per mm thickness of human toenails was 10·13 ± 3·12 (n = 10) while that for pig hoof slices was 8·84 ± 4·06 (n = 8). These were not significantly different from each other (P = 0·44, two-tailed Student's t-test).
For the second group of the porcine hoof cultures infected by immersion in T. rubrum suspensions (Table 1, cultures 8–14), sterilization was achieved in two of four (50%) cultures when a much lower fluence of UVC (72 J cm−2) had been delivered. When the UVC exposure reached 144 J cm−2 or above, sterilization was achieved in two of three cultures tested. The culture that was not sterilized at 144 J cm−2 was the one with the greatest thickness (1·66 mm).
Discussion
In the present study, four dermatophyte species, T. rubrum, T. mentagrophytes, E. floccosum and M. canis, were investigated in vitro. For different dermatophytes, the cellular morphology of suspensions is quite different. The cell suspensions of T. rubrum and T. mentagrophytes are composed mainly of microconidia with the size of 2–3 × 3–5 μm, while the c.f.u. of M. canis and E. floccosum exist mostly in the forms of hyphae and macroconidia with sizes of 10–15 × 35–110 μm and 7–12 × 20–40 μm, respectively. As a result, for the same cell density (c.f.u. mL−1), the ODs of different dermatophyte suspensions are different due to the difference in light scattering from different-sized cells. We compared the in vitro susceptibilities to UVC under the same OD for different dermatophytes vs. control for penetration of UVC into the suspension.
If microorganisms are not killed but injured by UVC irradiation, DNA mutations can be produced in the injured organisms. UVC irradiation of microorganisms causes DNA damage by inducing the formation of mutagenic DNA photoproducts, such as cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6–4) photoproducts.17 Mutations are produced by those UV-induced photoproducts that are not repaired before DNA replication. If the mutations lead to a competitive advantage they will spread to the whole microbial population. This is in fact largely responsible for the emergence of drug resistance in clinical therapy. DNA excision repair systems, which can recognize a broad range of DNA lesions including UVC-induced CPDs, play an important role in preventing UVC mutagenesis.18 In vitro data presented in this study showed that T. rubrum resistance to UVC did not increase after five cycles of subtotal UVC inactivation, indicating the successful maintenance of the genomic integrity of T. rubrum cells by the DNA excision repair system.
Due to limited availability of human nail samples, porcine hoof was used as an ex vivo model for human nail in the present study. As nail and hoof are based on a keratin matrix, it is reasonable to use hoof as a model for nail.19 Such a model has also been used by other investigators.20,21 Complete inactivation of dermatophytes within the nail plate requires that therapeutically sufficient UVC fluence be delivered to the site of fungal infection. Most dermatophyte species invade the ventral layer of the nail plate first;22 as a result, sufficient UVC fluence should be delivered through the whole nail plate, i.e. from the dorsal layer to the ventral layer. The penetration of UVC in human nail is determined by the nail optical properties including absorption coefficient, scattering coefficient, and anisotropy factor. UVC radiation is dramatically attenuated along its beam path within the nail plate mainly due to strong optical scattering of dermatophyte-invaded human nails and strong absorption of 254-nm radiation by protein molecules such as keratin. This attenuation is demonstrated by comparison between the inactivation UVC exposures in vitro and in vivo. Three logs of cell inactivation of T. rubrum can be produced in vitro when 105 mJ cm−2 UVC is delivered (Fig. 1), while to achieve an inactivation depth of 850 μm ex vivo a UVC exposure of 567 J cm−2 may be required (almost 5000-fold increase over the in vitro inactivation exposure) (Fig. 4). In recent years, an optical clearing technique to give transient reduction of the optical scattering of human skin has been widely investigated in phototherapy or photodiagnosis of skin diseases.23,24 By topically applying chemical agents such as dimethyl sulphoxide or glycerol to human skin, refractive index match among different components within skin is achieved: as a result, light scattering of skin is reduced and light penetration increased. In a future study, we will test this technique to improve the UVC inactivation efficacy of dermatophytes in nails.
It is well known that UV radiation is damaging to human tissue and particularly damaging to skin. UVB irradiation of skin has been particularly well studied, and is accepted as the main cause of skin cancer. UVC irradiation of human skin has been much less studied, but is also known to cause the same kind of damage.25 In proposing to employ UVC irradiation to treat onychomycosis, it is clearly of crucial importance to minimize or avoid UVC exposure to skin either surrounding the nails (perionychium, hyponychium and cuticle) or to the tissue underneath the nail (nail bed). The former can be prevented by careful masking of the area with UVC-opaque adhesive material, while the latter must be minimized by careful dosimetry calculated with reference to individual patients' nail thickness and optical properties. A phase I clinical trial of UVC irradiation for onychomycosis has been recently completed in our institution and results will be submitted for publication.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (STTR Grant R41-AI069641 to M.R.H. and W.E.C.). The authors thank Dr Evan Mojica, Dr John Branda and Ms Victoria Hamrahi for providing the clinical isolates of dermatophytes, Mr Bill Farinelli for providing the porcine hooves, and Drs Ana Castano and Pawel Mroz, Ms Tatyana Demidova-Rice, and Drs Dieter Manstain, Qiqi Mu, Noemi R. Romero and Robert W. Redmond for their advice and assistance in the experiments.
Footnotes
Conflicts of interest: W.E.C. is CEO of Keraderm LLC, a start-up company intending to commercialize this technology.
