Abstract
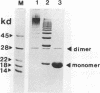
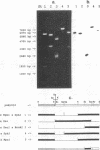
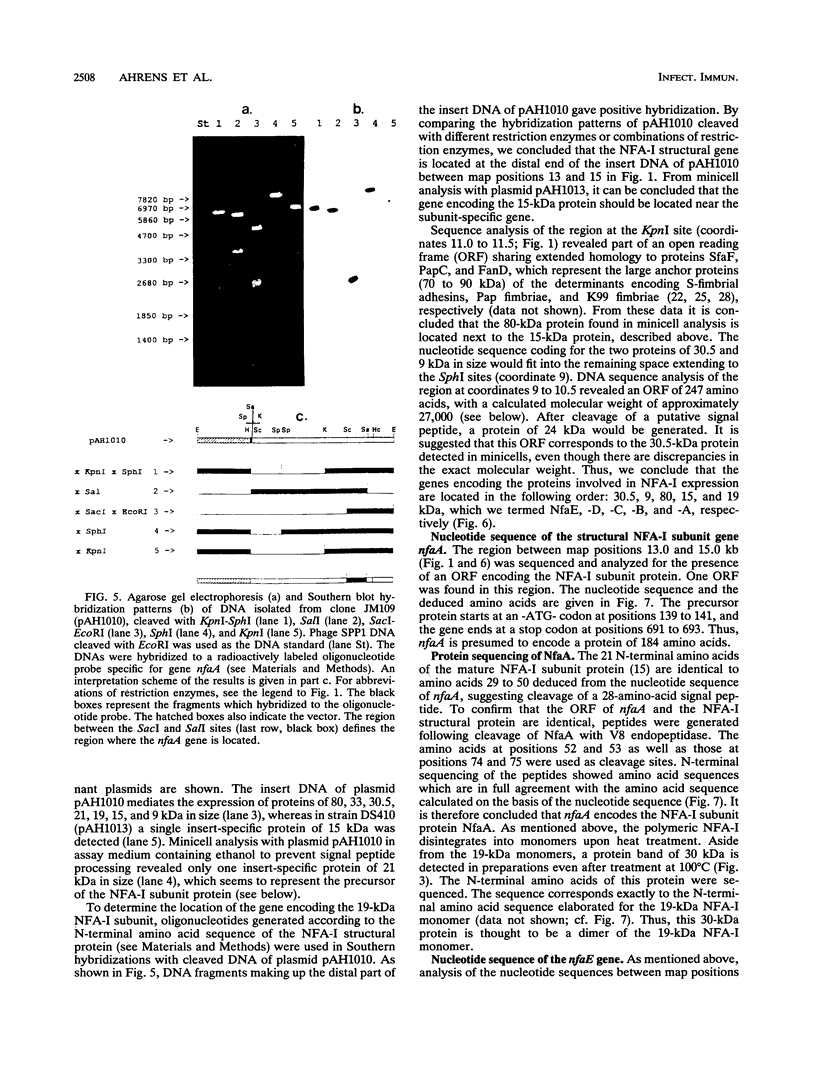
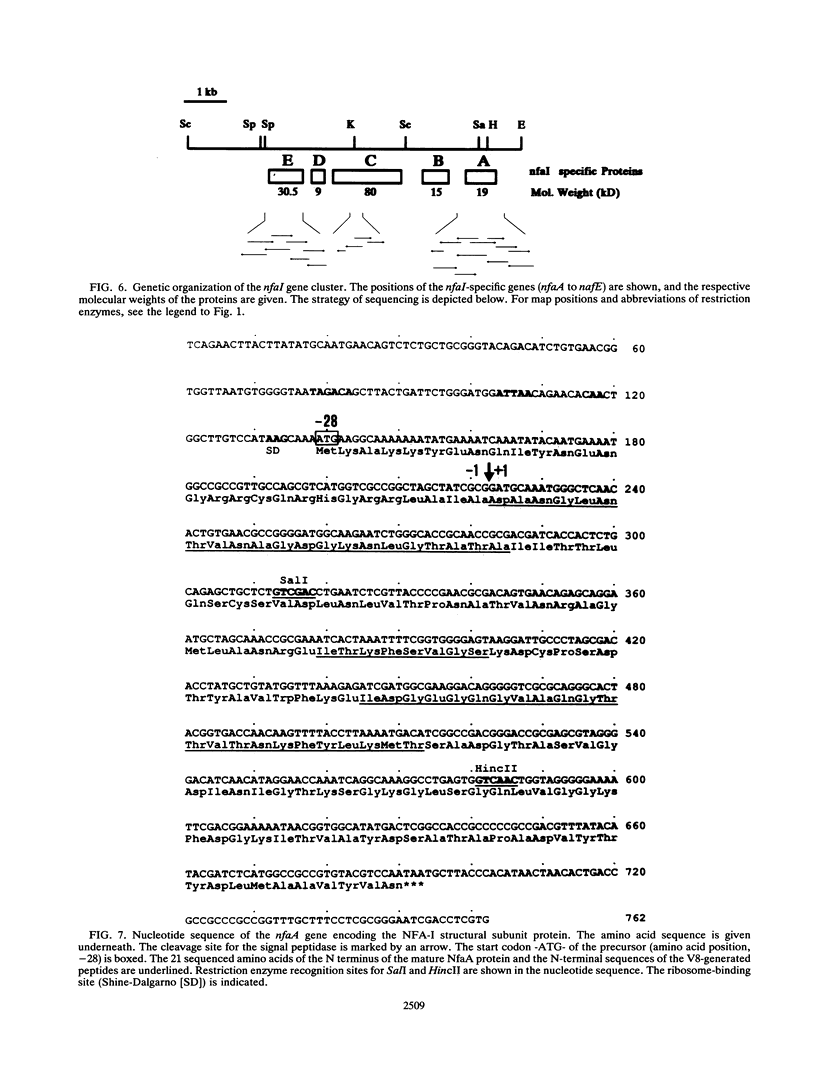
The chromosomally encoded nonfimbrial adhesion I (NFA-I) from Escherichia coli urinary tract isolate 827 (O83:K1:H4) mediates agglutination of human erythrocytes. Subclones were constructed from an NFA-I-expressing recombinant E. coli K-12 clone, derived from a genomic library of E. coli 827. Minicell analysis and nucleotide sequencing revealed that proteins of 30.5, 9, 80, 15, and 19 kDa encoded on a stretch of approximately 6 kb are involved in the expression of NFA-I. NFA-I exhibits a polymeric structure, which disintegrates with elevated temperature into a 19-kDa monomer but with some relatively stable dimers. By using gold-conjugated monoclonal antibodies directed against NFA-I in electron microscopy, the adhesin could be localized on the outer surface of the recombinant E. coli K-12 bacteria. The nucleotide sequence of the nfaA gene encoding the monomeric structural subunit of the adhesin was determined. An open reading frame of 184 amino acids encoding the NfaA precursor, which is processed to the mature protein, was found; it consisted of 156 amino acids with a calculated molecular weight of 16,000. Peptide sequencing of the NFA-I subunit protein confirmed that this open reading frame corresponds to the NfaA coding locus. Furthermore, the nucleotide sequence of the open reading frame termed NfaE, located at the proximal part of the DNA stretch responsible for NFA-I expression, was elaborated. NfaE consists of 247 amino acids, including a presumptive 29-amino-acid signal peptide, leading to a molecular weight of 24,000 for the mature protein. The nfaE sequence shares homology with the 27-kDa CS3 protein, which is involved in the assembly of CS3 fibrillae, and might encode the 30.5-kDa protein, detected in minicells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Eckerskorn C., Mewes W., Goretzki H., Lottspeich F. A new siliconized-glass fiber as support for protein-chemical analysis of electroblotted proteins. Eur J Biochem. 1988 Oct 1;176(3):509–519. doi: 10.1111/j.1432-1033.1988.tb14308.x. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Silhavy T. J. Sequence information required for protein translocation from the cytoplasm. J Bacteriol. 1987 Dec;169(12):5339–5342. doi: 10.1128/jb.169.12.5339-5342.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhar J., Perry R., Golecki J. R., Hoschutzky H., Jann B., Jann K. Nonfimbrial, mannose-resistant adhesins from uropathogenic Escherichia coli O83:K1:H4 and O14:K?:H11. Infect Immun. 1987 Aug;55(8):1837–1842. doi: 10.1128/iai.55.8.1837-1842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhar J., Yavzori M., Keisari Y., Ofek I. Phagocytosis of Escherichia coli mediated by mannose resistant non-fimbrial haemagglutinin (NFA-1). Microb Pathog. 1991 Sep;11(3):171–178. doi: 10.1016/0882-4010(91)90047-e. [DOI] [PubMed] [Google Scholar]
- Hacker J. Genetic determinants coding for fimbriae and adhesins of extraintestinal Escherichia coli. Curr Top Microbiol Immunol. 1990;151:1–27. doi: 10.1007/978-3-642-74703-8_1. [DOI] [PubMed] [Google Scholar]
- Hales B. A., Beverley-Clarke H., High N. J., Jann K., Perry R., Goldhar J., Boulnois G. J. Molecular cloning and characterisation of the genes for a non-fimbrial adhesin from Escherichia coli. Microb Pathog. 1988 Jul;5(1):9–17. doi: 10.1016/0882-4010(88)90076-9. [DOI] [PubMed] [Google Scholar]
- Jalajakumari M. B., Thomas C. J., Halter R., Manning P. A. Genes for biosynthesis and assembly of CS3 pili of CFA/II enterotoxigenic Escherichia coli: novel regulation of pilus production by bypassing an amber codon. Mol Microbiol. 1989 Dec;3(12):1685–1695. doi: 10.1111/j.1365-2958.1989.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Jann K., Hoschützky H. Nature and organization of adhesins. Curr Top Microbiol Immunol. 1990;151:55–70. doi: 10.1007/978-3-642-74703-8_3. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Falkow S. Distribution and degree of heterogeneity of the afimbrial-adhesin-encoding operon (afa) among uropathogenic Escherichia coli isolates. Infect Immun. 1988 Mar;56(3):640–648. doi: 10.1128/iai.56.3.640-648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Schmidt M. A., Walz W., Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985 Jun;162(3):1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel A., Rdest U., Springer W., Goebel W. Plasmid cistrons controlling synthesis and excretion of the exotoxin alpha-haemolysin of Escherichia coli. Mol Gen Genet. 1979 Oct 1;175(3):343–350. doi: 10.1007/BF00397234. [DOI] [PubMed] [Google Scholar]
