Abstract
The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) was funded by the National Institute on Aging in 1986 to develop standardized, validated measures for the assessment of Alzheimer’s disease (AD). The present report describes the measures that CERAD developed during its first decade, and their continued use in their original and translated forms. These measures include clinical, neuropsychological, neuropathological and behavioral assessments of AD, and also assessment of family history and parkinsonism in AD. An approach to evaluating neuroimages did not meet the standards desired. Further evaluations which could not be completed because of lack of funding (but where some materials are available), include evaluation of very severe AD, and of service use and need by patient and caregiver. The information that was developed in the U.S. and abroad permits standardized assessment of AD in clinical practice, facilitates epidemiological studies, and provides information valuable for individual and public health planning. CERAD materials and data remain available for those wishing to use them.
Keywords: Consortium to Establish a Registry for Alzheimer’s Disease, CERAD, Alzheimer’s disease, clinical assessment, neuropsychological assessment, neuropathological assessment, norms, prevalence, incidence
1. Background
Dementing disorders have long been recognized,1 with the identification of Alzheimer’s disease (AD) typically dated back to Alzheimer’s century-old paper.2 Although considerable attention has been paid to Alzheimer’s disease and substantial progress has been made in identifying its characteristics, nevertheless much remains unclear. As diagnostic procedures improve, the complexities of this disease become more apparent, and the threat it imposes becomes increasingly evident. In the population 65 years of age and older, both the incidence and prevalence of this disorder double every succeeding five years,3 with estimated prevalence as high as 40% among those over the age of 85. AD, not recognized as a leading cause of death in 1980, was recognized as the fifth leading cause of death in 2003 among persons 65 years of age and older.4
There is presently no cure and inadequate amelioration for this condition. It can not only strip personality and capability, but is demanding on family members, seriously disrupting their lives and their work. It is expensive for the long term care system, where about half of the residents may suffer from dementia, a substantial proportion of whom can no longer afford their own care. Although people are now reaching their older years in better health,5,6 it remains to be seen whether there will be a decrease in the incidence of AD. Currently the fastest growing element of the population is among those 85 years of age and older, the age group where the incidence of AD is greatest.
A major step in the management of a disease lies in accurate diagnosis. Relevant to current work, clinical diagnostic criteria for dementia and AD were specified about 25 years ago, and then further refined by the Diagnostic and Statistical Manual of Mental Disorders (DSM),7–9 the International Classification of Diseases,10,11 and the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA).12 Neuropathological criteria for AD were proposed by Khachaturian in1984.13
None of these clinical diagnostic criteria specified how the behaviors at issue were to be examined. Working within the general guidelines that these criteria present, different investigators could legitimately use very different measures, or the same measures but with different cut-points, to reach a diagnosis. Each investigator could be right within the parameters chosen, but there might be little agreement across investigators. Indeed, comparison of these alternative clinical diagnostic criteria showed little agreement in identifying dementia,14 and a review of studies of the prevalence and incidence of AD carried out by the U.S. General Accounting (now Accountability) Office excluded a major U.S. survey because it appeared to be a substantial outlier.15 Comparing or aggregating information under such circumstances has questionable legitimacy, and progress is hindered.
In 1986, the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) was funded by the National Institute on Aging (NIA) to address such concerns. Its mandate was to develop standardized, reliable and valid assessments of AD for use by all Alzheimer Disease Centers (ADCs) established by the NIA, provide training in their use, and aggregate CERAD-based data from carefully evaluated patients and controls.
Ultimately, CERAD developed standardized and validated clinical, neuropsychological, and neuropathological assessments that were used by the ADCs.16,17 CERAD also prepared a standardized protocol for neuroimaging in AD, but did not promote it because inter-site agreement on the rating of these neuroimages was considered to be inadequate.18 Additional evaluated assessments that were developed included a Behavior Rating Scale for Dementia to assess psychopathological behavior in AD,19,20 a family history assessment for investigators interested in familial aggregation studies and the possible genetics of AD,21 and since parkinsonism may be manifest with AD, a scale to assess extrapyramidal dysfunction.22 CERAD also prepared information for ADCs to use to facilitate brain donation; and simply written educational material in English and Spanish, describing the early symptoms of AD, and the appropriate steps to take to handle this condition (see Table 1).
Table 1.
The main CERAD instruments and ancillary materials
| Instrument | Brief description of contents |
|---|---|
| Clinical battery | Demographic data on subjects and informants; clinical history; Blessed Dementia Rating Scale, end points in late stage dementia, (screen for Behavior Rating Scale for Dementia (patients only); Short Blessed; calculation, clock, language; physical exam; neurological exam – overall and extrapyramidal dysfunction; report of laboratory and neuroimaging studies; Clinical Dementia Rating scale; diagnostic impression including AD, AD with secondary contributing disorder, extrapyramidal dysfunction in AD; nonAD dementias |
| Neuropsychology battery | Verbal Fluency (animal naming), Boston Naming (15 items), Mini-Mental State Exam (serial 7s omitted), Word List Learning, Constructional Praxis, Word List Recall, Word List Recognition (10 original words, 10 foils), Constructional Praxis recall |
| Neuropathology battery | Demographic data; History; Gross examination; Cerebrovascular disease, gross findings; Microscopic vascular findings; Major nonvascular microscopic findings; Microscopic evaluation; Hippocampus, neocortex; Assessment of neurohistologic findings; Neuropathologic diagnosis; Final assessment. |
| Neuroimaging battery | Technical protocol on acquisition of MR scans; general information; cerebral atrophy (perceptual ratings); white matter lesions, excluding hemorrhages and infarcts; cerebral vascular disease (infarcts and lacunes, parenchymal hemorrhages). Extensively illustrated. |
| Behavior Rating Scale for Dementia | 46-item scale assessing presence and frequency of behaviors on 6 subscales: depressive symptoms, inertia, vegetative symptoms, irritability/aggression, behavioral dysregulation, psychotic symptoms. An abbreviated 17-item scale has also been developed. Scales, manual, training video, and scoring guide available. |
| Family history assessment | Mapping of first and second degree kin and determining likely presence of AD, Parkinson’s disease, and Down syndrome |
| Services assessment | Evaluation of in-home and community-based service use and need, including institutionalization and hospitalization. Material not fully evaluated. |
| Autopsy resources | Materials to facilitate autopsy recruitment by sites (guidelines, forms, sample letters, informational brochures) |
| Educational brochures | Information on early symptoms of AD and steps to take, in 6th. grade level English, and simple Spanish |
Here we briefly describe each of the batteries and scales, the training procedures used, and the development of a database. We also touch on the many uses occasioned by the availability of these measures. These include use in the clinic, in epidemiological surveys, and in drug intervention trials. The data are relevant for planning at the individual and public health levels. Norms have been developed for clinic patients, community residents, and various racial/ethnic groups, and the measures have been translated into the major European and Asian languages (with further validation in several of these), permitting international comparison. The availability of the measures, and the development of a clean, substantial, and accessible database, has facilitated identification of possible clinical, neuropsychological, and neuropathological subtypes of AD, and determination of the characteristics of natural disease progression. It has also encouraged the development of statistical procedures appropriate for handling complex longitudinal data.
2. Organization of CERAD
Organizationally, CERAD consisted of three critical elements: an administrative core, headed by the Principal Investigator, who supervised the entire project; a methodology and data management center, which, among other activities, constructed all the data gathering forms, developed data input procedures, encouraged participating sites to abide by the protocol-prescribed followup evaluation intervals, and handled data cleaning and analyses; and a series of task forces, each headed by an acknowledged specialist in the area of interest, and external advisory committees. In developing their assessments, the task forces focussed on measures that were basic to the assessment of AD, familiar, and brief. Underlying CERAD’s success was the invaluable collaboration with each participating site, where those engaged included the site director (typically a clinician), administrative and data entry personnel, neuropsychologist and psychometrician, neuropathologist and neuroimaging specialists, as well as faculty with particular interests in areas such as genetics. Throughout the CERAD years, participating sites were kept informed by newsletters, mailings, fax, telephone, and, as technology advanced, by e-mail, in addition to face-to-face meetings, typically held at the annual meetings of professional societies. Starting with 15 ADCs, eventually 29 university sites and 9 special focus sites (e.g., high minority enrollment, non-English-speaking) were eligible to submit data on patients with AD and control subjects who met inclusion/exclusion criteria.
The clinical and neuropsychology task forces were the first to be established. Following the successful development of the clinical and neuropsychology batteries, additional task forces to address neuropathology and neuroimaging were created. As interest increased, and need was recognized, subtask forces, affiliated with the clinical task force, were instituted to develop evaluated assessments for behavioral pathology, extrapyramidal dysfunction, and family history. Finally, CERAD broadened its scope yet further to include consideration of the non-institutional services used and needed by patients with AD and their care givers.
At the encouragement of NIA, and with international interest, measures (in particular the clinical and neuropsychological batteries, and the Behavior Rating Scale for Dementia) were translated. Following the successful development and increasing use of the CERAD measures, consideration turned to broadening the clinical scope of CERAD to include a broader array of dementias, and in particular to examine dementia in its very early stages.
