Abstract
Context:
Arthrogenic muscle inhibition is an important underlying factor in persistent quadriceps muscle weakness after knee injury or surgery.
Objective:
To determine the magnitude and prevalence of volitional quadriceps activation deficits after knee injury.
Data Sources:
Web of Science database.
Study Selection:
Eligible studies involved human participants and measured quadriceps activation using either twitch interpolation or burst superimposition on patients with knee injuries or surgeries such as anterior cruciate ligament deficiency (ACLd), anterior cruciate ligament reconstruction (ACLr), and anterior knee pain (AKP).
Data Extraction:
Means, measures of variability, and prevalence of quadriceps activation (QA) failure (<95%) were recorded for experiments involving ACLd (10), ACLr (5), and AKP (3).
Data Synthesis:
A total of 21 data sets from 18 studies were initially identified. Data from 3 studies (1 paper reporting data for both ACLd and ACLr, 1 on AKP, and the postarthroscopy paper) were excluded from the primary analyses because only graphical data were reported. Of the remaining 17 data sets (from 15 studies), weighted mean QA in 352 ACLd patients was 87.3% on the involved side, 89.1% on the uninvolved side, and 91% in control participants. The QA failure prevalence ranged from 0% to 100%. Weighted mean QA in 99 total ACLr patients was 89.2% on the involved side, 84% on the uninvolved side, and 98.5% for the control group, with prevalence ranging from 0% to 71%. Thirty-eight patients with AKP averaged 78.6% on the involved side and 77.7% on the contralateral side. Bilateral QA failure was commonly reported in patients.
Conclusions:
Quadriceps activation failure is common in patients with ACLd, ACLr, and AKP and is often observed bilaterally.
Keywords: arthrogenic muscle inhibition, voluntary activation, twitch interpolation, superimposed burst, central activation ratio
Key Points.
Patients with anterior cruciate ligament deficiency or reconstruction and those with anterior knee pain commonly experience bilateral quadriceps muscle activation failure.
Prospective, controlled trials are warranted to help us understand the natural history of quadriceps activation failure and to guide interventions to improve outcomes.
Persistent quadriceps weakness after knee injury or surgery is frequently reported in the literature.1,2 Quadriceps strength and endurance are of vital importance for normal knee joint function, so restoring normal quadriceps function after knee joint injuries is an essential component of rehabilitation. Persistent posttraumatic quadriceps weakness presents a difficult clinical dilemma for the treating clinician. An important underlying factor contributing to persistent, perhaps rehabilitation-resistant posttraumatic quadriceps weakness is arthrogenic muscle inhibition (AMI), which remains understudied in current clinical outcomes research in patients with knee joint injury.
Arthrogenic muscle inhibition is an ongoing, reflex response after joint injury. The term describes the inability to completely contract a muscle despite no structural damage to the muscle or innervating nerve.3 It is considered a reflex response to joint injury because it is beyond conscious, voluntary control. Clinically, athletic trainers and other medical practitioners see quadriceps AMI manifested as posttraumatic weakness and muscle atrophy that may persist for a long time after the original injury. Although AMI is most likely a protective mechanism after joint injury, it may become a limitation during rehabilitation.3 Therefore, it is important for clinicians to understand the prevalence and clinical effects of AMI in order to devise strategies to overcome this impairment.
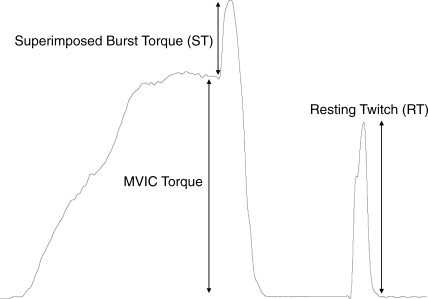
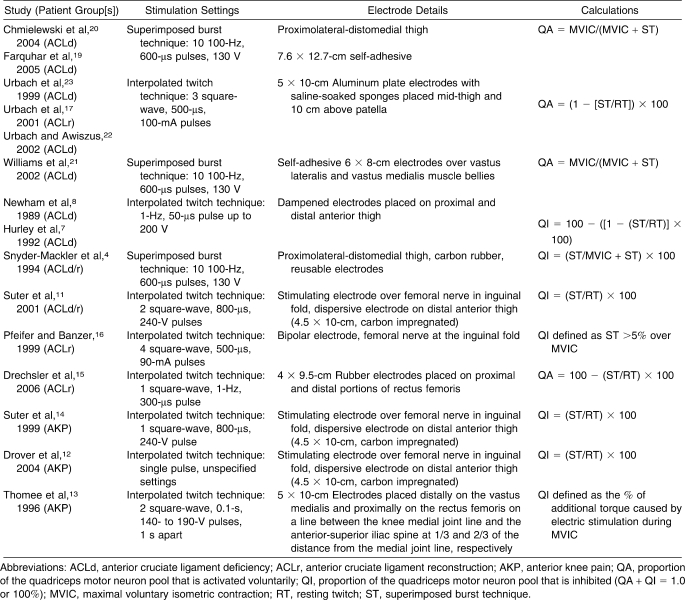
Condensing published findings regarding AMI after knee joint injury is difficult given the variety of measurement techniques and formulas used for calculating quadriceps AMI. Force-based measures of quadriceps activation have been used to determine the proportion of the quadriceps motor neuron pool that can be volitionally activated. Force-based measurement techniques include the superimposed burst technique (SIB)4 and the interpolated twitch technique (ITT).5 These techniques use a supramaximal, percutaneous electric stimulation during a maximal, voluntary isometric knee extension contraction to calculate the central activation ratio (CAR; Figure 1).6 In theory, when using SIB or ITT, if an individual is able to contract all motor units in the quadriceps, electric stimulation will not cause any increase in force.7,8 Similarly, if a portion of the quadriceps is inhibited, external stimulation will cause a force-producing contraction that is greater than the volitional contraction. People with more than 95% volitional activation have been defined as being fully activated.7,8 Regardless of the measurement technique, various formulas exist for calculating muscle activation or inhibition based on the force measures obtained with the SIB or ITT method.
Figure 1.
Sample force tracing showing components for calculating central activation ratio. Abbreviations: MVIC, maximal voluntary isometric contraction; ST, superimposed burst torque; RT, resting twitch.
