Abstract
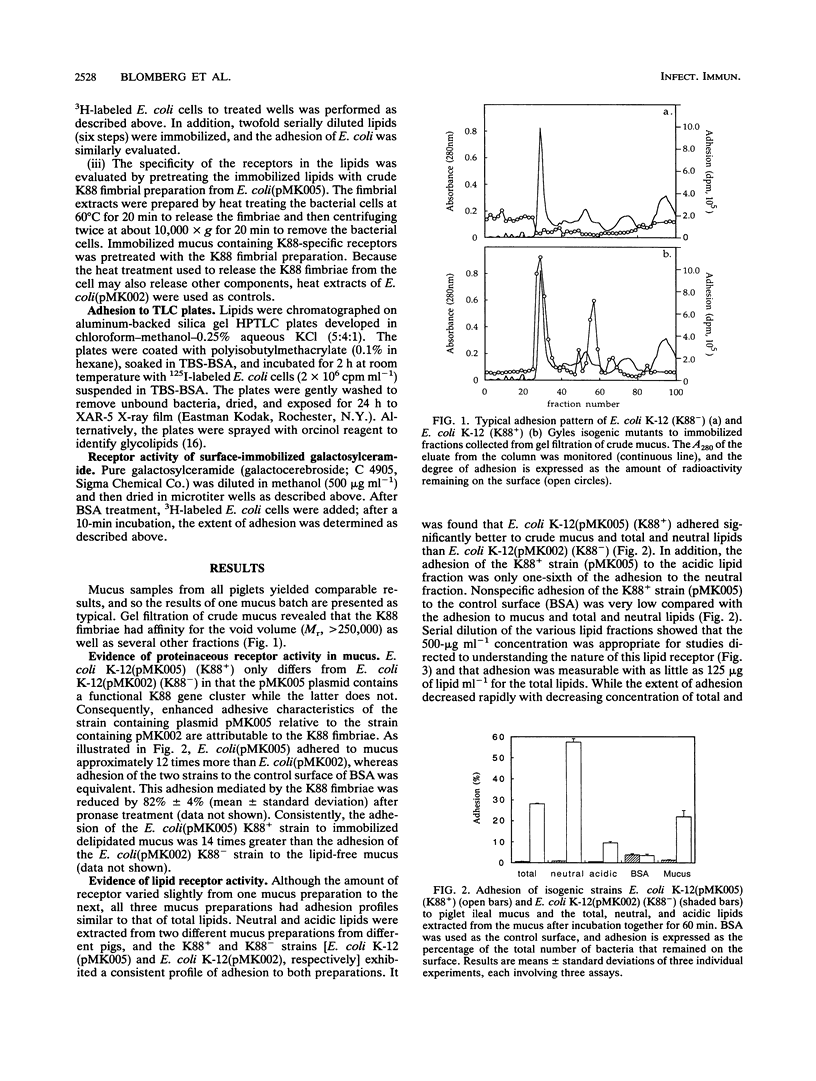
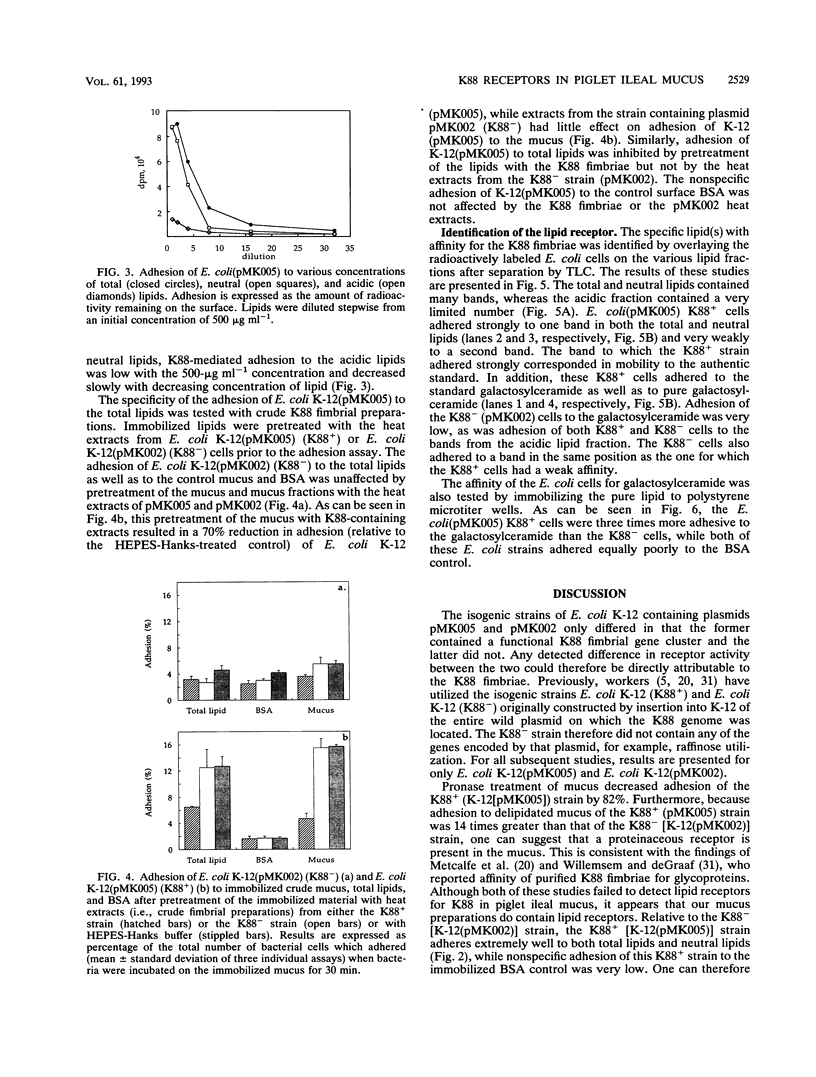
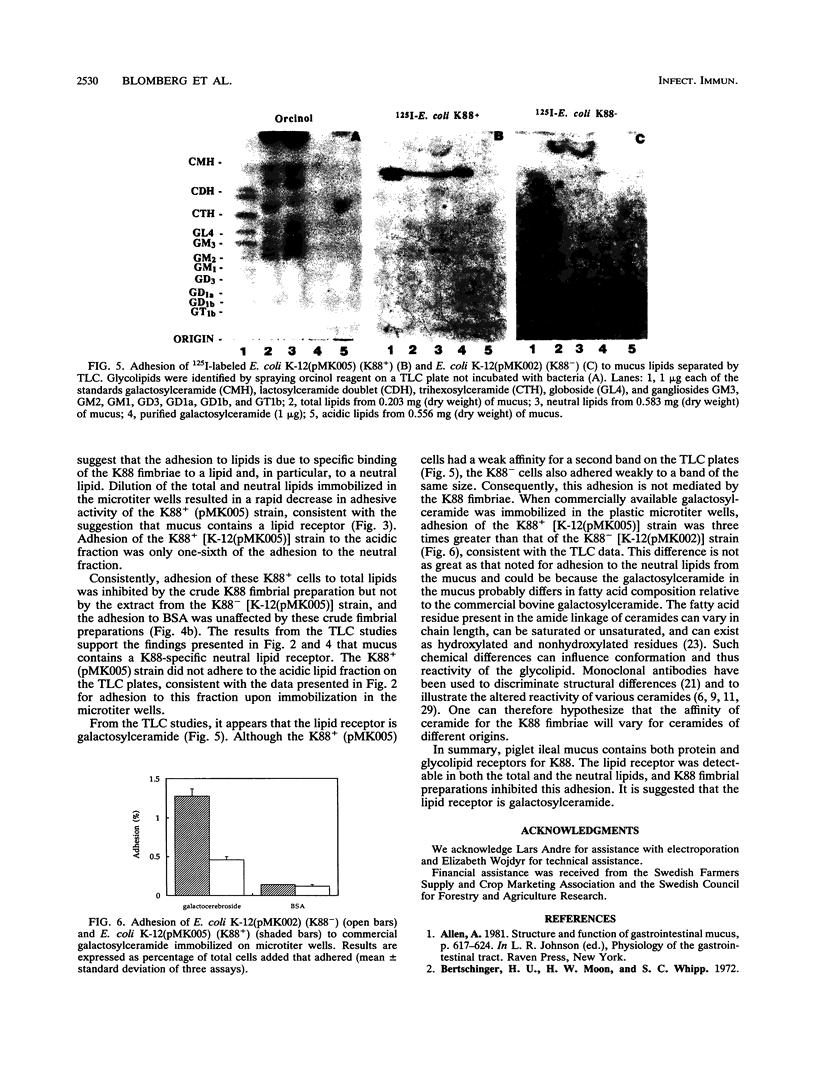
The aim of this study was to characterize the Escherichia coli K88-specific receptors in mucus from the small intestines of 35-day-old piglets with the isogenic strains E. coli K-12(pMK005) (K88+) and E. coli K-12(pMK002) (K88-). These strains differed only in that the latter one cannot produce intact K88 fimbriae because of a deletion in the gene coding for the major fimbrial subunit. Adhesion was studied by incubating 3H-labeled bacteria with crude mucus, pronase-treated whole mucus, mucus fractionated by gel filtration, delipidated mucus, or extracted lipids immobilized in microtiter wells. In addition, E. coli strains were tested for adhesion to glycolipids extracted from mucus by overlaying glycolipid chromatograms with 125I-labeled bacteria. The recently reported finding that K88 fimbriae bind to glycoproteins in mucus from the piglet small intestine was confirmed in two ways. Pronase treatment of immobilized mucus reduced adhesion by 82%, and adhesion to delipidated mucus was 14 times greater for the K88+ than for the K88- strain. E. coli K88+ adhered to several of the fractions collected after gel filtration of crude mucus, including the void volume (M(r), > 250,000). Receptor activity specific for the K88 fimbriae was demonstrated in the lipids extracted from mucus, as the neutral lipids contained six times as much receptor activity as the acidic lipid fraction. Specificity was confirmed by demonstrating that adhesion to the total lipids could be inhibited by pretreatment of the immobilized lipids with K88 fimbriae. Relative to K-12 (K88-), the K-12 (K88+) bacterial cells bound more avidly to galactosylceramide when the neutral lipids were separated on thin-layer chromatography plates. No adhesion to lipids in the acidic fraction separated on thin-layer plates was detected. Relative to adhesion of K-12 (K88-), adhesion of K-12 (K88+) to commercially available galactosylceramide immobilized in microtiter wells