3. Eligibility of subjects for the CERAD database
Entrants had to be 50 years of age or older. No maximum age was set. AD patients had to meet NINCDS/ADRDA criteria12 modified in two ways: memory loss had to be for a minimum of 12, rather than 6 months, and age could be greater than 90 years. Probable AD patients were not permitted to have other health conditions that could contribute to dementia (i.e., no history of stroke; severe hypertension; Parkinson’s disease; serious renal, metabolic, or toxic disorders; major neurological illness; alcoholism, cardiac disease, etc.). Some cases of possible AD were accepted where other potentially contributing disorders were present but AD was judged to be the primary cause of dementia. Patients had to be cooperative, ambulatory, understand and speak English (or the language of testing), and have no sensory disorder that precluded testing. Initially, score on the Mini-Mental State Examination (MMSE)23 had to fall between 10 and 24, or between 6 and 24 on the Short Blessed Test.24 The lower levels were relaxed later to permit inclusion of more impaired patients, as long as they could respond to at least 2 of the neuropsychology measures. Patients had to have an informant or caregiver who knew them well and could report observations confirming that the patient had experienced cognitive and functional decline relative to premorbid abilities. Patient characteristics were checked closely to ensure compliance with entry requirements. Thus, CERAD patients represent a very “clean” group with AD. Control subjects had to meet the same standards as patients with the exception of the MMSE/Short Blessed criteria; they could show no evidence of dementia, and could not be the kin of a patient. To reduce potential site effects, sites were initially limited to submitting 40 cases and 30 control subjects. All measures were administered to both cases and controls, with the exception of the informant-derived Blessed Dementia Rating Scale.25 Controls were not required to have informants. In longitudinal analysis this facilitated identification of changes caused by disease as compared to those attributable to aging. Sites were expected to gather data annually, but some were more attuned to this than were others, as indicated by variable dropout. Dropout also varied by race,26 and by severity of disease, the less severely impaired being more likely to drop out.27
4. Data available
The clinical and neuropsychological batteries were administered to 1094 patients with AD (890 White, 204 Black), and 463 control subjects (429 White, 34 Black). Of these entrants, 197 cases and 38 controls provided only baseline data, while 639 cases and 368 controls were evaluated on 3 or more annual occasions, with some followed for up to 8 years. Over the course of the study 47% of the patients, but only 4% of the controls, entered a nursing home, and 411 patients and 25 control subjects died. Brain autopsy data are available on 202 patients and 8 controls. De-identified clinical, neuropsychological, and neuropathological information on these subjects is on deposit at the National Alzheimer’s Coordinating Center at the University of Washington (www.alz.washington.edu). The database, batteries, and additional material are also available on CD from the CERAD administrative office (http://cerad.mc.duke.edu).
4.1 Uses of the database
The CERAD database was developed before the major advent of antidementia agents. Few enrolled in CERAD took such drugs (those who did were noted). Thus, the CERAD data provide an ethical control group, making it possible to examine the natural history of AD, and possibly helping to determine the impact of pharmacological or other interventions (there are, however, problems associated with the use of historical control groups). In regard to the natural history of AD, papers have been published on depression, insight, weight change, the time needed to reach selected endpoints (and their stability), and transition time from one stage to another.28–32 The clock drawing test is one of a set of brief objective measures included in the clinical battery to facilitate the clinical diagnosis of AD without reliance on the neuropsychological test results, thus permitting the neuropsychology battery to be evaluated independently. CERAD’s straightforward scoring of this measure (clocks are scored on a 4-point scale: normal, mild, moderate, severe impairment, with examples provided for each level), has been found to be reliable, and has been included in brief screens of dementia.33
5. Battery content, reliability and validity
(See Table 1 for an outline of the information gathered by each measure.)
5.1 Clinical assessment
The clinical battery sought information in areas relevant to determination of dementia and AD, doing so without recourse to information on the neuropsychology battery to permit independent assessment of each. To ensure that information was obtained and scored in a uniform manner across sites, an instruction manual detailed the diagnostic criteria, use of the clinical and neuropsychological batteries, and the scoring of the measures.16 In addition, training for site clinicians was held at the annual meetings of the American Academy of Neurology and the American Neurological Association. Video-taped “gold standard” cases evaluated with the clinical battery were shown, and administration and scoring were discussed and reviewed to ensure common agreement. Sites were permitted to enter data into CERAD only after submitting videotaped assessments of cases that were reviewed and approved by the chairs of the clinical and neuropsychological task forces.
At the sites, forms completed on patients and controls selected for CERAD were entered electronically in a double data entry system that was programmed to check for out-of-range values, and reduce missing entries. Special attention was paid to the staging of dementia severity with the Clinical Dementia Rating.34 The entire scale with full descriptors was included to improve agreement on use.35
The validity of the clinical assessment was determined using neuropathological diagnosis as the gold standard. Examination of autopsy brains derived from 201 patients clinically diagnosed as having probable or possible AD confirmed the diagnosis of AD as the primary dementing illness in 176 decedents (87.6%). In this group of confirmed AD cases, coexistent cerebrovascular lesions were present in 32%, and concomitant pathology of Parkinson’s disease (PD) in 23%. The primary dementing disorders present in the remaining 25 patients included PD-related pathology (n = 9), hippocampal and entorhinal sclerosis (n = 4), miscellaneous neurodegenerative and other disorders (n = 6), and no significant changes (n = 6).36 Thus, in a multisite setting which included experienced neurologists, the clinical diagnosis of AD based on the CERAD clinical battery (without reliance on the neuropsychological test results) was found to have substantial accuracy. At the same time it was obvious that concomitant pathologies could be present with AD, and that at least in some cases, clinically identified AD might be attributable to other conditions.
5.2 Neuropsychological assessment
The neuropsychology measures chosen were those recognized as assessing cognitive functions implicated in AD. Although the original intent was to permit staging of AD, later study found that some of the measures, in particular delayed recall of the word list, could efficiently distinguish persons with dementia from those with normal cognition.
To facilitate administration and scoring of the neuropsychology measures, directions for administration, if brief, were printed directly on the scoring page. If longer, they were printed on the facing page, together with explicit information on scoring. In addition, a training video was prepared (in English and French), demonstrating appropriate administration of the neuropsychology measures. Prior to permitting sites to enter data, they had to submit acceptable audio recordings and correctly scored “hard copy” of two cases. Spot checks were carried out throughout the study, by selecting five entries at random each month, and rescoring them. Modifications needed were transmitted to the sites. Few problems occurred.
One-month test-retest reliability was determined based on data from 632 patients with mild or moderate AD, and 394 control subjects. For AD patients, correlations ranged from 0.80–0.91 for verbal fluency, abbreviated Boston Naming, MMSE, Word List Learning, and Constructional Praxis. Correlations were lower for Word List Recall (r = 0.56), due to a floor effect, and for Word List Recognition (original words: r = 0.53; foils: r = 0.60), due to ceiling effects. Test-retest reliability was not determined for Constructional Praxis Recall, since it was a later addition to the battery. On all measures, correlations were lower for control subjects than for AD patients because of ceiling effects.37 Inter-rater reliability was high, with intraclass correlation coefficients ranging from 0.92 (Constructional Praxis), to 1.0 (Word List Recall).16
In regard to validity, cross-sectionally, average level of performance was poorer as stage of AD increased, with some measures reaching floor before others.38 These findings have been confirmed in nonclinical settings, and by other investigators.39 Decline in performance was also identified longitudinally, the rate of decline depending on initial level of performance.40
5.3 Neuroimaging assessment
Considerable attention was given to developing standardized procedures for administering and scoring magnetic resonance imaging (MRI) in AD, and in modifying the protocol in response to pre-testing. Nevertheless, inter-rater agreement among 14 raters rating 28 MRI scans of elderly patients was, overall, disappointing. Accordingly, while the protocol is available it has not been recommended for use in multicenter studies, although use at any one site may be satisfactory.18 More sophisticated means of evaluating neuroimages have since been developed, which should produce improved agreement across sites. Nevertheless, use in one study of 20 CERAD patients with neuropathologically ascertained definite AD, found a significant correlation between neuroimaging evidence of temporal horn enlargement, and autopsy-identified hippocampal atrophy, as well as between severity of cerebral atrophy determined by neuroimaging and MMSE score closest in time to the scan.41
5.4 Neuropathology assessment
The components of the neuropathology assessment are indicated in Table 1. Required microscopic sections included hippocampus and amygdala as well as frontal, temporal, parietal, and occipital neocortex. Since previous work indicated that quantitative assessment was problematic, CERAD neuropathologists opted to use a semiquantitative approach for assessing the frequency of senile plaques (neuritic and diffuse), neurofibrillary tangles, and other changes. The semiquantitative assessment of the frequency of neuritic plaques was related to patient age. Together with clinical history, this age-related plaque score indicated levels of certainty of the diagnosis of AD, i.e., definite, probable, or possible AD, or no evidence of AD. To facilitate accurate neuropathological diagnosis of AD, a primer for pathologists was published.42 Neuropathologists were asked to apply the routine stains of their choice to these sections and to assess each case using semiquantitative and quantitative measures. There was good inter-rater agreement on the relative severity of AD cases, and agreement on plaque and tangle frequencies was significantly greater for semiquantitative than quantitative assessment.
