Abstract
Objectives of this study were to determine the maximum tolerated dose and to characterize the side-effect profile and pharmacokinetics of lenalidomide in patients with advanced refractory solid tumors. Patients were treated on a modified Fibronacci dose-escalation scheme with an oral daily dose of lenalidomide. A total of 45 patients with 8 different tumor types were accrued. Doses administered included 5, 10, and 20 mg continuous daily doses, every 28 days (n=15); later modified to intermittent doses of 15, 20, 25, 30, 35, and 40 mg, 21 days-on and 7 days off schedule, due to observed side effects. Lenalidomide exhibited a linear pharmacokinetics over a wide range doses with the mean half-life of 3.9 hours. The renal function affected lenalidomide clearance, resulting in 50% reduction in patients with mild renal impairment compared wiht patients with normal function (CL/F = 257 mL/min). Stable disease was documented in 12 of 44 evaluable patients, of whom 9 patients had prostate cancer. Most frequent grade 1 and 2 toxicities included fatigue, nausea, pruritus/rash, neutropenia, and neuropathy. Grade 3/4 events were predominantly hematologic. Lenalidomide was well tolerated up to 35 mg/day intermittent dosing schedule at doses higher than previously indicated for hematologic malignancies.
Keywords: androgen-independent prostate cancer, angiogenesis, CC-5013, lenalidomide, refractory cancer
Introduction
Angiogenesis is increasingly recognized as essential in the growth and metastasis of human tumors (1). Thalidomide has been shown to inhibit vasculature in a rat cornea model after D’Amato et al. hypothesized that congenital anomalies brought about by thalidomide were secondary to its effect on vasculogenesis (2). While the precise mechanism by which thalidomide exerts its anti-angiogenic effects remain to be elucidated, thalidomide has been shown to have promising results in a variety of cancers (3, 4), with multiple myeloma at the forefront. However, thalidomide has associated side effects, including peripheral neuropathy, fatigue, thrombosis, sedation, constipation, and mood changes (5, 6). Therefore, alternative analogues with similar or better potency, absence of teratogenicity, and fewer adverse events are actively being sought.
Lenalidomide (Revlimid®), CC-5013, is an (α -(3-aminophthalimido) glutarimide)) that has anti-angiogenic properties and has demonstrated in vitro potency in a HUVEC (human umbilical vein endothelial cells) proliferation and tube formation assay (7). It has also been shown to inhibit tumor growth (8), and exhibited more potent immunomodulatory effects than thalidomide, with potent inhibition of tumor necrosis factor-alpha (TNF-α) in lipopolysaccharide induced peripheral blood mononuclear cell assays (9, 10). It has current approval for low risk myelodysplasia and previously treated multiple myeloma (11) and it has significant activity in lymphoma (12). To this end, we conducted a phase I trial of lenalidomide, assessing the safety and tolerability in refractory cancers.
Materials and Methods
Patients
Informed consent was obtained from all patients prior to participation and the consent form was approved by the National Cancer Institute Institutional Review Board. The study was conducted at the National Cancer Institute (NCI) and Clinical Center of the National Institutes of Health (NIH), Bethesda, MD, in compliance with Good Clinical Practice, guidelines of the International Conference on Harmonization, and the Declaration of Helsinki.
Eligible patients had to have a histopathological diagnosis prior to study entry of refractory solid tumors or lymphoma for which standard therapy has failed or there was no standard treatment, an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2, life expectancy of > 3 months, adequate bone marrow and organ function as defined by a granulocyte count ≥ 1500/µL, platelet count of ≥ 100,000/ µL, total bilirubin within normal institutional limits, ALT and AST ≤ 2.5x upper limit of normal, and creatinine within normal limits or measured creatinine clearance ≥ 60 ml/min if creatinine is > 1.5 mg/dL. Patients must have been off prior chemotherapy or radiotherapy for at least 4 weeks prior to starting therapy and have no lingering side-effects from such therapy. Patients who had a seizure disorder requiring anticonvulsant therapy, brain metastases, unstable angina, or recent myocardial infarction, were excluded. Although it is unclear which enzymes are responsible for metabolizing lenalidomide, thalidomide undergoes CYP3A4 metabolism (13). Hence, concomitant use of medications known to influence the expression or function of CYP3A4 were excluded from the study.