References
- 1.Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172–3. [PubMed] [Google Scholar]
- 2.Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641–8. doi: 10.1067/mjd.2000.107754. [DOI] [PubMed] [Google Scholar]
- 3.Robbins JM. Treatment of onychomycosis in the diabetic patient population. J Diabetes Complications. 2003;17:98–104. doi: 10.1016/s1056-8727(02)00199-x. [DOI] [PubMed] [Google Scholar]
- 4.Elewski BE, Hay RJ. Update on the management of onychomycosis: highlights of the Third Annual International Summit on Cutaneous Antifungal Therapy. Clin Infect Dis. 1996;23:305–13. doi: 10.1093/clinids/23.2.305. [DOI] [PubMed] [Google Scholar]
- 5.Clayton YM. Clinical and mycological diagnostic aspects of onychomycoses and dermatomycoses. Clin Exp Dermatol. 1992;17(Suppl 1):37–40. doi: 10.1111/j.1365-2230.1992.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 6.Crawford F, Young P, Godfrey C, et al. Oral treatments for toenail onychomycosis: a systematic review. Arch Dermatol. 2002;138:811–16. doi: 10.1001/archderm.138.6.811. [DOI] [PubMed] [Google Scholar]
- 7.Elewski BE. A full ‘cure’ for onychomycosis is not always possible. Arch Dermatol. 1999;135:852–3. doi: 10.1001/archderm.135.7.852. [DOI] [PubMed] [Google Scholar]
- 8.Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415–29. doi: 10.1128/cmr.11.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta AK. Ciclopirox nail lacquer: a brush with onychomycosis. Cutis. 2001;68:13–16. [PubMed] [Google Scholar]
- 10.Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31–46. doi: 10.1111/j.1529-8019.2007.00109.x. [DOI] [PubMed] [Google Scholar]
- 11.Arrese JE, Pierard GE. Treatment failures and relapses in onychomycosis: a stubborn clinical problem. Dermatology. 2003;207:255–60. doi: 10.1159/000073086. [DOI] [PubMed] [Google Scholar]
- 12.Katz HI. Drug interactions of the newer oral antifungal agents. Br J Dermatol. 1999;141(Suppl 56):26–32. doi: 10.1046/j.1365-2133.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 13.McInnes BD, Dockery GL. Surgical treatment of mycotic toenails. J Am Podiatr Med Assoc. 1997;87:557–64. doi: 10.7547/87507315-87-12-557. [DOI] [PubMed] [Google Scholar]
- 14.Grover C, Bansal S, Nanda S, et al. Combination of surgical avulsion and topical therapy for single nail onychomycosis: a randomized controlled trial. Br J Dermatol. 2007;157:364–8. doi: 10.1111/j.1365-2133.2007.08014.x. [DOI] [PubMed] [Google Scholar]
- 15.Conant M. New treatment options for onychomycosis. AIDS Patient Care. 1995;9(Suppl 1):S19–21. [PubMed] [Google Scholar]
- 16.Jett BD, Hatter KL, Huycke MM, et al. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–50. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong JD, Kunz BA. Photoreactivation implicates cyclobutane dimers as the major promutagenic UVB lesions in yeast. Mutat Res. 1992;268:83–94. doi: 10.1016/0027-5107(92)90086-h. [DOI] [PubMed] [Google Scholar]
- 18.Pfeifer GP, You YH, Besaratinia A. Mutations induced by ultraviolet light. Mutat Res. 2005;571:19–31. doi: 10.1016/j.mrfmmm.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly RF, McCarron PA, Lightowler JM, et al. Bioadhesive patch-based delivery of 5-aminolevulinic acid to the nail for photodynamic therapy of onychomycosis. J Control Release. 2005;103:381–92. doi: 10.1016/j.jconrel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Lee CH, Choi HK. A method to measure the amount of drug penetrated across the nail plate. Pharm Res. 2001;18:1468–71. doi: 10.1023/a:1012265125158. [DOI] [PubMed] [Google Scholar]
- 21.Mertin D, Lippold BC. In-vitro permeability of the human nail and of a keratin membrane from bovine hooves: prediction of the penetration rate of antimycotics through the nail plate and their efficacy. J Pharm Pharmacol. 1997;49:866–72. doi: 10.1111/j.2042-7158.1997.tb06127.x. [DOI] [PubMed] [Google Scholar]
- 22.Rashid A, Scott E, Richardson MD. Early events in the invasion of the human nail plate by Trichophyton mentagrophytes. Br J Dermatol. 1995;133:932–40. doi: 10.1111/j.1365-2133.1995.tb06929.x. [DOI] [PubMed] [Google Scholar]
- 23.Choi B, Tsu L, Chen E, et al. Determination of chemical agent optical clearing potential using in vitro human skin. Lasers Surg Med. 2005;36:72–5. doi: 10.1002/lsm.20116. [DOI] [PubMed] [Google Scholar]
- 24.Moulton K, Lovell F, Williams E, et al. Use of glycerol as an optical clearing agent for enhancing photonic transference and detection of Salmonella typhimurium through porcine skin. J Biomed Opt. 2006;11:054027. doi: 10.1117/1.2363366. [DOI] [PubMed] [Google Scholar]
- 25.Trevisan A, Piovesan S, Leonardi A, et al. Unusual high exposure to ultraviolet-C radiation. Photochem Photobiol. 2006;82:1077–9. doi: 10.1562/2005-10-27-ra-728. [DOI] [PubMed] [Google Scholar]