The CERAD guidelines have been recommended by the Autopsy Committee of the College of American Pathologists,43,44 and forms a basis for the consensus guidelines on the autopsy diagnosis of dementia with Lewy bodies.45 A consensus conference held jointly by the National Institute on Aging and the Reagan Institute largely adopted the CERAD system but additionally upgraded the significance of neurofibrillary tangle distribution and frequency in reaching a level of likelihood that AD accounts for the dementia.46
Among the CERAD batteries, the neuropathology protocol is the one most cited, and may be the one most used. It has been critically compared with the Braak and Braak,47 Khachaturian,13 NIA-Reagan Institute,46 and the Tierney A3 criteria.48–51 The CERAD neuropathology criteria have been and continue to be used in a substantial number of studies, both in the U.S., and abroad.
Some have expressed concerns that the CERAD protocol fails to take into account significant pathological and biochemical factors such as estimations of soluble amyloid load, aberrant tau accumulation, and synaptic density.52 Nor has the protocol been modified to encompass changes reflecting our latest understanding of certain other dementias (e.g. frontotemporal dementias, dementia with Lewy bodies, and vascular dementia). Although the CERAD protocol requires documentation of gross and microscopic changes of cerebrovascular disease, the significance of any vascular pathology is not assessed. Finally, unlike other CERAD batteries, the neuropathology battery has not been translated, since most users have a working knowledge (or better) of English, and no response is required of subjects.
6. CERAD’s limitations
In common with other studies, CERAD also has limitations. A preferred sampling design would have selected matched controls from the same standard metropolitan statistical areas as cases, instead of the still current approach of a sample of convenience. Further, while informants were required for cases, they were not required for the control subjects. Normative data from controls could be contaminated by unrecognized cases of very mild dementia. Very few controls, however, changed from CDR 0 to CDR 0.5, and their data can be examined separately. Urgency for brevity creates problems. Inquiry into medications is severely restricted, and a short, validated depression measure might have been preferable to the unevaluated depression items present. The neuropsychology battery is brief, nevertheless, alternative statistical approaches, such as item response theory, could have been used to select items to identify a broader range of disease status, with less cultural bias. We had considered, but never followed through on developing a neuropsychology profile that might have helped to distinguish patients with AD from patients with other dementing disorders. Overall, there was a strict focus on medical/psychiatric/neuropsychological aspects of AD; social and societal implications were largely ignored.
7. Main uses and findings
Review of publications that use the CERAD measures indicates that CERAD has had two major effects: (1) it has provided accepted standards for the clinical, neuropsychological, and neuropathologic diagnosis of AD; and (2) it has provided validated, normed measures that have been broadly used and that permit comparison across studies and settings. Tables 2 through 5 summarize the main studies in which the CERAD measures have been used. In addition, the neuropsychological battery in whole or in part, continues to be used by the ADCs in their ongoing clinical and research studies (personal communication, K. Welsh-Bohmer (6 Dec 2006), J.C. Morris (7 Dec 2006)).
Table 2.
Epidemiological studies using CERAD batteries
| Clinical Battery and Neuropsychology Battery used |
|
Black/White dementia study P.Is.: A. Heyman, G. G. Fillenbaum Study based on participants of the Duke Established Populations for Epidemiologic Studies of the Elderly. Stratified random sample of 4,136 community residents (54% African American), age 65+, used to determine 3-year incidence of dementia; and 2-stage sample of those age 68+ to determine prevalence of dementia.66 |
|
Honolulu-Asia Aging Study (HAAS) P.I.: L. White Survey of 3,734 community and institutional resident Japanese-American men age 71–83, 80% of the survivors of the Honolulu Heart Program.67 |
|
Prevalence of Alzheimer’s Disease in Province (PREMAP) Random sample of 1062 residents (community and institution) age 70+ in south-eastern France. Evaluation of persons with MMSE<24 using CERAD batteries identified 177 cases of dementia (9.2%), including 82 cases of AD (5.5%). Prevalence of AD increased significantly with age and was higher among women (Odds Ratio: 4.24) and persons with no formal education (Odds Ratio: 2.47).68 |
| Neuropsychology Battery only (entire battery, unless otherwise indicated) |
|
Aging, Demographics, and Memory Study (ADAMS) P.Is.: R. Willis, B. Plassman First nationally representative, population-based, study in the U.S., designed to provide data on the antecedents, prevalence, outcomes and costs of dementia and of “cognitive impairment, not demented”. Sample of 856 participants in the Health and Retirement Study (age 70+).69 |
|
Cache County Study on Memory, Health and Aging P.Is.: J. Breitner, K. Welsh-Bohmer Continuing longitudinal study of 5,677 persons age 65+ who were permanent residents of Cache County, Utah, in order to ascertain the prevalence and incidence of dementia, and the development and impact of cognitive impairment.70–72 |
|
Chicago Health and Aging Project (CHAP) P.I.: D. A. Evans Longitudinal study of >6,000 residents (age 65+, 61% female, 62% African American, mean education 12 years), of three adjacent neighborhoods in Chicago. Data collection at ~3 year intervals, those who have reached age 65 since the previous interview date are then eligible to enroll, so maintaining the relevance of the sample.73 |
|
Duke Twins Study P.Is.: J. Breitner, B. Plassman Ongoing study of intact pairs of veteran twins in the National Academy of Sciences--National Research Council Registry aged 62–73 years in 1990–1991, to ascertain concordance for AD in twins as a function of age and apolipoprotein E genotype.74–75 |
|
Indianapolis-Ibadan Dementia Project P.Is.: H. C. Hendrie, K. Hall Sample of 2,494 Yoruba in Ibadan, Nigeria (age estimated from historic landmarks) and 2,318 community-resident and institutionalized African Americans in Indianapolis, Indiana, age 65+. Followup waves to identify incident dementia were conducted 2 and 5 years later, and are ongoing.76,77 |
|
Indo-US Cross-National Dementia Epidemiology Study P.I.: M. Ganguli Rural population (N=5,126) age 50+, 73% illiterate, of Ballabgarh, Northern India. (CERAD neuropsychology measures adapted to the rural Indian experience.)78 |
|
Kame project P.Is.: E.B. Larson, A.B. Graves (Borenstein) Survey of 1,985 community and institutional residents of King County, Washington State, age 65+. Nearly all 100% Japanese heritage, the remainder with minimum of 50% Japanese heritage.79 |
| Korea Stratified cluster sample of 706 people age 65+ in Dong district of Gwangju, Korea, an urban area. (CERAD Word List tasks used to help identify presence of dementia.)80 |
|
Monongahela Valley Independent Elders Study (MoVIES) P.I.: M. Ganguli Longitudinal study of a random sample (N=1,350) of community residents age 65+ (6th grade education, except for very elderly), in rural area outside Pittsburgh, PA.81 |
|
Nun Study P.I.: D. A. Snowdon Longitudinal study of the School Sisters of Notre Dame (N=678; age 75–102 at entry). (Used selected CERAD neuropsychology measures.)82,83 |
|
Religious Orders Study P.I.: D. A. Bennett Nearly 1,000 older Catholic nuns, priests or brothers (mean age 76 years, mean education 18 years, 32% male, 11% minority), volunteers from about 40 groups in 12 states and the District of Columbia, who agreed to annual clinical evaluation and brain donation. (Used selected CERAD neuropsychology measures.)84 |
|
Rush Memory and Aging Project P.I.: D. A. Bennett More than 1,000 volunteers, mean age 81 years, 8% minority, 28% male, mean education 14 years (but a third had <12 years education), drawn from a variety of living arrangements, who agreed to detailed, repeated assessments, and donation of neurological material and muscle at death. (Used selected CERAD neuropsychology measures.)85 |
| Taiwan Two-phase study of a stratified random sample of 2915 inhabitants age 65+ in southern Taiwan. Following initial screening, subjects were administered CERAD Neuropsychological Battery and neurobehavioral examination.86 |
|
Veterans Study of Memory in Aging P.I.: B. Plassman Carefully selected group of World War II Navy and Marine veterans hospitalized during military service with head injury, and controls. Complete information on 548 with and 1228 without head injury, used to ascertain risk for dementia as a function of head injury and apolipoprotein E status.87 |
| Neuropathology battery |
| A review of population-based studies indicates that the CERAD Neuropathology Protocol appears to be the generally used standard.52 |
| CERAD Neuropathology Protocol has been adopted by the College of American Pathologists for practice guidelines on autopsy pathology.44 |
| The CERAD Neuropathology Protocol is the foundation for recommended diagnostic protocol for dementia with Lewy bodies.45 |
|
BrainNet Europe Consortium Comparison of inter-laboratory rating reliability based on the CERAD, Braak and Braak, and NIA-Reagan Institute neuropathologic criteria.88 |
|
Oxford Project to Investigate Memory and Aging (OPTIMA) Study in which by 1997, 200 patients with cognitive impairment or dementia had been referred by their physicians, and by 2002, 158 cognitively intact community residents age 60–91 had enrolled. CERAD neuropathological (and other criteria) were used.89 |
|
Religious Orders Study P.I.: D. A. Bennett (Used CERAD, NIA-Reagan, and Braak criteria.)84 |
|
U.K. Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Neuropathology data come from decedents who participated in a longitudinal study of stratified random samples of residents age 65+ (>18,000 individuals) selected from 6 centers (including urban and rural areas) in the U.K.90 |
| Behavior Rating Scale for Dementia |
| Alzheimer Disease Cooperative Study Representative studies: To examine emergence of behavioral pathology.91 Included, among other measures, to assess behavioral pathology.92 Incuded in the Alzheimer’s Disease Cooperative Study (ADCS) Instrument Development Project.93 |
| Taiwan – survey of the prevalence of dementia Both the CERAD neuropsychology battery and the Behavior Rating Scale for Dementia were used in this survey.86 |
Table 5.