Study Design
This was a phase I, single-center, open-label, dose-escalation study conducted at the Warren Grant Magnuson Clinical Center at the NIH. The primary objectives of the study included (a) determination of the maximum tolerated dose (MTD) and dose limiting toxicity (DLT) of lenalidomide in patients with metastatic solid tumors who were refractory to known standard therapy; (b) characterization of the pharmacokinetic (PK) profile of lenalidomide in patients; (c) determination of any PK correlations with clinical activity, biologic activity or toxicity; and (d) characterization of side-effect profile of lenalidomide. Secondary objectives of the trial included assessment of biologic markers including: basic fibroblast growth factor (bFGF), tumor necrosis factor-alpha (TNF-α), interferon-γ (IFN-γ), and cytokines such as interleukin (IL)-2, 4, 6, 10, 12p70, 13 in relation to their clinical relevance in metastatic tumors.
Drug administration and treatment
Pre-treatment history, physical examination, laboratory work and radiographic scans (CT scan, MRI or bone scans – deemed appropriate for following disease progression) was obtained at study entry. Lenalidomide was supplied by Celgene Corporation (Summit, NJ) as an off-white powder in gelatinous capsules in doses of 5 mg and 25 mg. Based on xenograft studies and prior clinical experience with lenalidomide in healthy male volunteers, single, daily oral dose of 5 mg starting dose was determined (14). This capsule was to be taken with a glass of water following overnight fasting, repeated every 28 days. The doses investigated were 5, 10, 15, 20, 25, 30, 35, and 40 mg/day. Three patients were to be accrued for each dose level (Table 1). If one of three patients had a ≥ Grade 3 non-hematologic or Grade 4 hematologic toxicity at a particular dose level, that group was expanded to a total of six patients. If at least 2 of these 6 patients demonstrated ≥ Grade 3 non-hematologic or Grade 4 hematologic toxicities, the dose level below was to be considered the MTD. MTD was defined as the dose at which less than 2 of 6 patients treated for at least 1 treatment cycle did not experience Grade 3 or greater non-hematologic toxicity or Grade 4 or greater hematologic toxicity. DLT was defined for only the first cycle of therapy (4 weeks). Due to the DLTs observed on the continuous daily dosing schedule, the protocol was amended to change the dosing from continuous daily dosing of every 28 days to daily dosing for 21 days followed by 7 days off drug, repeated every 28 days.
Table 1.
Dose escalation schema
| Dose level | Dose (mg) | Dosing schedule (every 28 days per cycle) | |
|---|---|---|---|
| 1 | 5 | 28 days ON | Continuous |
| 2 | 10 | 28 days ON | Continuous |
| 3 | 20 | 28 days ON | Continuous |
| 4 | 15 | 21 days ON; 7 days OFF | Intermittent |
| 5 | 20 | 21 days ON; 7 days OFF | Intermittent |
| 6 | 25 | 21 days ON; 7 days OFF | Intermittent |
| 7 | 30 | 21 days ON; 7 days OFF | Intermittent |
| 8 | 35 | 21 days ON; 7 days OFF | Intermittent |
| 9 | 40 | 21 days ON; 7 days OFF | Intermittent |
Cycle 1 day 1 was the first dose of the drug administered for all patients, which was followed by pharmacokinetic sampling after 24 hours. The second and subsequent doses began on cycle 2 day 1, approximately 4 – 10 days following the first dose administered.
Patients with measurable disease were assessed by standard criteria using Response Evaluation Criteria in Solid Tumors (RECIST) Criteria and radiographic studies were performed at the end of cycle 3 and every other cycle thereafter. Treatment continued until disease progression or toxicity was encountered.
Pharmacokinetic study
Sample collection and analysis
Blood samples (7 mL) were collected during each clinic visit, approximately every 4 weeks following the initial cycle 1 day 1 and day 15 visits. Serum samples (red top tubes) were collected for the determination of angiogenic markers and plasma samples (green tubes) were collected for PK analysis. The serums or plasmas were separated by centrifugation at 2400 rpm for 5 minutes and stored at −70°C until assay.
Blood samples for PK evaluation were obtained before drug administration as well as at 0.5, 1, 2, 4, 6, 9, 12, 24, 32, and 48 hours after the dose on day 1 of cycle 1. Urine samples were collected at every 4-hour period for 24 hours from patients in dose groups of 30 mg/day (n=3), 35 mg/day (n=4) and 40 mg/day (n=2). During 4-hour collection period, urine samples were placed at 0–4°C and stored at −70°C afterwards, prior to analysis. Concentrations of lenalidomide in plasma and urine were determined by using the validated liquid chromatographic-mass spectrometer method with a lower limit of quantification of 5 ng/ml (7). Briefly, lenalidomide was extracted from plasma or urine using a liquid-liquid extraction method into ethyl acetate followed by evaporation, reconstituted with mobile phase solvents consisting of acetonitrile:water:acetic acid (20:80:0.1), and detected by a single quadrupole mass spectrometer using electrospray ionization.
