Abstract
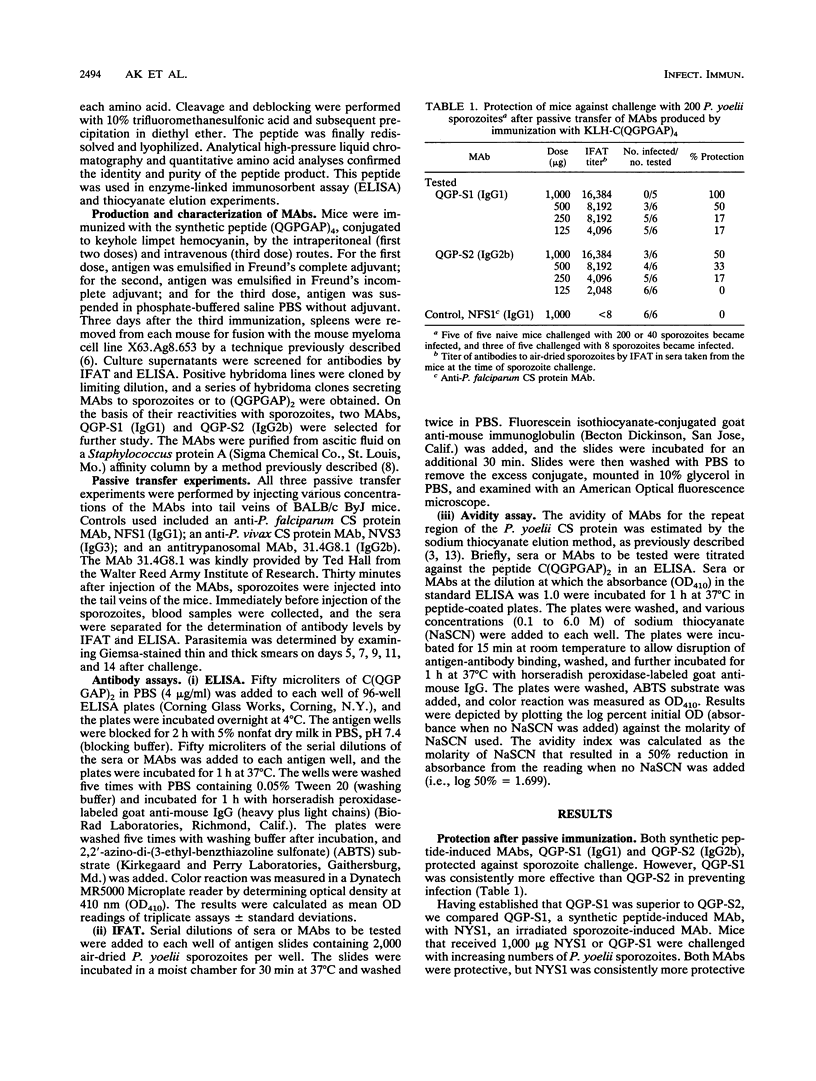
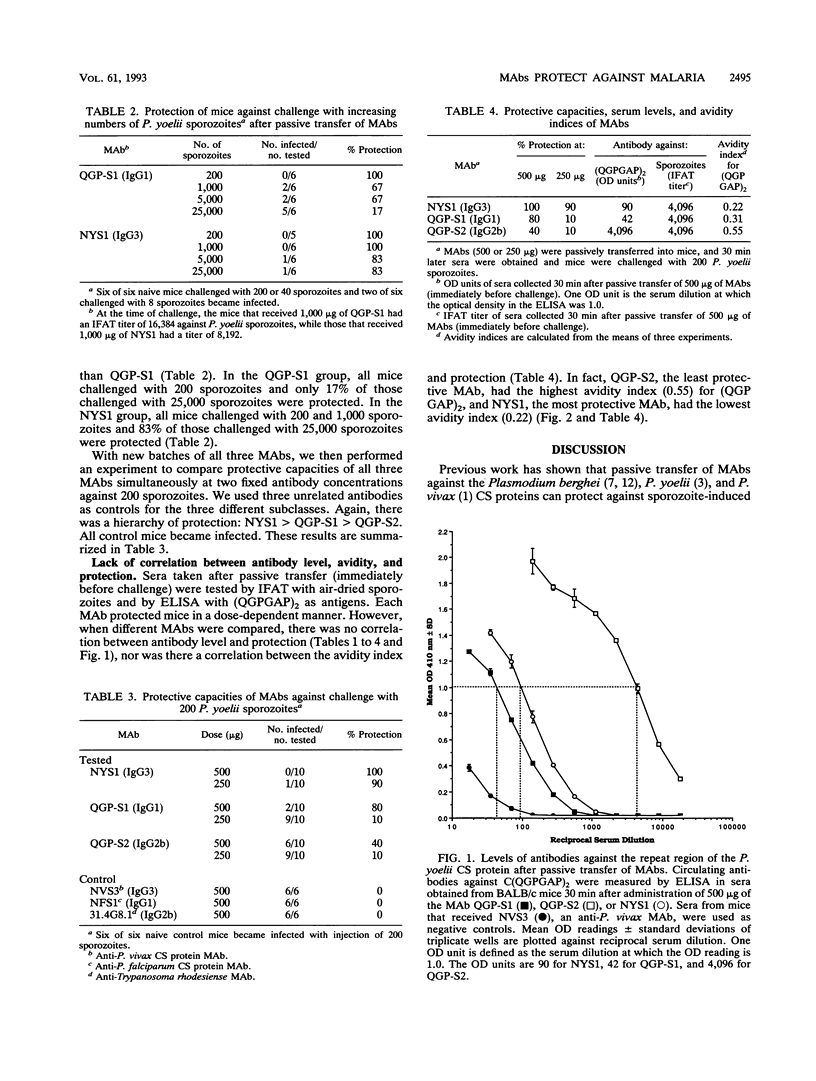
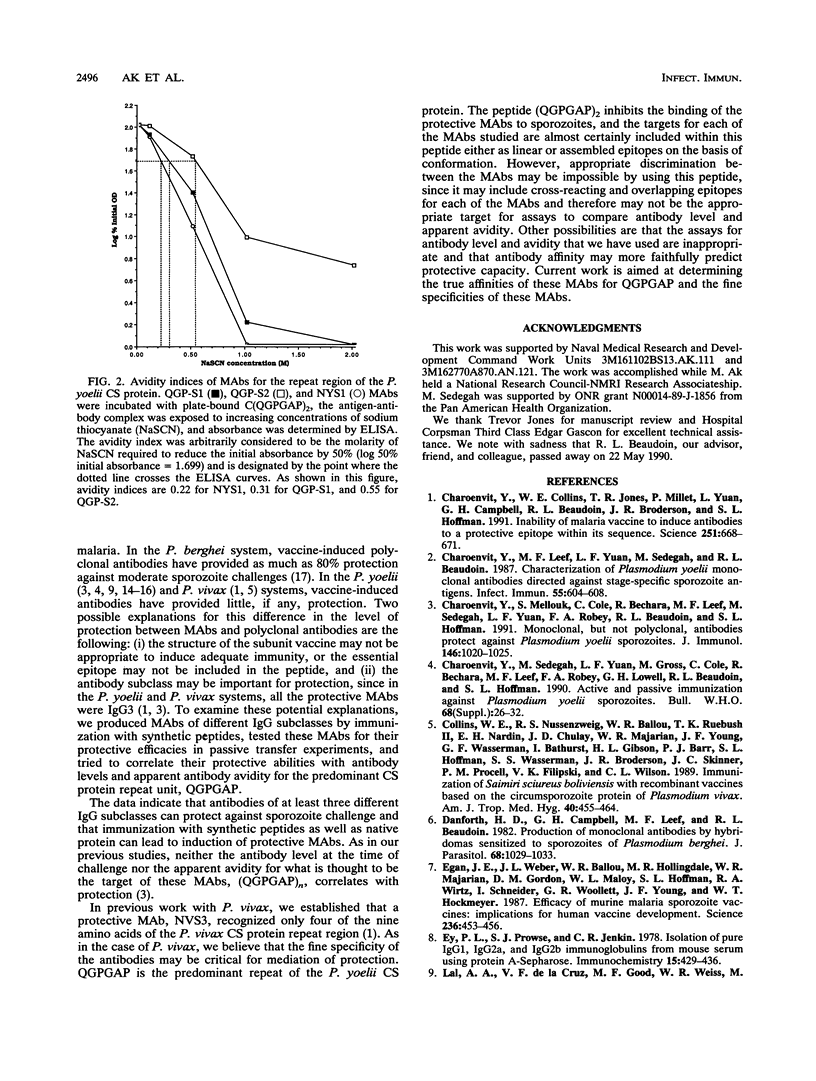
Passive transfer of monoclonal antibodies (MAbs) against malaria circumsporozoite (CS) proteins protects animals against malaria. Active immunization with synthetic or recombinant peptides induces a level of polyclonal antibodies to sporozoites comparable to those found after passive immunization but does not provide comparable protection. In the Plasmodium yoelii system, synthetic or recombinant peptide-induced antibodies have never been shown to protect. The current studies were designed to determine whether immunogen structure (native protein versus synthetic peptide) or immunoglobulin G (IgG) subclass of antibodies was responsible for the absolute differences between protective, passively transferred MAbs and nonprotective, actively induced polyclonal antibodies. In this study we produced two MAbs, QGP-S1 (IgG1) and QGP-S2 (IgG2b), by immunization with a synthetic peptide based on the P. yoelii CS major repeat, (QGPGAP)4, conjugated to keyhole limpet hemocyanin. These MAbs were compared tp NYS1 (IgG3), an anti-CS protein MAb previously produced by immunization with irradiated P. yoelii sporozoites, which recognizes (QGP GAP)2. QGP-S1 and QGP-S2 passively transferred protection. However, when compared with NYS1, there was a hierarchy of protection, NYS1 > QGP-S1 > QGP-S2. There was no correlation between antibody level at challenge as determined by immunofluorescent antibody test against sporozoites or enzyme-linked immunosorbent assay against (QGPGAP)2 or apparent antibody avidity for (QGPGAP)2 by sodium thiocyanate elution assay. The data demonstrate that a synthetic peptide can induce protective antibodies and that a specific antibody subclass is not required for protection. Work to determine whether antibody affinity or fine specificity can explain the hierarchy of protection among the MAbs is under way.