Languages into which CERAD measures have been translated
| Language | Clinical battery | Neuro-psychological battery | Behavior Rating Scale for Dementia | Individual measures only |
|---|---|---|---|---|
| Arabic | + | |||
| Bulgarian | + | + | ||
| Chinese | + | + | ||
| French | + | + | + | |
| Finnish | + | |||
| German | + | |||
| Hebrew | + | Forthcoming | ||
| Italian | + | + | ||
| Japanese | + | + | + | |
| Korean | + | |||
| Norwegian | + | |||
| Russian | Planned | |||
| Spanish | + | + | ||
| Swedish | Word List | |||
| Portuguese | + |
The clinical, neuropsychology, and neuropathology batteries, and the Behavior Rating Scale for Dementia have been used in major epidemiological studies with diverse racial/ethnic groups (Table 2). Such uniform use in a number of different countries, and within the same country with different population groups, permits direct comparison of prevalence and incidence rates, and facilitates assessment of alternative risk factors. By using uniformly operationalized diagnostic criteria, and comparable assessment measures (we recognize that measures may need to be adapted to the cultural experience of those who are evaluated), any differences found are likely to be true differences, or at least not artifacts of the assessment itself.
The clinical and neuropsychology batteries have also been used in clinical trials (Table 3), yielding information of national and international importance on the impact of hormone replacement therapy, and (provided the study is funded), on the impact of selenium and vitamin E in preventing AD. We have not included drug trials carried out by pharmaceutical companies. While we know that specific tests included in the CERAD neuropsychology battery have been used, we are not privy to the intervention being examined, or to the findings. Such use is, however, an indication of the perceived value of these measures. Finally, some entrepreneurs have selected tests in the neuropsychology battery for on-line outreach, inviting persons concerned about their memory to be evaluated by telephone, with a potential diagnosis given in short order. Evaluation under medical supervision is also available. The appropriateness and impact of this approach has not been determined.
Table 3.
Major clinical trials that have used CERAD materials
|
The Women’s Health Initiative Memory Study (WHIMS) WHIMS was a randomized, double-blind, placebo-controlled clinical trial of 4532 (92.6%) of the 4894 postmenopausal women free of probable dementia, aged 65 years or older, who were enrolled in the Women’s Health Initiative (WHI) estrogen plus progestin trial in May 1996. Participants received either 1 daily tablet of 0.625 mg of conjugated equine estrogen plus 2.5 mg of medroxyprogesterone acetate (n = 2229), or a matching placebo (n = 2303). Incidence of probable dementia (primary outcome) and mild cognitive impairment (secondary outcome) were identified through a structured clinical assessment.94–97 |
| Prevention of Alzheimer’s Disease by Vitamin E and Selenium (PREADVISE), add-on study to Selenium and Vitamin E Prostate Cancer Prevention Trial (SELECT) (LMTS) PREADVISE has enrolled >5,200 men (18% minority; age 62+ if white, age 60+ if African American or Hispanic), all of whom are participants in the SELECT trial, running in over 400 clinics. All are administered a brief cognitive screen, developed from the measures of the CERAD neuropsychological battery and the CERAD database, with additional assessment, if warranted.98,99 |
| Research into Memory, Brain function and Estrogen Replacement (REMEMBER), Pilot study, based on a random sample of 428 women age 60+ in Adelaide, performed prior to initiation of major study. Used CERAD word list, category fluency (Animals), and 15-item Boston Naming Test.100 |
Table 4 lists the types of samples for which norms have been developed. They include clinic-based samples of diverse race/ethnicity in the U.S., and clinic-based norms for German-speaking countries. Norms have also been developed for internationally distributed community-based samples. The presence of such norms facilitates appropriate comparison: newly evaluated community residents can be compared with other community residents of comparable age, race/ethnicity, and education; patients at tertiary medical care centers can be compared with other patients at the same types of centers.
Table 4.
Norms -- representative selection
| Clinic-based data (representative references) |
|
United States White (CERAD data),101,102 White (ADC clinic, age 85+)103 African American (CERAD data)38,104 White and Native American53 Spanish-speaking105 |
| German-speaking countries – clinics can compare their information on patients with community-based norms by going to: www.memoryclinic.ch and following the directions there (a performance profile and z-scores are provided; site developed by A.H. Monsch) |
| Community-based, including epidemiological studies |
|
Australia Sample: Healthy elderly, n = 243106 |
|
Brazil CERAD Neuropsychological Battery administered to 85 normal controls, 31 AD patients at CDR 1, and 12 AD patients at CDR 2. Performance of controls was similar to that of a U.S. control sample.107 |
|
Finland In a sample of 40 cognitively normal individuals age 58–85, education effects, but no age effects, were found.108 Comparison of 15 cognitively normal, 15 amnestic MCI cases, and 15 mild probable AD cases.109 |
|
Jamaica Norms, and ability to discriminate between normal and demented persons, based on 72 cognitively normal people and 12 people with dementia age 65+.110 |
|
Korea 618 healthy, cognitively normal volunteers. Norms provided for four overlapping age groups (60–74, 65–79, 70–84, 75–90), three levels of education (0–3 years, 4–6 years, 7+ years), and by gender.111 |
|
Nigeria (Yoruba) 100 normal, healthy adults age 65+.112 |
|
Switzerland Norms based on 617 participants in Basel Study on the Elderly (Project BASEL), 185 women, 432 men, age 53–92.113 |
|
United Kingdom (African-Caribbean) African Caribbean residents (n = 285, age 55–75) of south London, U.K. CERAD measures: Verbal Fluency (animals), Boston Naming, Word List.114 |
|
United States White (from Black/White dementia study and MoVIES study)38,81 African American (from Black/White dementia study and African Americans in Indianapolis)38,115 Japanese heritage (from Kame Project and HAAS study)116,117 |
The CERAD neuropsychology battery (sometimes in whole, sometimes in part), has been used with various groups who, in addition to those listed in Tables 2–4, include Native Americans,53 older Israelis (in Hebrew),54,55 elderly in Colombia,56,57 and older persons in India, China, Southeast Asia, Latin America and the Caribbean, and Africa.58,59
The clinical battery has been referenced as a standard by several clinical studies that indicate that they used NINCDS/ADRDA and CERAD criteria for AD. In those instances, however, it is difficult to know whether the CERAD clinical battery or the CERAD clinical criteria were used. Compared to NINCDS/ADRDA, CERAD clinical criteria are stricter regarding duration of memory loss, but more lenient regarding older age.
Table 5 shows the languages into which CERAD measures have been translated. The multiple translations (typically done in the conventionally approved manner with back translation) facilitate testing of patients within countries such as the U.S. and Canada that have a multilingual population, as well as facilitating cross-national comparisons. We have not included English as spoken in Australia in this table, but we would mention that, even when ostensibly the same language is spoken in two different locations, it may be important to evaluate the measure in each location if there are indications that terms are differently understood in the different locations, or may be understood differently by different residents of the same location. To make sure that the measures remain acceptable (and hopefully equivalent), item order has sometimes been changed (e.g., for the Behavior Rating Scale for Dementia in Arabic), items have been substituted (e.g., in the abbreviated Boston Naming task used in Finland), and different words used (e.g., in the “American” vs. “European” Spanish translations of the Word List).
8. Current status of CERAD
At the end of the first 10 years, CERAD had developed standardized versions of the basic assessments needed to evaluate AD. They were being used in the memory disorders clinics of major tertiary care medical centers nationwide, and in translation in clinical centers in Brazil, French-speaking Canada, France, Italy, Portugal, and Spain. The CERAD measures had also been adopted for use in major national and international epidemiological surveys. Hitherto, each epidemiological study of dementia in the U.S. had used its own assessments, creating disbelief in findings.15
Continued funding depended on competitive renewal through NIH’s standard research funding mechanism. This mechanism is designed well for evaluating hypothesis-driven research, but CERAD’s mandate was not to develop or test hypotheses. The name itself was misleading, for CERAD was not a registry as that term may generally be understood.60,61 CERAD’s mandate was to produce measures that would facilitate hypothesis-driven research. A pedestrian but crucial requirement for scientific communication is the existence of commonality of terms and standardization of definitions. CERAD filled this requirement, and was prepared to do more. Such activities, however, are rarely recognized as fundable under a research aegis.