Pharmacokinetic analysis
Individual lenalidomide concentration versus time profiles were analyzed using WinNonlin (version 5; Pharsight Corp., CA) to obtain non-compartmental parameters such as the area under the concentration-time curves (AUC0-∞), apparent total clearance (CL/F), apparent volume of distribution (V/F), and half-life (T1/2). Peak concentration (Cmax) and corresponding time (Tmax) were recorded as observed values. Renal clearance (CL/F_renal) were determined by dividing the amount of unchanged drug excreted in urine within 24 hr of dosing (Ae,0–24) by the area under the plasma concentration-time curve from 0 to 24 hr (AUC0–24).
A nonlinear mixed-effect modeling approach was also employed to characterize population PK (PopPK) of lenalidomide using the NONMEM software system (Version VI 2.0; Globomax, MD) (15). NONMEM was complied by Intel Visual Fortran (Version 10; Intel Corporation, CA) on a Window XP operating system. A transform-both-side approach was used by taking the logarithm of observed concentrations and of model predictions. A one-compartment model with the first-order absorption and first-order elimination rate constant, parameterized in terms of CL/F, V/F, and ka, was initially used to fit lenalidomide concentration-time data. Between-subject variability (BSV) for the PK parameters was modeled using an exponential error model and the residual variability was expressed as an additive error model. A combined additive and proportional error model was also evaluated. The model parameters were estimated with a combination of the first-order conditional estimation (FOCE) and first-order estimation (FO) methods by using HYBRID option in NONMEM. The parameter ALAG was estimated by the FO method. Covariance between the random effects was also evaluated using a BLOCK covariance matrix.
After establishing an appropriate structural PK model that would best describe observed lenalidomide disposition, the impact of patient characteristics such as age, weight, body surface area (BSA), sex, and creatinine clearance (CLCr) on lenalidomide PK was examined following a stepwise forward inclusion process. Inclusion of a covariate was on the basis of the likelihood ratio test and accepted if the objective function value decreases at least 10.83 (p < 0.001). Visual inspection of diagnostic plots, clinical relevance, and precision of parameter estimates were also used to discriminate among alternative models. CLCr was estimated via the method described by Cockcroft and Gault (16).
Predictive performance was assessed by a visual predictive check based on at least 6000 virtual patients. Parameter uncertainty was evaluated by a non-parametric bootstrap procedure using the bootstrap option in Wings for NONMEM (Version 614, http://wfn.sourceforge.net). One thousand sets of parameter estimates obtained from the bootstrap data were then used to compute the bootstrap 95% confidence interval (CI).
Toxicity assessment and dose modifications
Toxicities were reported using the Common Toxicity Criteria for Adverse Events (CTCAE) version 2. Patients were eligible for retreatment if they did not experience ≥ grade 3 non-hematologic or grade 4 hematologic toxicity lasting for ≤ 14 days. However, if patients developed the aforementioned toxicities, dose would be reduced at subsequent cycles if the toxicities decreased to ≤ grade 1 within 14 days.
Results
Patient Characteristics
A total of 45 patients were accrued from April 2002 to December 2005 at 9 different doses ranging from 5 mg to 40 mg per day. Patients’ characteristics are shown in Table 2. The predominant tumor histology was prostate cancer (n=35) followed by adrenocortical cancer, ACC (n=3). Majority of patients had ECOG status of 1 (n=34). Patients were pre-treated with a median of 3 prior therapies that included hormonal therapy, docetaxel, mitoxantrone, thalidomide, platinum agents, and irinotecan. Median prostate specific antigen (PSA) value for patients with prostate cancer was 82.3 µg/L (range: 5.1 – 2143).
Table 2.