Recognizing AMI and understanding its potential effects on long-term joint health in the posttraumatic knee are of vital clinical importance and will lead to a better understanding of likely mechanisms for muscle weakness, altered gait patterns, and the risks for reinjury and joint degeneration. Therefore, our purpose was to describe the magnitude and prevalence of volitional quadriceps activation deficits after acute knee injury or surgery.
METHODS
We performed an online search using Web of Science from 1970 to September 1, 2008. We had 809 initial hits using the search terms quadriceps activation and knee, quadriceps inhibition and knee, superimposed burst and quadriceps, interpolated twitch technique and quadriceps, and central activation ratio and quadriceps. Because of the types of injuries that were found with the initial search, we decided to add the search terms quadriceps activation and anterior cruciate ligament, quadriceps inhibition and anterior cruciate ligament, quadriceps activation and meniscus, quadriceps inhibition and meniscus, quadriceps activation and anterior knee pain, and quadriceps inhibition and anterior knee pain, which provided 262 additional hits, for a total of 1071 hits. In addition, we cross-referenced the reference lists of articles found by this method for further relevant articles. Only original research articles that were written in English, involved human participants, and reported means and SDs were included. We limited our search to studies evaluating quadriceps voluntary activation or inhibition in young, active patients with acute knee injuries or surgery (excluding osteoarthritis, knee arthroplasty, and models of artificial knee joint effusion). All studies had to include a force-based measure of quadriceps activation, such as the SIB or ITT.
Included Studies and Quality Assessment
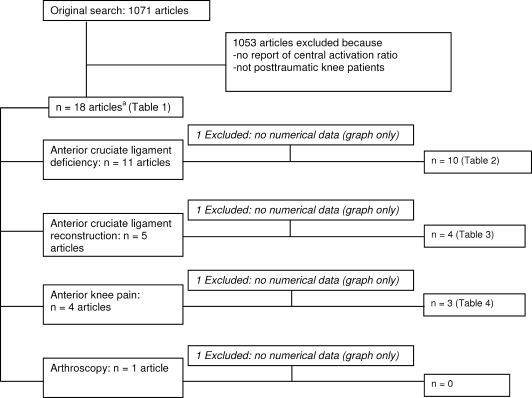
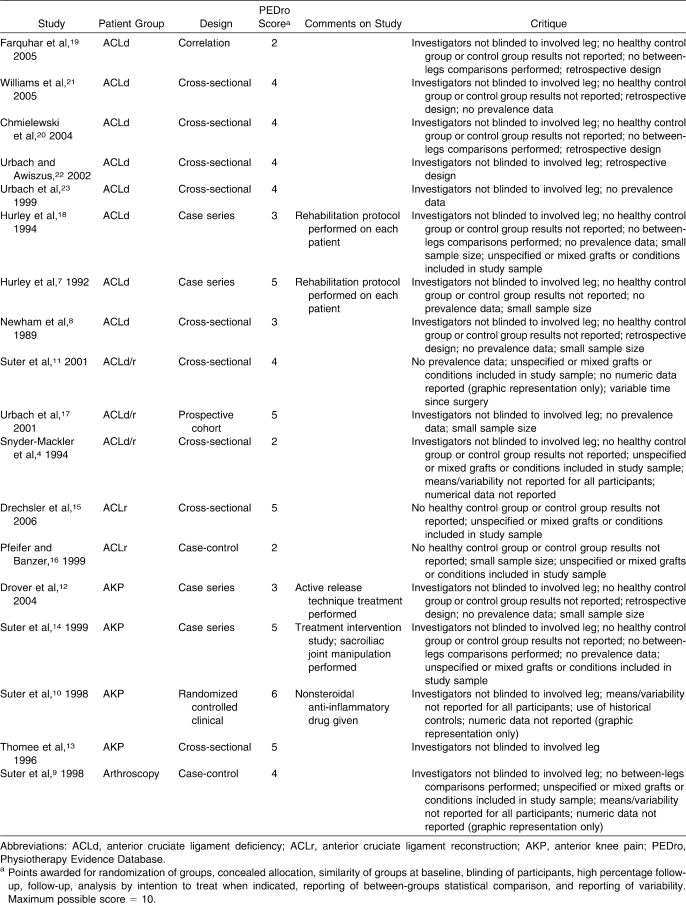
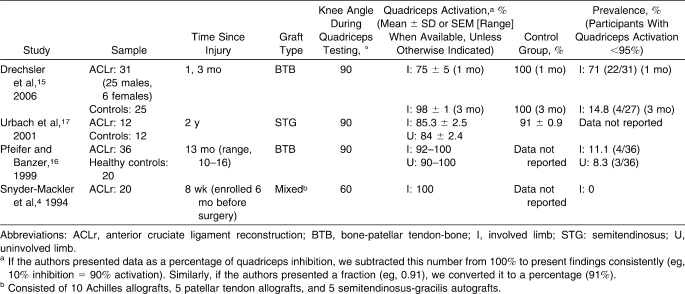
We initially identified 21 data sets from 18 studies4,7–23 that met the inclusion criteria (Figure 2). Data from 3 research papers (1 paper11 reporting data for both anterior cruciate ligament deficiency [ACLd] and anterior cruciate ligament reconstruction [ACLr], 1 reporting on anterior knee pain [AKP],10 and 1 postarthroscopy paper9) were excluded from the primary analyses because only graphical data were provided. Therefore, we included 17 data sets from 15 papers published in peer-reviewed journals4,7,8,12–23 that evaluated quadriceps function in ACLd patients (n = 10),4,7,8,17–23 ACLr patients (n = 4),4,15–17 and AKP patients (n = 3).12–14 Each study was independently evaluated with the Physiotherapy Evidence Database (PEDro) scale24 for methodologic quality (Table 1).
Figure 2.
Selection process for studies included in this review. a Three articles described both anterior cruciate ligament deficiency and anterior cruciate ligament reconstruction patients (Table 1).
Table 1.
Descriptions and Critiques of Reviewed Studies
Data Extraction and Analysis
Prevalence of activation failure was determined by reporting the percentage of study participants below 95% voluntary activation.25–27 Times of testing with respect to the onset of injury as well as follow-up testing intervals were also included. Because joint angle has been reported28 to affect quadriceps activation, we included these data in the results. The data were collected for the injured legs, uninjured legs, and control group (if provided). We calculated 95% confidence intervals from weighted means and weighted SDs to account for different sample sizes in the studies.