As investigation into AD progressed, it had become increasingly clear that there were subtypes of AD, which might, in fact, be distinct dementias that demanded unique diagnostic procedures and interventions (e.g., frontotemporal dementia, Lewy body disease). In addition, other dementing disorders were well known. CERAD had begun to develop diagnostic criteria, and was reviewing appropriate neuropsychological measures to assess these conditions. Completion of this task, in an environment which required no new start-up, could have facilitated identification and comparison of these conditions.
At the time that funding ceased, CERAD was developing measures to assess important endpoints for end-stage disease; service use and need throughout the course of the illness, and a brief assessment for use in primary care. Importantly, CERAD had a particular interest in potential dementia prodrome, a term coined to describe a very early phase in the dementing process, possibly a logical progression between normal cognition and dementia, particularly AD.62,63 CERAD had already selected clinical and neuropsychological assessments that seemed appropriate, and developed diagnostic criteria. Enrollment of such cases, and their eventual brain autopsy, could have furthered knowledge of the association (or lack of association) between clinical and neuropathological manifestation across the entire range of cognition. This has become recognized as an increasingly important area, in which significant problems persist because of multiple diagnostic criteria, and diversity in assessments.
Further development of the CERAD neuropathology protocol also ceased. As a consequence, the Neuropathology Task Force was unable to modify the battery to more appropriately reflect changes seen with other dementias (e.g. vascular dementia, frontotemporal dementias and dementia with Lewy bodies), and to incorporate the use of appropriate markers, e.g. alpha-synuclein immunohistochemistry. While the CERAD neuropathology protocol has become the standard for neuropathological assessment in epidemiological studies,52 the lack of ability to update the protocol has reduced the ability to aggregate standardized data from multiple center, mitigating the ability to glean important correlative and other information.
Careful consideration of funding for activities basic to sound research, but not necessarily addressing specific research topics (e.g., development of valid, reliable, and acceptable measures; new statistical approaches) is critical to sound, generalizable, research findings.
9. CERAD’s legacy
By the end of its first decade (1996/1997), seven CERAD-based theses or dissertations had been accepted, over 50 articles by CERAD investigators had been published or were in press, and achievements were summarized in a supplement to Neurology (Heyman et al., 1997).64 Now, 10 years later, approximately 400 articles have been published. Figure 1 shows the number of papers published, and Figure 2 the number of citations for 1992–2006. The vast majority has appeared in the fields of medicine and neuroscience, with substantial representation in psychology. CERAD has also made its way in genetics, the health professions, and pharmacology. According to the SCOPUS database (searched on “cerad” or “consortium to establish a registry for alzheimer’s disease”), CERAD-related papers have appeared in 117 different journals, involving 147 different authors.
Figure 1.
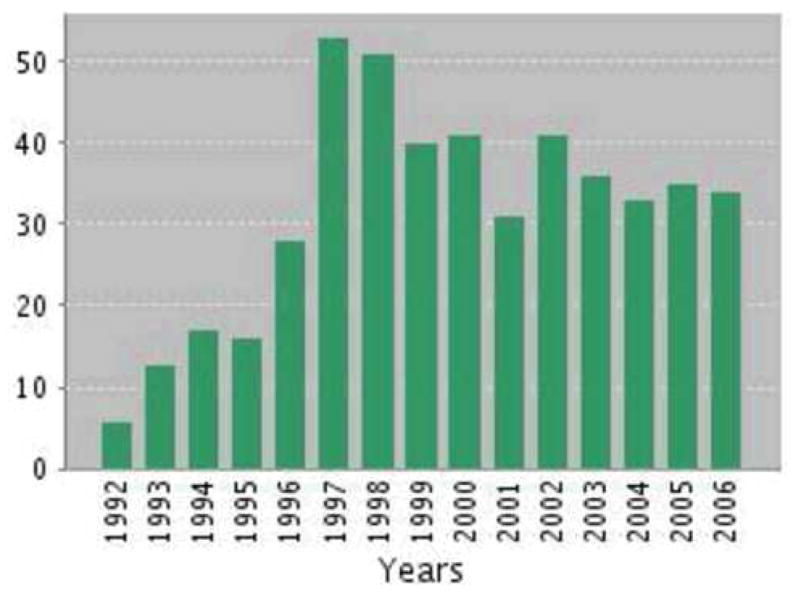
CERAD publications 1992–2006
Figure 2.
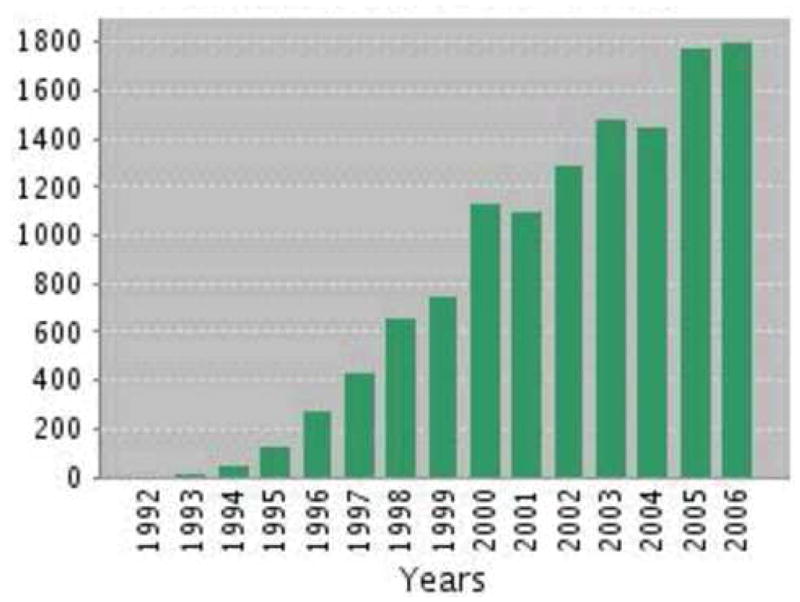
Citations to CERAD 1992–2006
CERAD, and its legacy, live on. We are pleased to see that, through the National Alzheimer Coordinating Center (NACC), AD centers are now strongly encouraged to use the set of carefully evaluated, predetermined measures selected for the Uniform Data Set.65 The CERAD materials remain in demand. The de-identified database has been archived with NACC. It is also available directly from the CERAD central office to users who can show that they are familiar with Alzheimer’s disease, and intend to use the data in a responsible manner – conditions placed on access by the participating sites. Information on CERAD is available through the CERAD web site http://cerad.mc.duke.edu and from the contacts listed there.
Acknowledgments
The Consortium to Establish a Registry for Alzheimer’s Disease was supported by NIA grant AG06790. The present paper was supported by NIA grant # 1P30 AG028716-01 Claude D. Pepper OAIC (Duke University) (Fillenbaum); NIA AG10124 (University of Pennsylvania ADC) (Clark); AG05128 and AG028377 (Bryan ADC, Duke University), and AG11380 (Cache County Memory Study, Duke University) (Welsh-Bohmer).
Individuals and sites: CERAD was made possible by the participation of clinics at 30 tertiary care sites (Albert Einstein College of Medicine, University of Maryland, Burke Rehabilitation Center, Baylor College of Medicine, Cornell University Medical Center, College of Physicians and Surgeons of Columbia University, University Hospitals of Cleveland, Duke University Medical Center, Emory University Medical Center, University of California San Francisco, Group Health Cooperative/University of Washington Alzheimer’s Disease Patient Registry, Indiana University School of Medicine, Johns Hopkins Medical Center, University of Kansas, Massachusetts General Hospital, University of Minnesota, Mount Sinai Medical Center, New York University Aging and Dementia Research Center, The Graduate Hospital – University of Pennsylvania, Rush Alzheimer’s Disease Center, University of Southern California School of Medicine, University of California San Diego, University of Texas Southwestern Medical Center, University of Alabama Hospitals Birmingham, University of Kentucky Medical Center, University of Michigan, University of Pittsburgh ADRC and ADPR, University of Rochester Medical Center, Veterans Administration Medical Center - Minneapolis, Washington University), 9 special focus sites (Emory University satellite – Dekalb; Elmhurst Hospital Center, New York; Philadelphia Inner City; University of California Los Angeles; Rush Medical College Satellite; Trover Clinic, Madisonville, KY; VA Medical Center, Dallas, TX; University of Washington satellite; Westside VA Medical Center, Chicago, IL), and 12 international associates (Hôpital d’Adultes Timone, Marseilles; McGill Centre for Studies in Aging, Montreal, Canada; Centre Hospitalier de Lachine, Lachine, Canada; Centre Hospitalier Régional et Universitaire de Lille, Lille, France; Hospital de Santa Maria, Lisbon, Portugal; Hôpital Maisonneuve-Rosemont, Montreal, Canada; Universidad de Navarra, Pamplona, Spain; Centre Paul Broca, Paris, France; St. James’s Hospital, Dublin, Ireland; Tokyo Metropolitan Institute of Gerontology, Tokyo, Japan; Victoria University of Manchester, Manchester, England; Hôpital d’Youville de Sherbrooke, Sherbrooke, Canada). The vast majority of these sites involved one or more clinic directors, clinicians, neuropathologists, neuropsychologists, neuroradiologists, psychometricians, project coordinators, minority coordinator, research nurses and other specialized staff. In addition, the task forces and their subcommittees frequently included major representatives of their disciplines located at other sites, whose names are not mentioned here but whose contributions were critical. At the risk of omitting many, we would acknowledge certain key players: Duane Beekly, with the Methodology and Data Management Center at the University of Washington, for the extraordinary ability and good will with which he handled numerous modifications to the assessment forms and maintained the complex database, and Jim Hughes, also there, who analyzed data for numerous CERAD investigators; Leonard Berg at Washington University, who consistently supported and played a critical role in the development of CERAD; Marian Patterson and Jim Mack of University Hospitals of Cleveland for their extensive work on the Behavior Rating Scale for Dementia, developed by the Behavioral Pathology Subcommittee; Marla Gearing at Emory School of Medicine, who played a critical role in the Neuropathology core. We would like to acknowledge CERAD’s External Advisory Committee (Drs. M. Albert, M.J. Ball, M. Conneally, A.E. George, H.R. Karp, R. Katzman, and P. Levy), and International Associates (Drs. C. Brayne, A. Degrand-Guillaud, G. Lafleche, I. McDowell, L. Ramos, K.A. Ritchie). Mary Strickland has been CERAD’s constant calm and most competent administrative secretary. Finally, none of CERAD’s achievements would have been possible without the vision, guidance, and constant encouragement of Teresa Radebaugh and Zaven Khatchaturian, CERAD’s project officers.