Patient demographics and characteristics
| Characteristics | No. of Patients |
|---|---|
| Total | 45 |
| Age, yrs | |
| Median | 68 |
| Range | 24–89 |
| Sex | |
| Female | 8 |
| Male | 37 |
| ECOG performance status | |
| 0 | 7 |
| 1 | 33 |
| 2 | 5 |
| Median | 1 |
| Primary Tumor type | |
| Prostate cancer | 35 |
| Adrenocortical carcinoma | 3 |
| Colon cancer | 2 |
| Bladder cancer | 1 |
| Renal cell cancer | 1 |
| Cholangiocarcinoma | 1 |
| Small intestinal cancer | 1 |
| Melanoma | 1 |
| Median number of prior therapy | 4 |
| Selected prior therapy | |
| Docetaxel | 24 |
| Estramustine | 5 |
| Hormonal therapy | 35 |
| Irinotecan | 3 |
| Ketoconazole | 21 |
| Mitoxantrone | 4 |
| Platinum agents | 5 |
| Thalidomide | 8 |
| Response | |
| Stable disease | 12 |
| Progressive disease | 32 |
| Not assessed | 1 |
Exposure to Study Medication
Fifteen patients received continuous daily dosing, every 28 days at the following dose levels: 5 mg (n=3), 10 mg (n=6), and 20 mg (n=6); and 30 patients received intermittent dosing with 21 days on-drug and 7 days off-drug, every 28 days, at the following dose levels: 15 mg (n=3), 20 mg (n=4), 25 mg (n=6), 30 mg (n=8), 35 mg (n=6), and 40 mg (n=3). All 45 patients received at least the PK dose (cycle 1 day 1). Patients were on-study for a median of 2 months (range 0.9 – 11).
Safety
Of the 15 patients who were treated continuously ranging from 5 mg to 20 mg daily dose, two patients from the 20 mg dose developed DLTs. One patient experienced grade 3 hypotension associated with diarrhea, nausea and vomiting, presumably from a viral gastroenteritis. Although he developed hypotension, this was transient in nature and responded to fluid therapy. Another patient developed grade 3 deep venous thrombosis (DVT), but this patient had a pre-existing history of a DVT and pelvic adenopathy secondary to prostate cancer. The MTD for lenalidomide in this trial was therefore determined to be 10 mg per day dose. Despite the documented events as DLTs, the aforementioned patients’ adverse events history was considered possibly not drug-related. Furthermore, simultaneous lenalidomide trials being conducted at the time suggested better tolerance with intermittent dosing of 21 days on, 7 days off (17, 18). Therefore, the protocol was amended to adopt an intermittent dosing schema as depicted in Table 1 and a less rapid dose-escalation scheme.
All patients who received any therapy were evaluable for toxicity. Table 3 lists the worst grade of toxicity per patient for grades 1 and 2 events occurring > 10 % of cases and any grade 3 or 4 events for the continuous daily dosing (n=15). Most common grade 1 events were nausea, diarrhea, neuropathy and myalgia. No grade 4 events were noted for the continuous dosing group while more grade 3 and observance of grade 4 neutropenia occurred in the intermittent dosing arm. Dose reductions occurred in 4 out of 15 patients (27%) receiving continuous doses and 7 out of 30 patients (23%) receiving intermittent doses. Of the 4 patients in the continuous dosing, two had one dose level reduction while two patients had doses held. Of the 7 patients in the intermittent dosing arm, one patient had a reduction of 4 dose levels, two patients had a 2 dose level reduction and 4 patients had one dose level reduction. Majority of patients tolerated the dosing schedule without need for further dose reduction. Although one patient who had been on-study the longest (11 months) commenced with 35 mg intermittent dosing schedule, had to be dose-reduced twice because of fatigue and neutropenia.
Table 3.
Treatment-related adverse events occurring > 10% for continuous dosing (n=15)
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Blood/Bone Marrow | ||||
| Anemia | 1 | |||
| Neutropenia | 1 | 3 | 2 | |
| Cardiovascular | ||||
| Hypotension | 2 | 1 | ||
| Thrombosis | 2 | |||
| Constitutional symptoms | ||||
| Fatigue | 4 | 1 | ||
| Dermatology/skin | ||||
| Rash/desquamation | 4 | |||
| Pruritus | 5 | 1 | ||
| Gastrointestinal | ||||
| Constipation | 2 | 1 | ||
| Diarrhea | 4 | |||
| Flatulence | 3 | |||
| Nausea | 4 | |||
| Metabolic/Laboratory | ||||
| SGPT | 2 | |||
| Neurology | ||||
| Dizziness | 3 | 1 | ||
| Neuropathic Sensory | 4 | |||
| Pulmonary | ||||
| Pleural effusion | 2 |
Response to Therapy
All patients who received any drug were evaluable for response except for one patient who was withdrawn at his request prior to re-staging evaluation. Stable disease (SD) was documented in twelve patients as best response, with a median of 5 months on-study (range 4 – 11). Of the 12 patients with SD, 9 patients had prostate cancer, 1 patient had cholangiocarcinoma, 1 with renal cell carcinoma, and another with adrenocortical cancer. No complete or partial response was noted, and the rest had progressive disease.