RESULTS
The PEDro scores and critiques for all studies included in this review are presented in Table 1. We summarized study findings separated by injury type in Tables 2 through 4. A review of measurement techniques is presented in Table 5 (Figure 2).
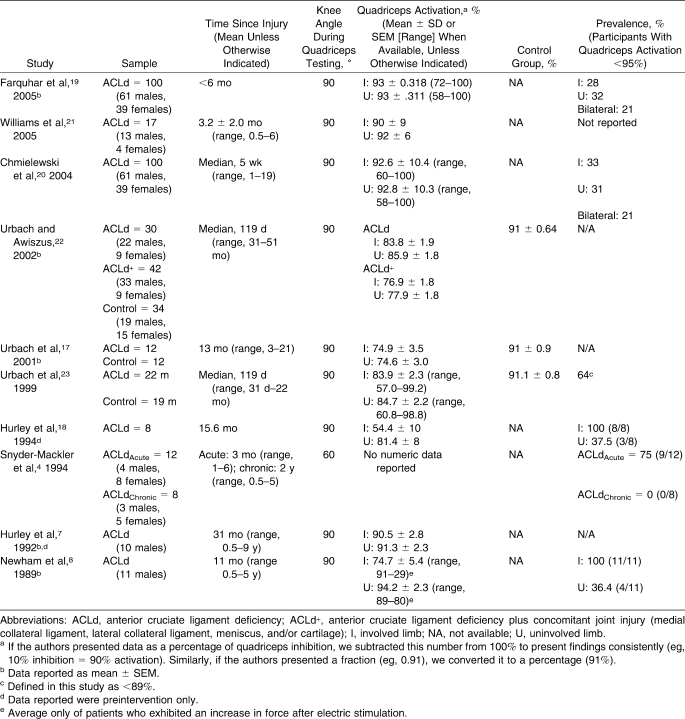
Table 2.
Studies Reporting Quadriceps Muscle Function in Anterior Cruciate Ligament–Deficient Patients
Table 3.
Studies Reporting Quadriceps Muscle Function in Anterior Cruciate Ligament Reconstruction Patients
Table 4.
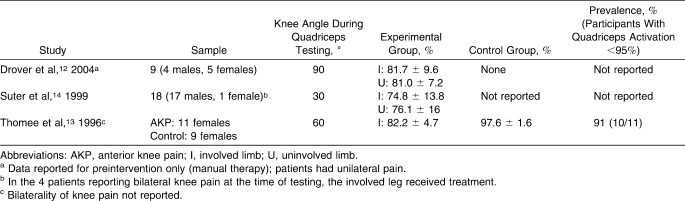
Studies Reporting Quadriceps Muscle Function in Patients With Unilateral Anterior Knee Pain
Table 5.
Measurement Techniques and Quadriceps Muscle Function Calculation Details for Reviewed Studies
Anterior Cruciate Ligament Deficiency
The 10 ACLd studies4,7,8,17–23 included in this review appear in Table 2. In a total of 352 patients, the overall weighted mean quadriceps activation (QA) was 87.3% (95% confidence interval [CI] = 85.4, 89.3) for the involved side, 89.1% (95% CI = 87.3, 90.8) for the uninvolved side, and 91.0% (95% CI = 89.3, 92.7) for control participants (Figure 3). The QA failure prevalence was 57.1% (range, 0%–100%) on the involved side and 34.2% (range, 31%–37.5%) on the uninvolved side. Finally, bilateral QA failure was reported for 21% of patients with ACLd. One group11 graphically reported QA data for 24 additional ACLd patients (mean = 44 months postinjury, measured at 30° of knee flexion using ITT). Extrapolating data from the graphs, ACLd patients exhibited approximately 80% and 82% QA for the involved and uninvolved sides, respectively, which would reduce overall weighted means for ACLd to 86.9% (involved side) and 88.6% (uninvolved side).
Figure 3.
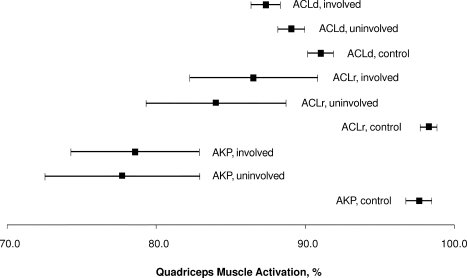
Average quadriceps activation data for the involved, uninvolved, and control limbs in all studies included in this review. Data points represent weighted averages, and error bars represent 95% confidence intervals. Abbreviations: ACLd, anterior cruciate ligament deficiency; ACLr, anterior cruciate ligament reconstruction; AKP, anterior knee pain.
Anterior Cruciate Ligament Reconstruction
Four groups4,15–17 evaluated volitional QA after ACLr (Table 3). Quadriceps neural activation was measured in 99 participants using SIB and ITT techniques (Table 5). The weighted mean was 86.5% (95% CI = 78.1, 94.9) for the involved side, 84.0% (95% CI = 74.8, 93.2) for the contralateral, uninvolved side, and 98.3% (95% CI = 97.2, 99.4) for control participants (Figure 3). The QA failure prevalence ranged from 0% to 71% (average = 24.2%) for the involved side and was reported by one group to be 8.3% on the uninvolved side. One set of investigators11 graphically reported QA data for 22 additional ACLr patients (mean = 22 months postsurgery, measured at 30° of knee flexion using ITT). Extrapolating data from the graphs, ACLr patients exhibited approximately 80% and 82% QA for the involved and uninvolved sides, respectively. This finding reduces the weighted mean QA for ACLr to 85.0% (involved side) and 82.3% (uninvolved side).
Anterior Knee Pain
Three groups12–14 evaluated QA in patients with AKP (Table 4). In 38 patients with AKP, weighted means were 78.6% (95% CI = 70.1, 87.0) on the involved side and 77.7% (95% CI = 67.5, 87.9) on the contralateral side, with only 1 set of authors reporting 97.6% (95% CI = 95.9, 99.3) QA in control participants (Figure 3). Only 1 group13 reported 91% QA failure prevalence in AKP patients. Using ITT, 2 additional sets of investigators9,10 reported QA graphically in 72 additional patients with AKP. Patients with AKP (n = 42) exhibited approximately 67% and 82% QA failure prevalence on their involved and uninvolved sides, respectively. In addition, when ITT was used before patellofemoral arthroscopy, patients with AKP (n = 30) exhibited approximately 62.5% and 70% QA failure prevalence on the involved and uninvolved sides, respectively.9 In this same study, patients exhibited approximately 64% (involved side) and 72% (uninvolved side) QA failure prevalence at 6 weeks and 68% (involved side) and 70% (uninvolved side) QA failure prevalence at 6 months after arthroscopy. These findings (excluding postarthroscopy data) reduce the weighted mean QA for AKP patients to 71.2% (involved side) and 77.1% (uninvolved side).