Abbreviations
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- NINCDS/ADRDA
National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association
- NIA
National Institute on Aging
Footnotes
With the exception of the senior author (Dr. Albert Heyman) who was the Principal Investigator of CERAD, and the first author, who was the Project Director, the order of authorship reflects the order of development of CERAD’s primary assessments. The Methodology and Data Management Center (Dr. van Belle), and the Clinical (Dr. Morris) and Neuropsychology (Dr. Mohs) batteries were developed simultaneously. These were followed by the Neuropathology protocol (Dr. Mirra), the Neuroimaging protocol (Dr. Davis), the Behavior Rating Scale for Dementia (Dr. Tariot), evaluation of family history (Dr. Silverman), and structured assessment of extrapyramidal dysfunction in AD (Dr. Clark).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foerstl H, Howard R, Burns A, Levy R. ‘The strange mental state of an old man who thought he would be slaughtered’ – an early report of dementia with delusion (1785) J Royal Soc Med. 1991;84:432–4. doi: 10.1177/014107689108400717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer A. Űber einer eigenartige Erkrankung der Hirnrinde. Allg Z Psychiatrie. 1907;64:146–8. [Google Scholar]
- 3.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–33. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 4.National Center for Health Statistics. Health, United States, 2005 with chartbook on trends on the health of Americans. Hyattsville, MD: U.S. Government Printing Office, Washington, DC 20402; [PubMed] [Google Scholar]
- 5.Freedman VA, Martin LG, Schoeni RF. Recent trends in disability and functioning among older adults in the United States: A systematic review. JAMA. 2002;288:3137–46. doi: 10.1001/jama.288.24.3137. [DOI] [PubMed] [Google Scholar]
- 6.Manton KG, Gu X, Lamb V. Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the U.S. elderly population. PNAS. 2006;103:18374–9. doi: 10.1073/pnas.0608483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 10.United States. Health Care Financing Administration. The International Classification of Diseases, 9th revision, Clinical Modification: ICD-9-CM. 2. U.S. Dept. of Health and Human Services, Public Health Service, Health Care Financing Administration; Washington DC, US GPO: 1980. [Google Scholar]
- 11.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization; 1993. [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 14.Erkinjuntii T, Østbye T, Steenhuis R, Hachinski V. The effect of different diagnostic criteria on the prevalence of dementia. N Engl J Med. 1997;337:1667–74. doi: 10.1056/NEJM199712043372306. [DOI] [PubMed] [Google Scholar]
- 15.U.S. General Accounting Office. Alzheimer’s disease: estimates of prevalence in the United States. Washington, DC: GAO/HEHS 98–16; 1998. [Google Scholar]
- 16.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part 1. Clinical and Neuropsychological Assessment of Alzheimer’s Disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 17.Mirra S, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 18.Davis PC, Gray L, Albert M, Wilkinson W, Hughes J, Heyman A, et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part III. Reliability of a standardized MRI evaluation of Alzheimer’s disease. Neurology. 1992;42:1676–1680. doi: 10.1212/wnl.42.9.1676. [DOI] [PubMed] [Google Scholar]
- 19.Mack JL, Patterson MB, Tariot PN. Behavior Rating Scale for Dementia (BRSD): Development of test scales and presentation of data for 555 individuals with Alzheimer’s disease. J Geriatr Psych Neurol. 1999;12:211–23. doi: 10.1177/089198879901200408. [DOI] [PubMed] [Google Scholar]
- 20.Tariot P, Mack JL, Patterson MB, Edland SD, Weiner MF, Fillenbaum G, et al. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1995;152:1349–57. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- 21.Silverman JM, Raiford K, Edland S, Fillenbaum G, Morris JC, Clark CM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part VI. Family history assessment: a multicenter study of first-degree relatives of Alzheimer’s disease probands and nondemented spouse controls. Neurology. 1994;44:1253–9. doi: 10.1212/wnl.44.7.1253. [DOI] [PubMed] [Google Scholar]
- 22.Clark CM, Ewbank D, Lerner A, Doody R, Henderson VW, Panisset M, et al. The relationship between extrapyramidal signs and cognitive performance in patients with Alzheimer’s disease enrolled in the CERAD Study. Neurology. 1997;49:70–5. doi: 10.1212/wnl.49.1.70. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: a practical method of grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–9. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 25.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 26.Fillenbaum GG, Beekly D, Edland SD, Hughes JP, Heyman A, van Belle G. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Development, data base structure and selected findings. Topics in Health Information Management. 1997;1:47–58. [PubMed] [Google Scholar]
- 27.Koss E, Peterson B, Fillenbaum GG. Determinants of attrition in a natural history study of Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1999;13:209–215. doi: 10.1097/00002093-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Galasko D, Edland SD, Morris JC, Clark C, Mohs R, Koss E. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part XI. Clinical milestones in patients with Alzheimer’s disease followed over 3 years. Neurology. 1995;45:1451–5. doi: 10.1212/wnl.45.8.1451. [DOI] [PubMed] [Google Scholar]
- 29.McDaniel KD, Edland SD, Heyman A. Relationship between level of insight and severity of dementia in Alzheimer disease. CERAD Clinical Investigators. Consortium to Establish a Registry for Alzheimer’s Disease. Alzheimer Dis Assoc Disord. 1995;9:101–4. doi: 10.1097/00002093-199509020-00007. [DOI] [PubMed] [Google Scholar]
- 30.Neumann PJ, Araki SS, Arcelus A, Longo A, Papadopoulos G, Kosik KS, et al. Measuring Alzheimer’s disease progression with transition probabilities: estimates from CERAD. Neurology. 2001;57:957–64. doi: 10.1212/wnl.57.6.957. [DOI] [PubMed] [Google Scholar]
- 31.Weiner MF, Doody RS, Sairam R, Foster B, Liao TY. Prevalence and incidence of major depressive disorder in Alzheimer’s disease: findings from two databases. Dementia & Geriatric Cognitive Disord. 2002;13:8–12. doi: 10.1159/000048627. [DOI] [PubMed] [Google Scholar]
- 32.White H, Pieper C, Schmader K, Fillenbaum G. Weight change in Alzheimer’s disease. J Am Geriatr Soc. 1996;44:265–72. doi: 10.1111/j.1532-5415.1996.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 33.Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451–4. doi: 10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- 34.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 35.Barnett M. Master’s thesis. Department of Biostatistics, University of Washington; Seattle, Washington State: 1992. Standardization of Clinical Dementia Rating (CDR) among 23 Alzheimer’s disease study centers. [Google Scholar]
- 36.Mirra S, Gearing M, Nash F. Neuropathologic assessment of Alzheimer’s disease. Neurology. 1997;49(suppl 3):S14–S16. doi: 10.1212/wnl.49.3_suppl_3.s14. [DOI] [PubMed] [Google Scholar]
- 37.Welsh-Bohmer KA, Mohs RC. Neuropsychological assessment of Alzheimer’s disease. Neurology. 1997;49(Suppl 3):S11–S3. doi: 10.1212/wnl.49.3_suppl_3.s11. [DOI] [PubMed] [Google Scholar]
- 38.Fillenbaum GG, Unverzagt FW, Ganguli M, Welsh-Bohmer KA, Heyman A. The CERAD Neuropsychological Battery: Performance of representative community and tertiary care samples of African American and European American elderly. In: Ferraro FR, editor. Minority and cross-cultural aspects of neuropsychological assessment. Swets and Zeitlinger; Lisse, NL: 2002. pp. 45–62. [Google Scholar]
- 39.Ganguli M, Ratcliff G, DeKosky ST. Cognitive test scores in community-based older adults with and without dementia. Aging & Mental Health. 1997;1:176–80. [Google Scholar]
- 40.Morris JC, Edland S, Clark C, Galasko D, Koss E, Mohs R, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part IV: rates of cognitive change, a longitudinal assessment of probable Alzheimer’s disease. Neurology. 1993;43:2457–65. doi: 10.1212/wnl.43.12.2457. [DOI] [PubMed] [Google Scholar]