Pharmacokinetics
Lenalidomide PK was evaluated in 45 patients with refractory metastatic cancers after the first dose on cycle 1. One patient at dose level of 5 mg was excluded from the noncompartmental analysis (NCA) because of sparse sampling. A patient at dose level of 20 mg received incorrect dose (100 mg) and thus was not included in calculation of NCA parameters for this dose group. However, these two patients were included for following PopPK analysis. A number of time points per patient varied between 3 and 11. A total 292 observations were used to develop PopPK models of lenalidomide.
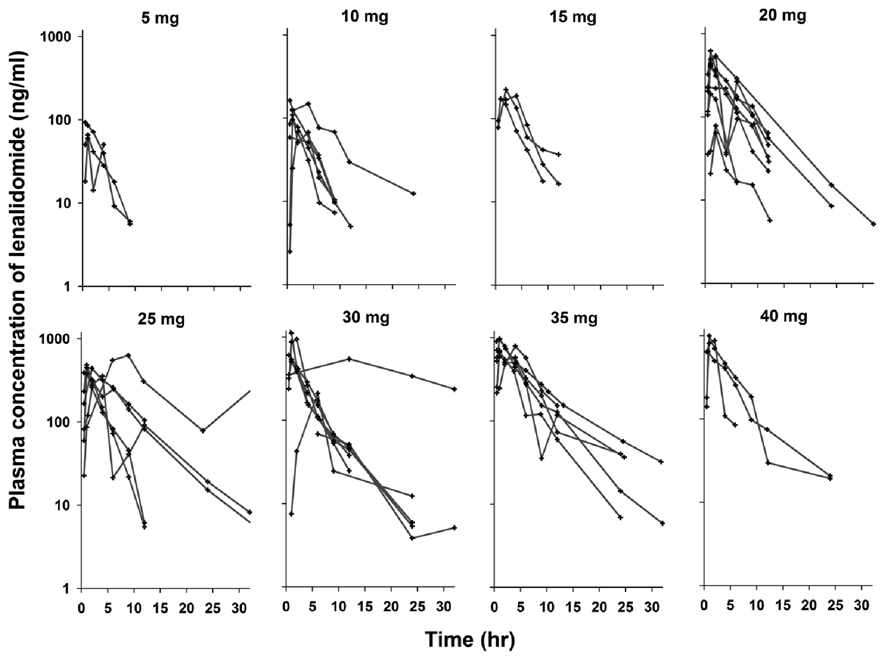
Plasma concentrations of lenalidomide following a single oral administration at various dosages are shown in Figure 1. After oral administration, lenalidomide was rapidly absorbed, peaked at around 1 hour, and decayed in a monoexponential manner. The NCA parameters of lenalidomide are summarized in Table 5. A systemic exposure of lenalidomide (e.g., AUC and Cmax) appeared to increase more than proportionally with increasing dose but it appeared to be attributed to two patients with exceptionally high AUC values at dose groups of 25 mg and 30 mg. However, the apparent volume of distribution (V/F) and terminal half-life (T1/2) were comparable across all doses. Similar to other previous studies showing a T1/2 of 3–4 hours (11, 19), the mean T1/2 was 3.9 hours. The amount of unchanged lenalidomide excreted into urine for 24 hours was about 45–55% of dose, which is slightly lower than two thirds reported in healthy volunteers (20), and was independent of dose over the range studied. The CL/F_renal estimated from 9 patients ranged 54 – 72 mL/min, accounting for about 50% of the total clearance.
Figure 1.
Observed concentrations of lenalidomide following oral administration at various dosages in patients. Each line indicates individual patients.
Table 5.