DISCUSSION
In this systematic review, we have described average QA in patient populations and the prevalence of QA failure (defined as CAR of <95%). The prevalence and magnitude of QA failure after traumatic knee injuries is a phenomenon with important clinical implications in persons rehabilitating from joint injury. Therefore, from this review of available literature, we can make the following clinically relevant statements:
On average, clinically meaningful deficits existed bilaterally in both ACLd and ACLr populations compared with control participants. Weighted mean quadriceps CAR was slightly higher in ACLd patients; however, the proportion of patients with QA failure (ie, CAR of <95%) is considerably higher in ACLd patients. This indicates an overall improvement in QA after reconstruction.
The magnitude of activation deficits in patients with AKP seems to be higher than in patients with ligament injuries.
Bilaterality of QA deficits may complicate side-to-side (within-patients or within-participants) comparisons, especially when used for return-to-play decision making. Concurrent controls seems to be the best comparison, because they are evaluated using the same technique and experimental setup. Blinding of the evaluator and data assessor may help improve the methodologic quality of clinical research studies using SIB and ITT to measure QA.
Interesting and contradictory findings from this review are the observations that the weighted mean CAR was higher in ACLd than in ACLr patients, whereas the prevalence of QA failure was more than double in ACLd patients compared with ACLr patients. Therefore, the prevalence of QA failure indicates successful quadriceps recovery after ACL reconstruction; however, weighted averages do not. One explanation for this is that only 3 data sets from 2 studies15,17 included means and variability measurements for quadriceps CAR in ACLr knees, and only 1 group17 reported quadriceps CAR in ACLr knees and only 1 group reported CAR for the uninvolved side in patients with ACLr knees. Therefore, the overall average CAR for the uninvolved side for ACLr patients is drawn from a single representative study. In addition, 2 of the 4 ACLr studies included in this review (which included 62/74 patients in this portion of the analysis) were conducted 1 to 3 months after the participants' ACL injuries; the other ACLr study reported on patients who were, on average, 24 months postreconstruction. The remaining study reported prevalence data only. Thus, this analysis may be weighted more toward a short-term outcome after ACL reconstruction. In the ACLd group, patients were an average of 9.8 months since injury (range, 3.2–31 months) and may have been more representative of mid-term consequences. In addition, we calculated the overall, weighted mean for all healthy participants included in this review: 94.8% ± 3.6%. This finding supports the existence of bilateral QA failure in both ACLd and ACLr individuals, which is evident in Figure 3: CIs for both limbs in injured persons (ACLd, ACLr, or AKP) do not cross CIs for the concurrent control participants.
Similarly, quadriceps CAR was higher on the involved side than the uninvolved side in AKP patients. In addition, the AKP patients in this review exhibited lower quadriceps CAR than did the ACLd and ACLr patients (Figure 3). One explanation is that patients with chronic AKP may experience bilateral symptoms resulting from bilateral quadriceps weakness or activation failure. Alternatively, this finding provides evidence of bilateral quadriceps central activation failure in the presence of a unilateral injury, which may predispose an asymptomatic knee to future injury. In addition, the 3 groups reporting quadriceps CAR in AKP patients used 3 different knee joint angles for measuring knee extension force. Interestingly, it appears that quadriceps CAR was lowest when measured at a more extended angle (30°; Table 4). This result agrees with previous findings29 of lower QA during isometric contractions when the knee is in a more extended position and is likely because the shortened quadriceps are at a mechanical disadvantage in generating knee extension force.30,31
Arthrogenic muscle inhibition is a typical consequence of joint injury when the body's protective, reflexive, and unconscious responses are to alter neural drive to the surrounding musculature. This reflexive “shut-down” of a joint's surrounding musculature is initially protective. Unfortunately, AMI prevents complete activation of a muscle and, thus, may impede recovery after injury. The exact mechanisms eliciting and controlling quadriceps AMI after knee joint injury are unclear. Hurley et al18 suggested that AMI is due to the altered afferent input originating from mechanoreceptors within the diseased joint reflexively reducing efferent output from quadriceps α motor neurons, thereby causing incomplete muscle activation. Other factors, such as pain and disuse, may also contribute to quadriceps inhibition after joint injury.3
Quadriceps activation failure after knee joint injury may be the cause of persistent weakness and resultant kinematic and kinetic changes during gait,32 which may compromise the ability of lower extremity muscles to appropriately respond to joint loading. Lewek et al33 showed that persons with ACLr knees who exhibited asymmetric quadriceps strength (less than 80% of the strength of the nonsurgical side) tended to display a quadriceps avoidance pattern, defined as lower knee flexion angles and reduced knee joint torques during the loading phase of walking and jogging gait. Excessive QA failure from AMI causes persistent quadriceps weakness, leading to altered kinetics and kinematics after knee injury. Ernst et al34 reported reduced knee joint torques after ACLr and increased reliance on the hip and ankle joints during a lateral step-down task. Webster et al35 reported reduced external knee flexion moments, indicating a QA gait in patients with reconstructed knees and occurring more often in patients who received patellar tendon autografts. In theory, a weak quadriceps from persistent posttraumatic AMI reduces the ability to generate force and thereby to provide efficient eccentric muscle control of joint loading during the loading phase of gait. The inability of dynamic stabilizers to efficiently absorb impact forces during gait may initiate joint surface damage.36 Animal data indicate that inhibited muscles may lead to joint degeneration through detriments to muscle force production and altered ground reaction forces during gait.37 Abnormal gait kinematics and kinetics are common after knee injuries such as ACL reconstruction38–40 and meniscectomy.41 Altered gait patterns after knee joint reconstruction may persist for 839 to 1242 months and for years after major joint trauma and reconstruction.43 Evidence also exists of altered lower extremity kinetics during a seated leg press44 and during gait32,45 after an artificial knee joint effusion. Altered knee joint movements46 and torques have been implicated as causative factors in the genesis of osteoarthritis in the patellofemoral47 and tibiofemoral joints.48 Given the extensive findings of persistent muscle weakness after knee joint trauma1,2 and the high likelihood of early-onset osteoarthritis,49,50 the development and implementation of therapies to treat the underlying cause of muscle weakness and altered gait patterns are of primary concern to clinicians.