confirmed the results with the thin-layer plates. It can be concluded that 35-day-old piglet mucus contains both protein and glycolipid receptors specific for K88 fimbriae, the latter being galactosylceramide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertschinger H. U., Moon H. W., Whipp S. C. Association of Escherichia coli with the small intestinal epithelium. I. Comparison of enteropathogenic and nonenteropathogenic porcine strains in pigs. Infect Immun. 1972 Apr;5(4):595–605. doi: 10.1128/iai.5.4.595-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway P. L., Welin A., Cohen P. S. Presence of K88-specific receptors in porcine ileal mucus is age dependent. Infect Immun. 1990 Oct;58(10):3178–3182. doi: 10.1128/iai.58.10.3178-3182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook S. J., Boggs J. M., Vistnes A. I., Koshy K. M. Factors affecting surface expression of glycolipids: influence of lipid environment and ceramide composition on antibody recognition of cerebroside sulfate in liposomes. Biochemistry. 1986 Nov 18;25(23):7488–7494. doi: 10.1021/bi00371a035. [DOI] [PubMed] [Google Scholar]
- Gibbons R. A., Jones G. W., Sellwood R. An attempt to identify the intestinal receptor for the K88 adhesin by means of a haemagglutination inhibition test using glycoproteins and fractions from sow colostrum. J Gen Microbiol. 1975 Feb;86(2):228–240. doi: 10.1099/00221287-86-2-228. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H. Behavior of Escherichia coli K antigens K88ab, K88ac, and K88ad in immunoelectrophoresis, double diffusion, and hemagglutination. Infect Immun. 1979 Mar;23(3):700–705. doi: 10.1128/iai.23.3.700-705.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itonori S., Hidari K., Sanai Y., Taniguchi M., Nagai Y. Involvement of the acyl chain of ceramide in carbohydrate recognition by an anti-glycolipid monoclonal antibody: the case of an anti-melanoma antibody, M2590, to GM3-ganglioside. Glycoconj J. 1989;6(4):551–560. doi: 10.1007/BF01053777. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R., Nudelman E., Hakomori S. Possible role of ceramide in defining structure and function of membrane glycolipids. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3470–3474. doi: 10.1073/pnas.79.11.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Kehoe M., Sellwood R., Shipley P., Dougan G. Genetic analysis of K88-mediated adhesion of enterotoxigenic Escherichia coli. Nature. 1981 May 14;291(5811):122–126. doi: 10.1038/291122a0. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Franklin D. P., Wang W., Laux D. C., Cohen P. S. Phosphatidylserine found in intestinal mucus serves as a sole source of carbon and nitrogen for salmonellae and Escherichia coli. Infect Immun. 1992 Sep;60(9):3943–3946. doi: 10.1128/iai.60.9.3943-3946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Kyogashima M., Ginsburg V., Krivan H. C. Escherichia coli K99 binds to N-glycolylsialoparagloboside and N-glycolyl-GM3 found in piglet small intestine. Arch Biochem Biophys. 1989 Apr;270(1):391–397. doi: 10.1016/0003-9861(89)90042-8. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. W., Krogfelt K. A., Krivan H. C., Cohen P. S., Laux D. C. Characterization and identification of a porcine small intestine mucus receptor for the K88ab fimbrial adhesin. Infect Immun. 1991 Jan;59(1):91–96. doi: 10.1128/iai.59.1.91-96.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakakuma H., Arai M., Kawaguchi T., Horikawa K., Hidaka M., Sakamoto K., Iwamori M., Nagai Y., Takatsuki K. Monoclonal antibody to galactosylceramide: discrimination of structural difference in the ceramide moiety. FEBS Lett. 1989 Dec 4;258(2):230–232. doi: 10.1016/0014-5793(89)81660-6. [DOI] [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., SOJKA W. J., WITTIG W. K ANTIGENS K88AB(L) AND K88AC(L) IN E. COLI. A NEW O ANTIGEN: 0147 AND A NEW K ANTIGEN: K89(B). Acta Pathol Microbiol Scand. 1964;62:439–447. doi: 10.1111/apm.1964.62.3.439. [DOI] [PubMed] [Google Scholar]
