Abstract
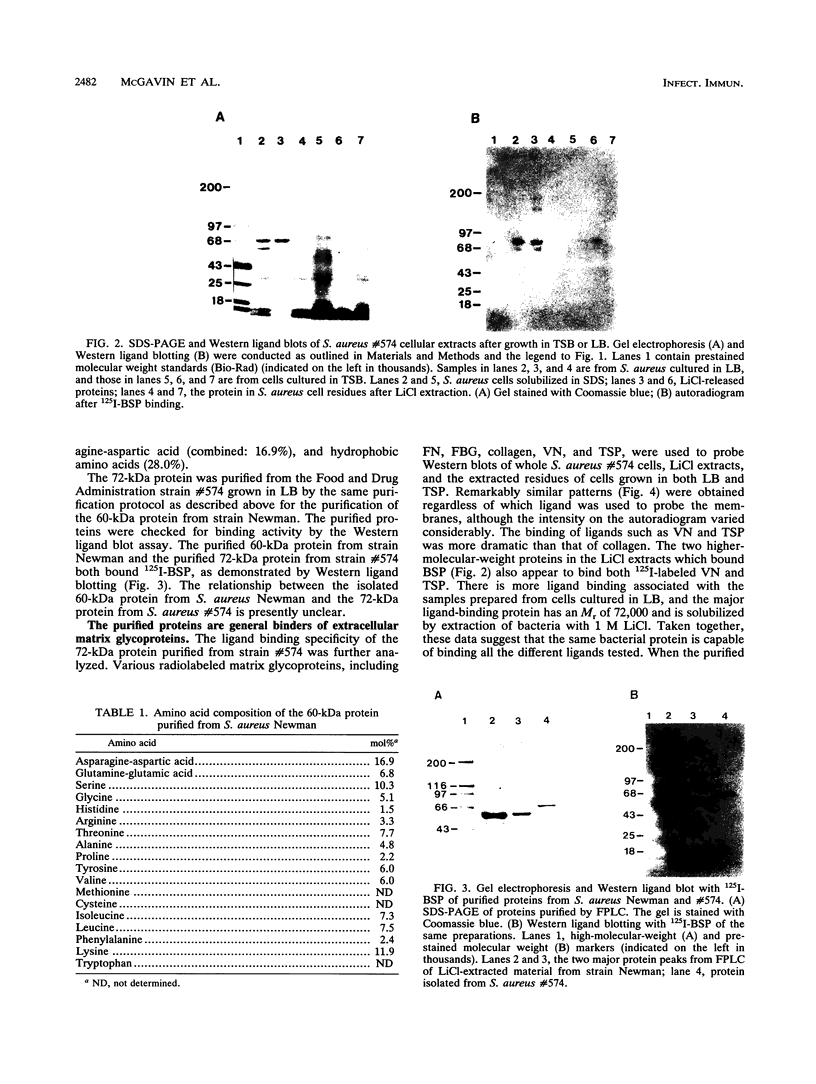
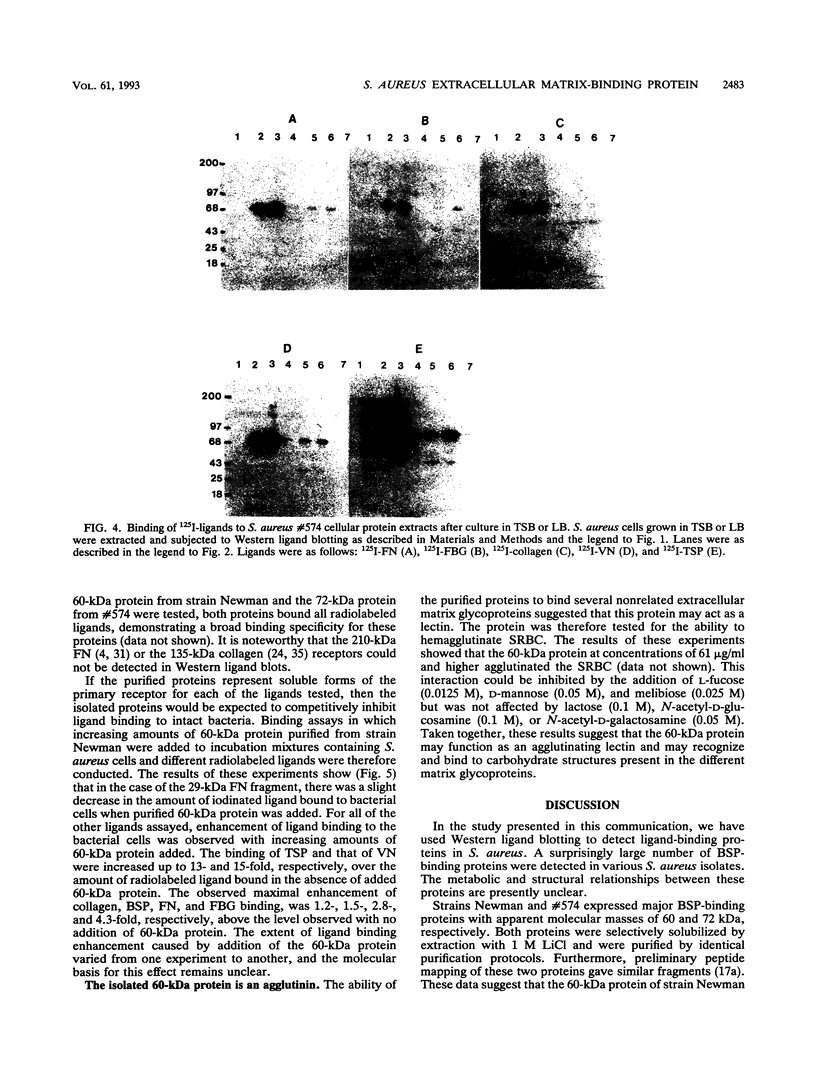
A staphylococal surface protein capable of binding several extracellular matrix glycoproteins was purified as a result of our attempts to identify a receptor(s) for bone sialoprotein (BSP) on Staphylococcus aureus cells. Proteins from different staphylococcal strains were solubilized in sodium lauryl sulfate, separated by polyacrylamide gel electrophoresis, blotted onto Immobilon P membranes, and probed with 125I-BSP. Several bacterial proteins bound the radiolabeled ligand, and various strains expressed different repertoirs of BSP-binding proteins. Major BSP-binding proteins with apparent M(r)s of 72,000 or 60,000 were present on most strains, and these proteins were further studied. The 72- and 60-kDa proteins were preferentially expressed when bacteria were cultured in Luria broth compared with when they were cultured on tryptic soy broth, and the abundance of the proteins could be correlated to an increased 125I-BSP binding. Both the 72-kDa and the 60-kDa proteins were solubilized by extraction of cells with 1 M LiCl and were purified by cation-exchange chromatography. Amino acid composition analysis of the purified 72-kDa protein indicated a high content of lysine (11.9%) and hydrophobic amino acids (28.0% combined). In Western ligand blotting (immunoblotting) experiments, the 72-kDa protein bound not only BSP but also radiolabeled fibronectin, fibrinogen, vitronectin, thrombospondin, and, to some extent, collagen. Addition of the purified 60-kDa protein to S. aureus cells did not inhibit binding of the different ligands but in some cases resulted in an augmentation of the binding of 125I-ligand. Purified 60-kDa protein could hemagglutinate sheep erythrocytes at a concentration of 61 micrograms/ml. The agglutination reaction was inhibited by high concentrations of fucose, mannose, or melibiose. These data suggest that the purified proteins may serve as bacterial receptors with broad specificity for matrix glycoproteins and that the proteins may act as carbohydrate-binding proteins.