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charoenvit Y., Collins W. E., Jones T. R., Millet P., Yuan L., Campbell G. H., Beaudoin R. L., Broderson J. R., Hoffman S. L. Inability of malaria vaccine to induce antibodies to a protective epitope within its sequence. Science. 1991 Feb 8;251(4994):668–671. doi: 10.1126/science.1704150. [DOI] [PubMed] [Google Scholar]
- Charoenvit Y., Leef M. F., Yuan L. F., Sedegah M., Beaudoin R. L. Characterization of Plasmodium yoelii monoclonal antibodies directed against stage-specific sporozoite antigens. Infect Immun. 1987 Mar;55(3):604–608. doi: 10.1128/iai.55.3.604-608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenvit Y., Mellouk S., Cole C., Bechara R., Leef M. F., Sedegah M., Yuan L. F., Robey F. A., Beaudoin R. L., Hoffman S. L. Monoclonal, but not polyclonal, antibodies protect against Plasmodium yoelii sporozoites. J Immunol. 1991 Feb 1;146(3):1020–1025. [PubMed] [Google Scholar]
- Charoenvit Y., Sedegah M., Yuan L. F., Gross M., Cole C., Bechara R., Leef M. F., Robey F. A., Lowell G. H., Beaudoin R. L. Active and passive immunization against Plasmodium yoelii sporozoites. Bull World Health Organ. 1990;68 (Suppl):26–32. [PMC free article] [PubMed] [Google Scholar]
- Collins W. E., Nussenzweig R. S., Ballou W. R., Ruebush T. K., 2nd, Nardin E. H., Chulay J. D., Majarian W. R., Young J. F., Wasserman G. F., Bathurst I. Immunization of Saimiri sciureus boliviensis with recombinant vaccines based on the circumsporozoite protein of Plasmodium vivax. Am J Trop Med Hyg. 1989 May;40(5):455–464. doi: 10.4269/ajtmh.1989.40.455. [DOI] [PubMed] [Google Scholar]
- Danforth H. D., Campbell G. H., Leef M. F., Beaudoin R. L. Production of monoclonal antibodies by hybridomas sensitized to sporozoites of Plasmodium berghei. J Parasitol. 1982 Dec;68(6):1029–1033. [PubMed] [Google Scholar]