- Sako F., Gasa S., Makita A. Characterization of neutral glycosphingolipids from porcine erythrocyte membranes. Int J Biochem. 1987;19(10):923–929. doi: 10.1016/0020-711x(87)90173-x. [DOI] [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Sellwood R. The interaction of the K88 antigen with porcine intestinal epithelial cell brush borders. Biochim Biophys Acta. 1980 Oct 1;632(2):326–335. doi: 10.1016/0304-4165(80)90090-2. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The influence of plasmid-determined and other characteristics of enteropathogenic Escherichia coli on their ability to proliferate in the alimentary tracts of piglets, calves and lambs. J Med Microbiol. 1978 Nov;11(4):471–492. doi: 10.1099/00222615-11-4-471. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Mansa B. Episome-carried surface antigen K88 of Escherichia coli. II. Isolation and chemical analysis. J Bacteriol. 1967 Feb;93(2):731–739. doi: 10.1128/jb.93.2.731-739.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington F. W., Bernstein I. D., Hakomori S. Monoclonal antibody specific for lactosylceramide. J Biol Chem. 1984 May 10;259(9):6008–6012. [PubMed] [Google Scholar]
- Teneberg S., Willemsen P., de Graaf F. K., Karlsson K. A. Receptor-active glycolipids of epithelial cells of the small intestine of young and adult pigs in relation to susceptibility to infection with Escherichia coli K99. FEBS Lett. 1990 Apr 9;263(1):10–14. doi: 10.1016/0014-5793(90)80693-d. [DOI] [PubMed] [Google Scholar]
- Willemsen P. T., de Graaf F. K. Age and serotype dependent binding of K88 fimbriae to porcine intestinal receptors. Microb Pathog. 1992 May;12(5):367–375. doi: 10.1016/0882-4010(92)90099-a. [DOI] [PubMed] [Google Scholar]
- Wilson I. B., Staley T. E., Bush L. J., Gilliland S. E. Recovery of intestinal membrane binding sites for K88 E. coli from pig mucosal organ cultures. Mol Cell Biochem. 1984 Apr;62(1):57–65. doi: 10.1007/BF00230078. [DOI] [PubMed] [Google Scholar]
- Wilson M. R., Hohmann A. W. Immunity to Escherichia coli in pigs: adhesion of enteropathogenic Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1974 Oct;10(4):776–782. doi: 10.1128/iai.10.4.776-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