- Norgren M., Båga M., Tennent J. M., Normark S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol Microbiol. 1987 Sep;1(2):169–178. doi: 10.1111/j.1365-2958.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Escherichia coli in extra-intestinal infections. J Hyg (Lond) 1985 Dec;95(3):551–575. doi: 10.1017/s0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M., Väisänen-Rhen V., Saraste M., Korhonen T. K. Organization of genes expressing the blood-group-M-specific hemagglutinin of Escherichia coli: identification and nucleotide sequence of the M-agglutinin subunit gene. Gene. 1986;49(3):351–360. doi: 10.1016/0378-1119(86)90371-9. [DOI] [PubMed] [Google Scholar]
- Roosendaal B., de Graaf F. K. The nucleotide sequence of the fanD gene encoding the large outer membrane protein involved in the biosynthesis of K99 fimbriae. Nucleic Acids Res. 1989 Feb 11;17(3):1263–1263. doi: 10.1093/nar/17.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T., Morschhäuser J., Ott M., Ludwig B., van Die I., Hacker J. Complete genetic organization and functional aspects of the Escherichia coli S fimbrial adhesion determinant: nucleotide sequence of the genes sfa B, C, D, E, F. Microb Pathog. 1990 Nov;9(5):331–343. doi: 10.1016/0882-4010(90)90067-z. [DOI] [PubMed] [Google Scholar]
- Svanborg Edén C., Hagberg L., Hanson L. A., Hull S., Hull R., Jodal U., Leffler H., Lomberg H., Straube E. Bacterial adherence--a pathogenetic mechanism in urinary tract infections caused by Escherichia coli. Prog Allergy. 1983;33:175–188. [PubMed] [Google Scholar]
- Swanson T. N., Bilge S. S., Nowicki B., Moseley S. L. Molecular structure of the Dr adhesin: nucleotide sequence and mapping of receptor-binding domain by use of fusion constructs. Infect Immun. 1991 Jan;59(1):261–268. doi: 10.1128/iai.59.1.261-268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- van Die I., Bergmans H. Nucleotide sequence of the gene encoding the F72 fimbrial subunit of a uropathogenic Escherichia coli strain. Gene. 1984 Dec;32(1-2):83–90. doi: 10.1016/0378-1119(84)90035-0. [DOI] [PubMed] [Google Scholar]