- Egan J. E., Weber J. L., Ballou W. R., Hollingdale M. R., Majarian W. R., Gordon D. M., Maloy W. L., Hoffman S. L., Wirtz R. A., Schneider I. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science. 1987 Apr 24;236(4800):453–456. doi: 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Lal A. A., de la Cruz V. F., Good M. F., Weiss W. R., Lunde M., Maloy W. L., Welsh J. A., McCutchan T. F. In vivo testing of subunit vaccines against malaria sporozoites using a rodent system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8647–8651. doi: 10.1073/pnas.84.23.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco N. D., Strome C. P., Mitchell F., Bawden M. P., Beaudoin R. L. Rapid, large-scale isolation of Plasmodium berghei sporozoites from infected mosquitoes. J Parasitol. 1979 Jun;65(3):414–417. [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen G. R., Fitzgerald M. G., Hosking C. S. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986 Jan 22;86(1):83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- Sedegah M., Beaudoin R. L., Majarian W. R., Cochran M. D., Chiang C. H., Sadoff J., Aggarwal A., Charoenvit Y., Hoffman S. L. Evaluation of vaccines designed to induce protective cellular immunity against the Plasmodium yoelii circumsporozoite protein: vaccinia, pseudorabies, and Salmonella transformed with circumsporozoite gene. Bull World Health Organ. 1990;68 (Suppl):109–114. [PMC free article] [PubMed] [Google Scholar]
- Sedegah M., Chiang C. H., Weiss W. R., Mellouk S., Cochran M. D., Houghten R. A., Beaudoin R. L., Smith D., Hoffman S. L. Recombinant pseudorabies virus carrying a plasmodium gene: herpesvirus as a new live viral vector for inducing T- and B-cell immunity. Vaccine. 1992;10(9):578–584. doi: 10.1016/0264-410x(92)90436-n. [DOI] [PubMed] [Google Scholar]
- Tam J. P., Clavijo P., Lu Y. A., Nussenzweig V., Nussenzweig R., Zavala F. Incorporation of T and B epitopes of the circumsporozoite protein in a chemically defined synthetic vaccine against malaria. J Exp Med. 1990 Jan 1;171(1):299–306. doi: 10.1084/jem.171.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]