- 41.Davis PC, Gearing M, Gray L, Mirra SS, Morris JC, Edland SD, et al. The CERAD experience, part VIII: neuroimaging-neuropathology correlates of temporal lobe changes in Alzheimer’s disease. Neurology. 1995;45:178–9. doi: 10.1212/wnl.45.1.178. [DOI] [PubMed] [Google Scholar]
- 42.Mirra S, Hart MN, Terry RD. Making the diagnosis of Alzheimer’s disease: a primer for practicing pathologists. Arch Pathol Lab Med. 1993;117:132–44. Note: no longer available from author or CERAD.) [PubMed] [Google Scholar]
- 43.Crain BJ, Mirra SS. The autopsy in cases of Alzheimer’s disease and other dementias. Chapter 21. In: Collins KA, Hutchins GM, editors. Autopsy Performance & Reporting. 2. College of American Pathologists; Northfield, IL: 2003. pp. 199–204. [Google Scholar]
- 44.Powers JM Autopsy Committee of the College of American Pathologists. Practice guidelines for autopsy pathology: autopsy procedures for brain, spinal cord, and neuromuscular system. Arch Pathol Lab Med. 1995;119:777–83. [PubMed] [Google Scholar]
- 45.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 46.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18(4 Suppl):S1–2. [PubMed] [Google Scholar]
- 47.Braak H, Braak E. Neuropathologic stageing of Alzheimer related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 48.Tierney MC, Fisher RH, Lewis AJ, Zorzitto ML, Snow WG, Reid DW, et al. The NINCDS-ADRDA work group criteria for the clinical diagnosis of probable Alzheimer’s disease: a clinicopathologic study of 57 cases. Neurology. 1988;38:359–64. doi: 10.1212/wnl.38.3.359. [DOI] [PubMed] [Google Scholar]
- 49.Murayama S, Saito Y. Neuropathologic diagnostic criteria for Alzheimer’s disease. Neuropathology. 2004;24:254–60. doi: 10.1111/j.1440-1789.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- 50.Nagi Z, Esiri MM, Hindley NJ, Joachim C, Morris JH, King EM, et al. Accuracy of clinical operational diagnostic criteria for Alzheimer’s disease in relation to different pathological diagnostic protocols. Dementia & Geriatric Cog Disord. 1998;9:219–26. doi: 10.1159/000017050. [DOI] [PubMed] [Google Scholar]
- 51.Newell KL, Hyman BT, Growdon JH, Hedley-White ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:1147–55. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Zaccai J, Ince P, Brayne C. Population-based neuropathological studies of dementia: design, methods and area of investigation – a systematic review. BMC Neurology. 2006;6:2. doi: 10.1186/1471-2377-6-2. available at: http://www.biomedcentral.com/1471-2377/6/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whyte SR, Cullum CM, Hynan LS, Lacritz LH, Rosenberg RN, Weiner MF. Performance of elderly Native Americans and Caucasians on the CERAD Neuropsychological Battery. Alzheimer Dis Assoc Disord. 2005;19:74–8. doi: 10.1097/01.wad.0000165508.67993.a3. [DOI] [PubMed] [Google Scholar]
- 54.Aharon-Peretz J, Daskovski E, Mashiach T, Kliot D, Tomer R. Progression of dementia associated with lacunar infarctions. Dementia & Geriatric Cognitive Disorders. 2003;16:71–7. doi: 10.1159/000070678. [DOI] [PubMed] [Google Scholar]
- 55.Aharon-Peretz J, Daskovski E, Mashiach T, Tomer R. Natural history of dementia associated with lacunar infarctions. J Neurological Sciences. 2002;203–204:53–5. doi: 10.1016/s0022-510x(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 56.Arango Lasprilla JC, Iglesias J, Lopera F. Neuropsychological study of familial Alzheimer’s disease caused by mutation E280A in the presenilin 1 gene. Am J Alzheimer’s Disease & Other Dementias. 2003;18:137–46. doi: 10.1177/153331750301800306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Ospina GP, Jimenez-Del Rio M, Lopera F, Velez-Pardo C. Neuronal DNA damage correlates with a positive detection of c-Jun, nuclear factor kB, p53 and Par-4 transcription factors in Alzheimer’s disease. Revista de Neurologia. 2003;36:1004–10. [PubMed] [Google Scholar]
- 58.Ganguli M, Chandra V, Gilby J, Ratcliff G, Sharma SD, Pandav R, et al. Cognitive test performance in a community-based nondemented elderly sample in rural India: the Indo-U.S. Cross-National Dementia Epidemiology Study. Int Psychogeriatr. 1996;8:507–24. doi: 10.1017/s1041610296002852. [DOI] [PubMed] [Google Scholar]
- 59.Prince M, Acosta D, Chiu H, Scazufca M Varghese M10/66 Dementia Research Group. Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet. 2003;361:909–17. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- 60.Barnhart RL, van Belle G, Edland SD, Kukull W, Borson S, Raskind M, et al. Geographically overlapping Alzheimer’s Disease registries: comparisons and implications. J Geriatr Psychiatry Neurol. 1995;8:203–8. doi: 10.1177/089198879500800401. [DOI] [PubMed] [Google Scholar]
- 61.Hughes JP, van Belle G, Kukull W, Larson EH, Teri L. On the uses of registries for Alzheimer Disease. Alzheimer Dis Assoc Disord. 1989;3:205–17. [PubMed] [Google Scholar]
- 62.Huppert FA, Brayne C, O’Connor DW. Dementia and normal aging. Cambridge, UK: Cambridge University Press; 1994. [Google Scholar]
- 63.Morris JC. Mild cognitive impairment is early stage Alzheimer’s disease. Time to revise diagnostic criteria. Arch Neurol. 2006;63:15–6. doi: 10.1001/archneur.63.1.15. editorial. [DOI] [PubMed] [Google Scholar]
- 64.Heyman A, Fillenbaum G, Nash F. Consortium to Establish a Registry for Alzheimer’s Disease: the CERAD experience. Neurology. 1997;49(suppl 3) [Google Scholar]
- 65.Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 66.Fillenbaum GG, Heyman A, Huber MS, Woodbury MA, Leiss J, Schmader KE, et al. The prevalence and three-year incidence of dementia in older Black and White community residents. J Clin Epidemiol. 1998;51:587–595. doi: 10.1016/s0895-4356(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 67.White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, et al. Prevalence of dementia in older Japanese-American men living in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–60. [PubMed] [Google Scholar]
- 68.Obadia Y, Rotily M, Degrand-Guillaud A, Guelain J, Ceccaldi M, Severo C, et al. The PREMAP Study: prevalence and risk factors of dementia and clinically diagnosed Alzheimer’s disease in Provence, France. Prevalence of Alzheimer’s Disease in Provence. Eur J Epidemiol. 1997;13:247–53. doi: 10.1023/a:1007300305507. [DOI] [PubMed] [Google Scholar]
- 69.Langa KM, Plassman BL, Wallace RB, Herzog RA, Heeringa GS, Ofstedal MB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiol. 2005;25:181–91. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 70.Breitner JCS, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, et al. APOE-ε4 count predicts age when prevalence of AD increases, then declines: The Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 71.Miech RA, Breitner JCS, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County study. Neurology. 2002;58:209–18. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- 72.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, Corcoran C, Green RC, Hayden K, et al. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006;67:229–34. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- 73.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP) J Alzheimers Dis. 2003;5:349–55. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 74.Breitner JC, Welsh KA, Gau BA, McDonald WM, Steffens DC, Saunders AM, et al. Alzheimer’s disease in the National Academy of Sciences-National Research Council Registry of Aging Twin Veterans. III. Detection of cases, longitudinal results, and observations on twin concordance. Arch Neurol. 1995;52:763–71. doi: 10.1001/archneur.1995.00540320035011. [DOI] [PubMed] [Google Scholar]
- 75.Plassman BL, Steffens DC, Burke JR, Welsh-Bohmer KA, Newman TN, Drosdick D, et al. Duke Twins Study of Memory in Aging in the NAS-NRC Twin Registry. Twin Research and Human Genetics. 2006;9(6) doi: 10.1375/183242706779462381. in press. [DOI] [PubMed] [Google Scholar]
- 76.Hendrie HC, Osuntokun BO, Hall KS, Ogunniyi AO, Hui SL, Unverzagt FW, et al. The prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 77.Hendrie HC, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Gureje O, et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;14285:739–47. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- 78.Chandra V, Ganguli M, Pandav R, Johnston J, Belle S, DeKosky ST. Prevalence of Alzheimer’s disease and other dementias in rural India: the Indo-US study. Neurology. 1998;51:1000–8. doi: 10.1212/wnl.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 79.Graves AB, Larson EB, Edland SD, Bowen JD, McCormick WC, McCurry SM, et al. Prevalence of dementia and its subtypes in the Japanese American population of King County, Washington State: the Kame Project. Am J Epidemiol. 1996;144:760–71. doi: 10.1093/oxfordjournals.aje.a009000. [DOI] [PubMed] [Google Scholar]