Strategic implementation of treatments aimed at removing AMI may be an appropriate strategy to overcome persistent postinjury muscle weakness.2 Certified athletic trainers and other medical and rehabilitation specialists should be aware of this clinical dilemma and focus rehabilitation interventions on removing AMI, thereby providing patients the benefit of exercising with a more complete motor neuron pool. Hurley et al7 reported that QA failure in ACLd patients remained unchanged after 4 weeks of traditional strengthening. This indicates the clinical importance of prescribing therapies aimed at removing AMI before rehabilitation exercises begin. Modalities such as manual therapy,14,51,52 focal knee joint cooling,53–56 transcutaneous electric nerve stimulation,55–58 and neuromuscular electric stimulation59,60 have been shown to improve quadriceps function and may be useful in patients experiencing posttraumatic AMI.
The included studies all involved variations of the SIB or ITT technique. These techniques have been used to artificially activate all, or close to all, motor units in the quadriceps motor neuron pool with a percutaneous electric stimulus or several stimuli delivered to the femoral nerve or directly to the quadriceps muscle.5,61–64 These torque-based measurements compare volitional recruitment, with the theoretical 100% recruitment achieved via electric stimulation. Because 100% voluntary activation is physiologically unlikely, the clinical interpretation of these measurements is limited. In addition, the potential for stimulus volume conduction to other adjacent muscle tissue via skin or other biologic tissue may confound force readings and may, therefore, result in underestimated QA from unintentional recruitment of muscles other than the targeted muscle.65 In the context of the current review, stimulus volume conduction to the hamstrings muscles may reduce the knee extension torque achieved during SIB testing.66 Despite the inherent limitations of these force-based measurements, SIB and ITT are currently the best methods for measuring voluntary activation.
The choice of grafts used for ACLr may have implications for quadriceps strength. Patellar tendon (bone-tendon-bone [BTB]) and quadrupled semitendinosis-gracilis (STG) are common choices. Several groups67–69 have shown greater extensor strength deficits in BTB than in STG reconstruction. This finding may be, in part, from donor site morbidity in BTB harvesting resulting in AKP.69 Muscle performance in patients with AKP after BTB ACLr showed limited isokinetic extensor muscle strength recovery when compared with performance in patients without AKP.69 This strength deficit may be due to neural quadriceps inhibition, which is exaggerated in the presence of AKP.10 In addition, AKP prevalence is greater with BTB graft than with STG graft.70 In one of the included studies,15 weakness and decreased activation were associated with AKP after ACLr with BTB. Altered biomechanics during gait35 are also more common in patients receiving BTB grafts. Furthermore, 2 recent groups, one studying patients at 6 years68 and the other studying patients at 10 years70 postoperatively, showed a higher incidence of radiographic osteoarthritis in patients with BTB grafts than in patients with STG grafts. Notably, osteoarthritis at 10 years was correlated with an abnormal hop test at 1 year, again indicating the link between abnormal neuromuscular function and subsequent degenerative changes.70 A causal relationship among graft site, pain, activation failure, persistent quadriceps weakness, altered gait, and, ultimately, joint degeneration is plausible. This relationship warrants further study, as such an association could affect the continuing evolution of indications for BTB versus hamstrings tendon grafts and may also help in the development of evidence-based, graft-specific rehabilitation strategies.
It is important to determine the existence of QA failure in patients with knee injuries. If left untreated, quadriceps inhibition may prevent restoration of preinjury quadriceps function, thereby leading to further joint damage or osteoarthritis71–73; thus, authors2,3 have strongly suggested that interventions specifically targeting AMI be used in rehabilitation. Restoring quadriceps function by removing neural inhibitory mechanisms may be essential in facilitating complete neuromuscular recovery after knee injury or surgery and a prompt, safe return to sport, while avoiding the development of osteoarthritis.3
Methodologic Concerns and Limitations
Traditionally, when using a force-based estimate of quadriceps CAR, activation failure is considered a CAR of less than 95%.25–27 However, force-based measurement techniques such as the SIB or ITT procedures may overestimate muscle activation,74 which make CAR data difficult to interpret if ratios are high. Although the SIB and ITT techniques are sensitive to changes in muscle activation after interventions or treatments, valid CAR estimates rely on the ability to effectively isolate knee extensors during a maximal voluntary isometric contraction. For example, improper patient positioning or poor control of trunk motion can confound isometric knee extension torque measurements, because muscles other than the quadriceps will contribute to generating maximal torque during a volitional effort. Electric stimulation to the femoral nerve or directly to the quadriceps muscle (ie, using SIB or ITT) is only intended to elicit contraction from the quadriceps. Therefore, force augmentation may be minimized and voluntary activation overestimated. Suboptimal electric stimulation settings may also cause invalid estimates of QA, especially if the stimulation fails to fully activate all motor units in a motor neuron pool. These factors, in addition to variations in technique, make comparison among studies difficult. Therefore, the extent of motor unit activation may vary from person to person and from protocol to protocol. The variations in measurement technique complicate the ability to compare the QA data reported in different studies. The techniques used to estimate voluntary activation for the studies included in this review are summarized in Figure 2 and Table 5.
This review is limited by the fact that the studies included were of low methodologic quality based on the level of evidence and the PEDro score. The PEDro scores in the included studies were low; however, this may be an inherent issue given the types of research design used and those that are appropriate to study AMI in patient populations. Future research designs should be prospective and should include methods to protect internal validity, such as matching and blinding. The critiques described in the included studies (Table 1) are largely inherent to clinical studies: random allocation of surgical patients is not feasible, and blinding is difficult. However, improved study design would provide better evidence to determine the incidence of quadriceps neural activation failure in populations with knee injuries. We suggest the need for prospective studies that include an adequate number of participants, a well-matched control group, and blinded data collection. Confounding factors such as time since injury or surgery, graft type, concomitant injury, and type of rehabilitation should be described and controlled.