Full text
PDF


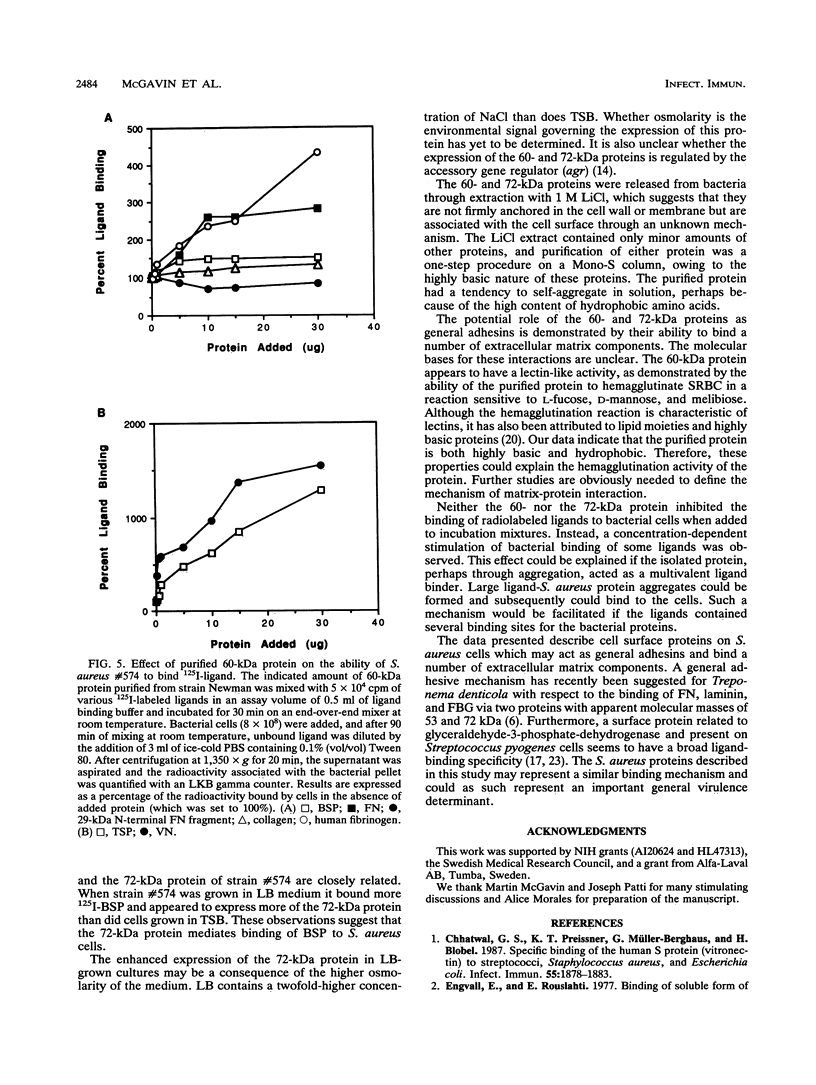

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chhatwal G. S., Preissner K. T., Müller-Berghaus G., Blobel H. Specific binding of the human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect Immun. 1987 Aug;55(8):1878–1883. doi: 10.1128/iai.55.8.1878-1883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985 Dec 15;232(3):715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Goldenberg D. L., Reed J. I. Bacterial arthritis. N Engl J Med. 1985 Mar 21;312(12):764–771. doi: 10.1056/NEJM198503213121206. [DOI] [PubMed] [Google Scholar]
- Haapasalo M., Müller K. H., Uitto V. J., Leung W. K., McBride B. C. Characterization, cloning, and binding properties of the major 53-kilodalton Treponema denticola surface antigen. Infect Immun. 1992 May;60(5):2058–2065. doi: 10.1128/iai.60.5.2058-2065.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Suchard S. J., Boxer L. A., Waldvogel F. A., Lew P. D. Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect Immun. 1991 Jan;59(1):279–288. doi: 10.1128/iai.59.1.279-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Holderbaum D., Hall G. S., Ehrhart L. A. Collagen binding to Staphylococcus aureus. Infect Immun. 1986 Nov;54(2):359–364. doi: 10.1128/iai.54.2.359-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Jönsson K., Signäs C., Müller H. P., Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991 Dec 18;202(3):1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lantz M. S., Switalski L. M., Kornman K. S., Hök M. Bacteroides intermedius binds fibrinogen. J Bacteriol. 1985 Aug;163(2):623–628. doi: 10.1128/jb.163.2.623-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottenberg R., Broder C. C., Boyle M. D., Kain S. J., Schroeder B. L., Curtiss R., 3rd Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J Bacteriol. 1992 Aug;174(16):5204–5210. doi: 10.1128/jb.174.16.5204-5210.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M. J., Raucci G., Gurusiddappa S., Hök M. Fibronectin binding determinants of the Staphylococcus aureus fibronectin receptor. J Biol Chem. 1991 May 5;266(13):8343–8347. [PubMed] [Google Scholar]
- Midura R. J., McQuillan D. J., Benham K. J., Fisher L. W., Hascall V. C. A rat osteogenic cell line (UMR 106-01) synthesizes a highly sulfated form of bone sialoprotein. J Biol Chem. 1990 Mar 25;265(9):5285–5291. [PubMed] [Google Scholar]