- 80.Shin HY, Chung EK, Rhee JA, Yoon JS, Kim JM. Prevalence and related factors of dementia in an urban elderly population using a new screening method. [Korean] J Preventive Med Public Health/Yebang Uihakhoe Chi. 2005;38:351–8. [PubMed] [Google Scholar]
- 81.Ganguli M, Ratcliff G, Huff FJ, Belle S, Kancel JJ, Fischer L, et al. Effects of age, gender and education on cognitive tests in a rural elderly community sample: Norms from the Monongahela Valley Independent Elders Survey. Neuroepidemiology. 1991;10:42–52. doi: 10.1159/000110246. [DOI] [PubMed] [Google Scholar]
- 82.Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life: Findings from the Nun Study. JAMA. 1996;275:528–532. [PubMed] [Google Scholar]
- 83.Snowdon DA. Aging and Alzheimer’s disease: Lessons from the Nun Study. Gerontologist. 1997;37:150–6. doi: 10.1093/geront/37.2.150. [DOI] [PubMed] [Google Scholar]
- 84.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 85.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–75. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 86.Lin RT, Lai CL, Tai CT, Liu CK, Yen YY, Howng SL. Prevalence and subtypes of dementia in southern Taiwan: impact of age, sex, education, and urbanization. J Int Neuropsychol Soc. 1998;160:67–75. doi: 10.1016/s0022-510x(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 87.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 88.Alafuzoff I, Pikkarainen M, Al-Sarraj S, Arzberger T, Bell J, Bodi I, et al. Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol. 2006;65:740–57. doi: 10.1097/01.jnen.0000229986.17548.27. [DOI] [PubMed] [Google Scholar]
- 89.Jobst KA, Barnetson LP, Shepstone BJ. Accurate prediction of histologically confirmed Alzheimer’s disease and the differential diagnosis of dementia: the use of NINCDS-ADRDA and DSM-III-R criteria, SPECT, X-ray CT, and Apo E4 in medial temporal lobe dementias. Oxford Project to Investigate Memory and Aging. Int Psychogeriatr. 1998;10:271–302. doi: 10.1017/s1041610298005389. [DOI] [PubMed] [Google Scholar]
- 90.Neuropathology Group of the UK. Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 91.Tractenberg RE, Gamst A, Thomas RG, Patterson M, Schneider LS, Thal LJ. Investigating emergent symptomatology as an outcome measure in a behavioral study of Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2002;14:303–10. doi: 10.1176/jnp.14.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Teri L, Logsdon RG, Peskind E, Raskind M, Weiner MF, Tractenberg RE, et al. Treatment of agitation in AD: a randomized, placebo-controlled clinical trial. Neurology. 2000;55:1271–8. doi: 10.1212/wnl.55.9.1271. Erratum in: Neurology. 2001;56:426. [DOI] [PubMed] [Google Scholar]
- 93.Patterson MB, Mack JL, Mackell JA, Thomas R, Tariot P, Weiner M, et al. A longitudinal study of behavioral pathology across five levels of dementia severity in Alzheimer’s disease: the CERAD Behavior Rating Scale for Dementia. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S40–4. [PubMed] [Google Scholar]
- 94.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 95.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 96.Shumaker SA, Legault C, Thal L, Wallace RB, Ockene JK, Hendrix SL, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 97.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 98.Kryscio RJ, Mendiondo MS, Schmitt FA, Markesbery WR. Designing a large prevention trial: statistical issues. Stat Med. 2004;23:285–96. doi: 10.1002/sim.1716. [DOI] [PubMed] [Google Scholar]
- 99.Mendiondo MS, Ashford JW, Kryscio RJ, Schmitt FA. Designing a brief Alzheimer screen. J Alzheimers Dis. 2003;5:391–8. doi: 10.3233/jad-2003-5506. [DOI] [PubMed] [Google Scholar]
- 100.MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, et al. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13:28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- 101.Welsh K, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer’s disease - use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol. 1992;49:448–52. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- 102.Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–14. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 103.Beeri MS, Schmeidler J, Sano M, Wang J, Lally R, Grossman H, et al. Age, gender, and education norms on the CERAD neuropsychological battery in the oldest old. Neurology. 2006;67:1006–10. doi: 10.1212/01.wnl.0000237548.15734.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Welsh KA, Fillenbaum G, Wilkinson W, Heyman A, Mohs RC, Stern Y, et al. Neuropsychological test performance in African-American and white patients with Alzheimer’s disease. Neurology. 1995;45:2207–11. doi: 10.1212/wnl.45.12.2207. [DOI] [PubMed] [Google Scholar]
- 105.Fillenbaum GG, Kuchibhatla M, Henderson VW, Clark CM, Taussig IM. Comparison of performance on the CERAD Neuropsychological Battery of Hispanic patients and cognitively normal controls at two sites. Clin Gerontologist. 2007 forthcoming. [Google Scholar]
- 106.Collie A, Shafiq-Antonacci R, Maruff P, Tyler P, Currie J. Norms and the effects of demographic variables on a neuropsychological battery for use in healthy ageing Australian populations. Aust N Z J Psychiatry. 1999;33:568–75. doi: 10.1080/j.1440-1614.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 107.Bertolucci PHF, Okamoto IH, Brucki SMD, Siviero MO, Neto JT, Ramos LR. Applicability of the CERAD Neuropsychological Battery to Brazilian elderly. Arq Neuropsiquiatr. 2001;59:532–6. doi: 10.1590/s0004-282x2001000400009. [DOI] [PubMed] [Google Scholar]
- 108.Karrasch M, Laine M. Age, education and test performance on the Finnish CERAD. Acta Neurol Scand. 2003;108:97–101. doi: 10.1034/j.1600-0404.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 109.Karrasch M, Sinerva E, Gronholm P, Rinne J, Laine M. CERAD test performances in amnestic mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand. 2005;111:172–9. doi: 10.1111/j.1600-0404.2005.00380.x. [DOI] [PubMed] [Google Scholar]
- 110.Unverzagt FW, Morgan OS, Thesiger CH, Eldemire DA, Luseko J, Pokuri S, et al. Clinical utility of CERAD neuropsychological battery in elderly Jamaicans. JINS. 1999;5:255–9. doi: 10.1017/s1355617799003082. [DOI] [PubMed] [Google Scholar]
- 111.Lee DY, Lee KU, Lee JH, Kim KW, Jhoo JH, Kim SY, et al. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J Int Neuropsychol Soc. 2004;10:72–81. doi: 10.1017/S1355617704101094. [DOI] [PubMed] [Google Scholar]
- 112.Guruje O, Unverzargt FW, Osuntokun BO, Hendrie HC, Baiyewu O, Ogunniyi A, et al. The CERAD Neuropsychological Test Battery: norms from a Yoruba-speaking Nigerian sample. West African J Med. 1995;14:29–33. [PubMed] [Google Scholar]
- 113.Berres M, Monsch AU, Bernasconi F, Thalmann B, Stahelin HB. Normal ranges of neuropsychological tests for the diagnosis of Alzheimer’s disease. Studies in Health Technology Information. 2000;77:195–9. [PubMed] [Google Scholar]
- 114.Stewart R, Richards M, Brayne C, Mann A. Cognitive function in UK community-dwelling African Caribbean elders: normative data for a test battery. Int J Geriatric Psychiatry. 2001;16:518–27. doi: 10.1002/gps.384. [DOI] [PubMed] [Google Scholar]
- 115.Unverzagt FW, Hall KS, Torke AM, Rediger JD, Mercado N, Gureje O, et al. Effects of age, education, and gender on CERAD neuropsychological test performance in an African–American sample. Clinical Neuropsychologist. 1996;10:180–90. [Google Scholar]
- 116.Fillenbaum GG, McCurry SM, Kuchibhatla M, Masaki KH, Borenstein AR, Foley DJ, et al. Performance on the CERAD neuropsychology battery of two samples of Japanese-American elders: norms for persons with and without dementia. J Int Neuropsychol Soc. 2005;11:192–201. doi: 10.1017/s1355617705050198. [DOI] [PubMed] [Google Scholar]
- 117.McCurry SM, Gibbons LE, Uomoto JM, Thompson ML, Graves AB, Edland SD, et al. Neuropsychological test performance in a cognitively intact sample of older Japanese American adults. Arch Clin Neuropsychol. 2001;16:447–459. [PubMed] [Google Scholar]