In conclusion, QA failure is common in patients with ACLd, ACLr, and AKP and is commonly observed bilaterally. The duration and magnitude of posttraumatic AMI have significant clinical effects, so prospective, controlled clinical trials are warranted. An understanding of the natural history of activation failure after ACLr will help to guide interventions aimed at improving outcomes and, possibly, reduce the incidence of osteoarthritis in this population.
REFERENCES
- 1.Ingersoll C. D., Grindstaff T. L., Pietrosimone B. G., Hart J. M. Neuromuscular consequences of anterior cruciate ligament injury. Clin Sports Med. 2008;27(3):383–404, vii. doi: 10.1016/j.csm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Palmieri-Smith R. M., Thomas A. C., Wojtys E. M. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27(3):405–424, vii–ix. doi: 10.1016/j.csm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins J. T., Ingersoll C. D. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9(2):135–159. [Google Scholar]
- 4.Snyder-Mackler L., De Luca P. F., Williams P. R., Eastlack M. E., Bartolozzi A. R., III Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994;76(4):555–560. doi: 10.2106/00004623-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Behm D. G., St-Pierre D. M., Perez D. Muscle inactivation: assessment of interpolated twitch technique. J Appl Physiol. 1996;81(5):2267–2273. doi: 10.1152/jappl.1996.81.5.2267. [DOI] [PubMed] [Google Scholar]
- 6.Kent-Braun J. A., Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19(7):861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Hurley M. V., Jones D. W., Wilson D., Newham D. J. Rehabilitation of quadriceps inhibited due to isolated rupture of the anterior cruciate ligament. J Orthop Rheumatol. 1992;5(3):145–154. [Google Scholar]
- 8.Newham D. J., Hurley B. F., Jones D. W. Ligamentous knee injuries and muscle inhibition. J Orthop Rheumatol. 1989;2:163–173. [Google Scholar]
- 9.Suter E., Herzog W., Bray R. C. Quadriceps inhibition following arthroscopy in patients with anterior knee pain. Clin Biomech (Bristol, Avon) 1998;13(4–5):314–319. doi: 10.1016/s0268-0033(98)00098-9. [DOI] [PubMed] [Google Scholar]
- 10.Suter E., Herzog W., De Souza K., Bray R. Inhibition of the quadriceps muscles in patients with anterior knee pain. J Appl Biomech. 1998;14(4):360–373. [Google Scholar]
- 11.Suter E., Herzog W., Bray R. Quadriceps activation during knee extension exercises in patients with ACL pathologies. J Appl Biomech. 2001;17(2):87–102. [Google Scholar]
- 12.Drover J. M., Forand D. R., Herzog W. Influence of active release technique on quadriceps inhibition and strength: a pilot study. J Manipulative Physiol Ther. 2004;27(6):408–413. doi: 10.1016/j.jmpt.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Thomee R., Grimby G., Svantesson U., Osterberg U. Quadriceps muscle performance in sitting and standing in young women with patellofemoral pain syndrome and young healthy women. Scand J Med Sci Sports. 1996;6(4):233–241. doi: 10.1111/j.1600-0838.1996.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 14.Suter E., McMorland G., Herzog W., Bray R. Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain. J Manipulative Physiol Ther. 1999;22(3):149–153. doi: 10.1016/S0161-4754(99)70128-4. [DOI] [PubMed] [Google Scholar]
- 15.Drechsler W. I., Cramp M. C., Scott O. M. Changes in muscle strength and EMG median frequency after anterior cruciate ligament reconstruction. Eur J Appl Physiol. 2006;98(6):613–623. doi: 10.1007/s00421-006-0311-9. [DOI] [PubMed] [Google Scholar]
- 16.Pfeifer K., Banzer W. Motor performance in different dynamic tests in knee rehabilitation. Scand J Med Sci Sports. 1999;9(1):19–27. doi: 10.1111/j.1600-0838.1999.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 17.Urbach D., Nebelung W., Becker R., Awiszus F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris: a prospective twitch interpolation study. J Bone Joint Surg Br. 2001;83(8):1104–1110. doi: 10.1302/0301-620x.83b8.11618. [DOI] [PubMed] [Google Scholar]
- 18.Hurley M. V., Jones D. W., Newham D. J. Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clin Sci (Lond) 1994;86(3):305–310. doi: 10.1042/cs0860305. [DOI] [PubMed] [Google Scholar]
- 19.Farquhar S. J., Chmielewski T. L., Snyder-Mackler L. Accuracy of predicting maximal quadriceps force from submaximal effort contractions after anterior cruciate ligament injury. Muscle Nerve. 2005;32(4):500–505. doi: 10.1002/mus.20366. [DOI] [PubMed] [Google Scholar]
- 20.Chmielewski T. L., Stackhouse S., Axe M. J., Snyder-Mackler L. A prospective analysis of incidence and severity of quadriceps inhibition in a consecutive sample of 100 patients with complete acute anterior cruciate ligament rupture. J Orthop Res. 2004;22(5):925–930. doi: 10.1016/j.orthres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Williams G. N., Buchanan T. S., Barrance P. J., Axe M. J., Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005;33(3):402–407. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]
- 22.Urbach D., Awiszus F. Impaired ability of voluntary quadriceps activation bilaterally interferes with function testing after knee injuries: a twitch interpolation study. Int J Sports Med. 2002;23(4):231–236. doi: 10.1055/s-2002-29074. [DOI] [PubMed] [Google Scholar]
- 23.Urbach D., Nebelung W., Weiler H. T., Awiszus F. Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Med Sci Sports Exerc. 1999;31(12):1691–1696. doi: 10.1097/00005768-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Sherrington C., Herbert R. D., Maher C. G., Moseley A. M. PEDro: a database of randomized trials and systematic reviews in physiotherapy. Man Ther. 2000;5(4):223–226. doi: 10.1054/math.2000.0372. [DOI] [PubMed] [Google Scholar]