- Morrison M., Bayse G. S. Catalysis of iodination by lactoperoxidase. Biochemistry. 1970 Jul 21;9(15):2995–3000. doi: 10.1021/bi00817a010. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Mosher D. F. Localization of thrombospondin in clots formed in situ. Blood. 1985 Nov;66(5):1098–1104. [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992 Aug 1;176(2):415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti J. M., Jonsson H., Guss B., Switalski L. M., Wiberg K., Lindberg M., Hök M. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J Biol Chem. 1992 Mar 5;267(7):4766–4772. [PubMed] [Google Scholar]
- Pelletier L. L., Jr, Petersdorf R. G. Infective endocarditis: a review of 125 cases from the University of Washington Hospitals, 1963-72. Medicine (Baltimore) 1977 Jul;56(4):287–313. [PubMed] [Google Scholar]
- Ramphal R. The role of bacterial adhesion in cystic fibrosis including the staphylococcal aspect. Infection. 1990 Jan-Feb;18(1):61–64. doi: 10.1007/BF01644188. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Mayne R. Minor collagens of chicken hyaline cartilage. Biochemistry. 1981 Sep 15;20(19):5443–5448. doi: 10.1021/bi00522a014. [DOI] [PubMed] [Google Scholar]
- Rydén C., Maxe I., Franzén A., Ljungh A., Heinegård D., Rubin K. Selective binding of bone matrix sialoprotein to Staphylococcus aureus in osteomyelitis. Lancet. 1987 Aug 29;2(8557):515–515. doi: 10.1016/s0140-6736(87)91830-7. [DOI] [PubMed] [Google Scholar]
- Rydén C., Yacoub A. I., Maxe I., Heinegård D., Oldberg A., Franzén A., Ljungh A., Rubin K. Specific binding of bone sialoprotein to Staphylococcus aureus isolated from patients with osteomyelitis. Eur J Biochem. 1989 Sep 15;184(2):331–336. doi: 10.1111/j.1432-1033.1989.tb15023.x. [DOI] [PubMed] [Google Scholar]
- Signäs C., Raucci G., Jönsson K., Lindgren P. E., Anantharamaiah G. M., Hök M., Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci U S A. 1989 Jan;86(2):699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Switalski L. M., Wadström T. Fibronectin binding to a Streptococcus pyogenes strain. J Bacteriol. 1984 Feb;157(2):420–427. doi: 10.1128/jb.157.2.420-427.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Raucci G., Visai L., Switalski L. M., Timpl R., Hök M. Binding of collagen to Staphylococcus aureus Cowan 1. J Bacteriol. 1986 Jul;167(1):77–81. doi: 10.1128/jb.167.1.77-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M. Isolation and characterization of a putative collagen receptor from Staphylococcus aureus strain Cowan 1. J Biol Chem. 1989 Dec 15;264(35):21080–21086. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel F. A., Papageorgiou P. S. Osteomyelitis: the past decade. N Engl J Med. 1980 Aug 14;303(7):360–370. doi: 10.1056/NEJM198008143030703. [DOI] [PubMed] [Google Scholar]