- 25.Stackhouse S. K., Stevens J. E., Johnson C. D., Snyder-Mackler L., Binder-Macleod S. A. Predictability of maximum voluntary isometric knee extension force from submaximal contractions in older adults. Muscle Nerve. 2003;27(1):40–45. doi: 10.1002/mus.10278. [DOI] [PubMed] [Google Scholar]
- 26.Stackhouse S. K., Dean J. C., Lee S. C., Binder-Macleod S. A. Measurement of central activation failure of the quadriceps femoris in healthy adults. Muscle Nerve. 2000;23(11):1706–1712. doi: 10.1002/1097-4598(200011)23:11<1706::aid-mus6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Stackhouse S. K., Stevens J. E., Lee S. C., Pearce K. M., Snyder-Mackler L., Binder-Macleod S. A. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001;81(5):1102–1109. [PubMed] [Google Scholar]
- 28.Pietrosimone B. G., Hammill R. R., Saliba E. N., Hertel J., Ingersoll C. D. Joint angle and contraction mode influence quadriceps motor neuron pool excitability. Am J Phys Med Rehabil. 2008;87(2):100–108. doi: 10.1097/PHM.0b013e31815882e0. [DOI] [PubMed] [Google Scholar]
- 29.Kubo K., Tsunoda N., Kanehisa H., Fukunaga T. Activation of agonist and antagonist muscles at different joint angles during maximal isometric efforts. Eur J Appl Physiol. 2004;91(2–3):349–352. doi: 10.1007/s00421-003-1025-x. [DOI] [PubMed] [Google Scholar]
- 30.Babault N., Pousson M., Ballay Y., Van Hoecke J. Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J Appl Physiol. 2001;91(6):2628–2634. doi: 10.1152/jappl.2001.91.6.2628. [DOI] [PubMed] [Google Scholar]
- 31.Babault N., Pousson M., Michaut A., Van Hoecke J. Effect of quadriceps femoris muscle length on neural activation during isometric and concentric contractions. J Appl Physiol. 2003;94(3):983–990. doi: 10.1152/japplphysiol.00717.2002. [DOI] [PubMed] [Google Scholar]
- 32.Torry M. R., Decker M. J., Viola R. W., O'Connor D. D., Steadman J. R. Intra-articular knee joint effusion induces quadriceps avoidance gait patterns. Clin Biomech (Bristol, Avon) 2000;15(3):147–159. doi: 10.1016/s0268-0033(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 33.Lewek M., Rudolph K., Axe M., Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2002;17(1):56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 34.Ernst G. P., Saliba E., Diduch D. R., Hurwitz S. R., Ball D. W. Lower extremity compensations following anterior cruciate ligament reconstruction. Phys Ther. 2000;80(3):251–260. [PubMed] [Google Scholar]
- 35.Webster K. E., Wittwer J. E., O'Brien J., Feller J. A. Gait patterns after anterior cruciate ligament reconstruction are related to graft type. Am J Sports Med. 2005;33(2):247–254. doi: 10.1177/0363546504266483. [DOI] [PubMed] [Google Scholar]
- 36.Hurley M. V. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999;25(2):283–298, vi. doi: 10.1016/s0889-857x(05)70068-5. [DOI] [PubMed] [Google Scholar]
- 37.Herzog W., Longino D., Clark A. The role of muscles in joint adaptation and degeneration. Langenbecks Arch Surg. 2003;388(5):305–315. doi: 10.1007/s00423-003-0402-6. [DOI] [PubMed] [Google Scholar]
- 38.Torry M. R., Decker M. J., Ellis H. B., Shelburne K. B., Sterett W. I., Steadman J. R. Mechanisms of compensating for anterior cruciate ligament deficiency during gait. Med Sci Sports Exerc. 2004;36(8):1403–1412. doi: 10.1249/01.mss.0000135797.09291.71. [DOI] [PubMed] [Google Scholar]
- 39.Knoll Z., Kiss R. M., Kocsis L. Gait adaptation in ACL deficient patients before and after anterior cruciate ligament reconstruction surgery. J Electromyogr Kinesiol. 2004;14(3):287–294. doi: 10.1016/j.jelekin.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Knoll Z., Kocsis L., Kiss R. M. Gait patterns before and after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(1):7–14. doi: 10.1007/s00167-003-0440-1. [DOI] [PubMed] [Google Scholar]
- 41.Magyar O. M., Illyes A., Knoll Z., Kiss R. M. Effect of medial meniscectomy on gait parameters. Knee Surg Sports Traumatol Arthrosc. 2008;16(4):427–433. doi: 10.1007/s00167-007-0430-9. [DOI] [PubMed] [Google Scholar]
- 42.Timoney J. M., Inman W. S., Quesada P. M., et al. Return of normal gait patterns after anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21(6):887–889. doi: 10.1177/036354659302100623. [DOI] [PubMed] [Google Scholar]
- 43.Hart J. M., Blanchard B. F., Hart J. A., Montgomery S. C., Schoderbek R., Miller M. D. Multiple ligament knee reconstruction clinical follow-up and gait analysis. Knee Surg Sports Traumatol Arthrosc. 2009;17(3):277–285. doi: 10.1007/s00167-008-0681-0. [DOI] [PubMed] [Google Scholar]
- 44.Hopkins J. T., Ingersoll C. D., Krause B. A., Edwards J. E., Cordova M. L. Effect of knee joint effusion on quadriceps and soleus motoneuron pool excitability. Med Sci Sports Exerc. 2001;33(1):123–126. doi: 10.1097/00005768-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Palmieri-Smith R. M., Kreinbrink J., Ashton-Miller J. A., Wojtys E. M. Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med. 2007;35(8):1269–1275. doi: 10.1177/0363546506296417. [DOI] [PubMed] [Google Scholar]
- 46.Stergiou N., Ristanis S., Moraiti C., Georgoulis A. D. Tibial rotation in anterior cruciate ligament (ACL)–deficient and ACL-reconstructed knees: a theoretical proposition for the development of osteoarthritis. Sports Med. 2007;37(7):601–613. doi: 10.2165/00007256-200737070-00004. [DOI] [PubMed] [Google Scholar]
- 47.Amin S., Baker K., Niu J., et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60(1):189–198. doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker K. R., Xu L., Zhang Y., et al. Quadriceps weakness and its relationship to tibiofemoral and patellofemoral knee osteoarthritis in Chinese: the Beijing osteoarthritis study. Arthritis Rheum. 2004;50(6):1815–1821. doi: 10.1002/art.20261. [DOI] [PubMed] [Google Scholar]
- 49.Roos E. M. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17(2):195–200. doi: 10.1097/01.bor.0000151406.64393.00. [DOI] [PubMed] [Google Scholar]
- 50.Roos H., Adalberth T., Dahlberg L., Lohmander L. S. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 51.Grindstaff T. L., Hertel J., Beazell J. R., Magrum E. M., Ingersoll C. D. Effects of lumbopelvic joint manipulation on quadriceps activation and strength in healthy individuals. Man Ther. 2009;14(4):415–420. doi: 10.1016/j.math.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Suter E., McMorland G., Herzog W., Bray R. Conservative lower back treatment reduces inhibition in knee-extensor muscles: a randomized controlled trial. J Manipulative Physiol Ther. 2000;23(2):76–80. [PubMed] [Google Scholar]
- 53.Palmieri-Smith R. M., Leonard-Frye J. L., Garrison C. J., Weltman A., Ingersoll C. D. Peripheral joint cooling increases spinal reflex excitability and serum norepinephrine. Int J Neurosci. 2007;117(2):229–242. doi: 10.1080/00207450600582702. [DOI] [PubMed] [Google Scholar]
- 54.Hopkins J. T. Knee joint effusion and cryotherapy alter lower chain kinetics and muscle activity. J Athl Train. 2006;41(2):177–184. [PMC free article] [PubMed] [Google Scholar]
- 55.Pietrosimone B. G., Hart J. M., Saliba S. A., Hertel J., Ingersoll C. D. Immediate effects of transcutaneous electric nerve stimulation and focal knee joint cooling on quadriceps activation. Med Sci Sports Exerc. 2009;41(6):1175–1181. doi: 10.1249/MSS.0b013e3181982557. [DOI] [PubMed] [Google Scholar]
- 56.Hopkins J., Ingersoll C. D., Edwards J., Klootwyk T. E. Cryotherapy and transcutaneous electric neuromuscular stimulation decrease arthrogenic muscle inhibition of the vastus medialis after knee joint effusion. J Athl Train. 2002;37(1):25–31. [PMC free article] [PubMed] [Google Scholar]
- 57.Cheing G. L., Hui-Chan C. W. Would the addition of TENS to exercise training produce better physical performance outcomes in people with knee osteoarthritis than either intervention alone? Clin Rehabil. 2004;18(5):487–497. doi: 10.1191/0269215504cr760oa. [DOI] [PubMed] [Google Scholar]
- 58.Iles J. F. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol. 1996;491(pt 1):197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petterson S., Snyder-Mackler L. The use of neuromuscular electric stimulation to improve activation deficits in a patient with chronic quadriceps strength impairments following total knee arthroplasty. J Orthop Sports Phys Ther. 2006;36(9):678–685. doi: 10.2519/jospt.2006.2305. [DOI] [PubMed] [Google Scholar]
- 60.Stevens J. E., Mizner R. L., Snyder-Mackler L. Neuromuscular electric stimulation for quadriceps muscle strengthening after bilateral total knee arthroplasty: a case series. J Orthop Sports Phys Ther. 2004;34(1):21–29. doi: 10.2519/jospt.2004.34.1.21. [DOI] [PubMed] [Google Scholar]
- 61.Shield A., Zhou S. Assessing voluntary muscle activation with the twitch interpolation technique. Sports Med. 2004;34(4):253–267. doi: 10.2165/00007256-200434040-00005. [DOI] [PubMed] [Google Scholar]
- 62.Suter E., Herzog W. Effect of number of stimuli and timing of twitch application on variability in interpolated twitch torque. J Appl Physiol. 2001;90(3):1036–1040. doi: 10.1152/jappl.2001.90.3.1036. [DOI] [PubMed] [Google Scholar]
- 63.Huber A., Suter E., Herzog W. Inhibition of the quadriceps muscles in elite male volleyball players. J Sports Sci. 1998;16(3):281–289. doi: 10.1080/026404198366812. [DOI] [PubMed] [Google Scholar]
- 64.Dousset E., Jammes Y. Reliability of burst superimposed technique to assess central activation failure during fatiguing contraction. J Electromyogr Kinesiol. 2003;13(2):103–111. doi: 10.1016/s1050-6411(02)00064-0. [DOI] [PubMed] [Google Scholar]
- 65.Sheffler L. R., Chae J. Neuromuscular electric stimulation in neurorehabilitation. Muscle Nerve. 2007;35(5):562–590. doi: 10.1002/mus.20758. [DOI] [PubMed] [Google Scholar]
- 66.Krishnan C., Williams G. N. Hamstrings activity during knee extensor strength testing: effects of burst superimposition. Iowa Orthop J. 2008;28:36–41. [PMC free article] [PubMed] [Google Scholar]
- 67.Gobbi A., Tuy B., Mahajan S., Panuncialman I. Quadrupled bone-semitendinosus anterior cruciate ligament reconstruction: a clinical investigation in a group of athletes. Arthroscopy. 2003;19(7):691–699. doi: 10.1016/s0749-8063(03)00685-6. [DOI] [PubMed] [Google Scholar]
- 68.Keays S. L., Bullock-Saxton J. E., Keays A. C., Newcombe P. A., Bullock M. I. A 6-year follow-up of the effect of graft site on strength, stability, range of motion, function, and joint degeneration after anterior cruciate ligament reconstruction: patellar tendon versus semitendinosus and gracilis tendon graft. Am J Sports Med. 2007;35(5):729–739. doi: 10.1177/0363546506298277. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi A., Higuchi H., Terauchi M., Kobayashi F., Kimura M., Takagishi K. Muscle performance after anterior cruciate ligament reconstruction. Int Orthop. 2004;28(1):48–51. doi: 10.1007/s00264-003-0502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinczewski L. A., Lyman J., Salmon L. J., Russell V. J., Roe J., Linklater J. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35(4):564–574. doi: 10.1177/0363546506296042. [DOI] [PubMed] [Google Scholar]
- 71.Becker R., Berth A., Nehring M., Awiszus F. Neuromuscular quadriceps dysfunction prior to osteoarthritis of the knee. J Orthop Res. 2004;22(4):768–773. doi: 10.1016/j.orthres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Slemenda C., Brandt K. D., Heilman D. K., et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127(2):97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 73.Slemenda C., Heilman D. K., Brandt K. D., et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41(11):1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 74.Kendall T. L., Black C. D., Elder C. P., Gorgey A., Dudley G. A. Determining the extent of neural activation during maximal effort. Med Sci Sports Exerc. 2006;38(8):1470–1475. doi: 10.1249/01.mss.0000228953.52473.ce. [DOI] [PubMed] [Google Scholar]