Pharmacokinetic parameters of lenalidomide obtained by noncompartmental analysis
| Dose (mg) |
n | Tmax (hr) | Cmax (ng/ml) | AUC (ng·hr/ml) | CL/F (mL/min) | V/F (L) | T1/2 (hr) | Urine excretion, %dose* |
|---|---|---|---|---|---|---|---|---|
| 5 | 2 | 0.75 (0.5, 1) | 80 (66, 93) | 302 (257, 346) | 282 (240, 324) | 63.7 (59.7, 67.7) | 2.7 (2.1, 3.3) | - |
| 10 | 6 | 1 (0.5– 4) | 107 (59–163) | 571 (256–1415) | 388 (118–650) | 108.9 (48.7–236) | 3.1 (2.4–4.2) | - |
| 15 | 3 | 2 (1–4) | 195 (174–224) | 1090 (712–1391) | 248 (180–352) | 76.6 (57.4–99) | 2.9 (1.9–2.9) | - |
| 20 | 9 | 1 (0.5–6.1) | 343 (64–621) | 1901 (239–4093) | 351 (82–1395) | 90.4 (32.1–346) | 3.1 (1.9–7.8) | - |
| 25 | 6 | 1.5 (0.5–8.9) | 451 (311–631) | 3820 (1081–11363) | 192 (37–385) | 57.9 (39.4–91.1) | 5.3 (1.6–15.9) | - |
| 30 | 8 | 1.5 (0.5–6) | 593 (69–1315) | 4040 (844–15445) | 224 (32–593) | 95.2 (30.3–268) | 4.0 (3.2–14.2) | 47.0 (36.3–62.0) |
| 35 | 6 | 1 (0.5–4) | 782 (594–969) | 5309 (3172–7062) | 117 (83–183) | 70.6 (33.0–95.3) | 6.7 (3.7–14.5) | 54.6 (48.3–63.1) |
| 40 | 3 | 1 (0.75–2) | 842 (648–1002) | 3926 (3281–4656) | 173 (143–203) | 62.1 (21.7–92.7) | 4.1 (1.8–6.2) | 44.9 (43.8, 46.0) |
Values are presented as mean with the range in the bracket
Number of subjects used in calculation of urine excretion: 30 mg (n=3), 35 mg (n=4), and 40 mg (n=2)
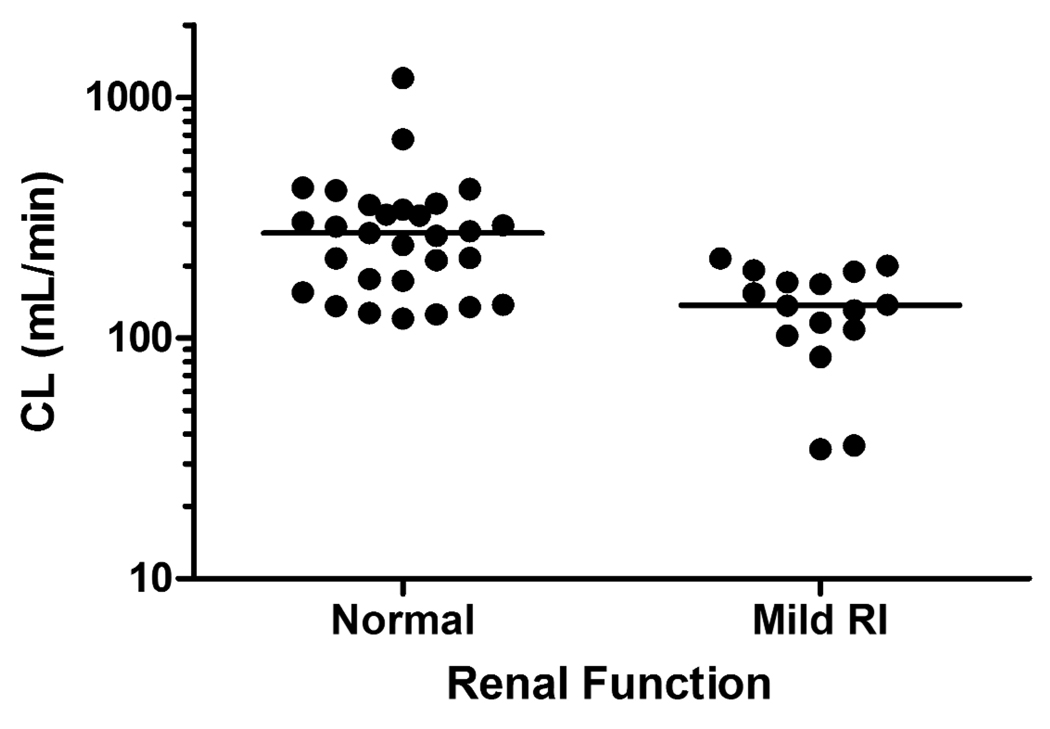
The PopPK analysis showed that the disposition of lenalidomide was adequately described by a one-compartment model with a linear elimination as has previously been observed (19, 21). Given that the results from the noncompartmental assessment a nonlinear model in a form of the Michaelis-Menten equation (e.g., Vmax, Km) instead of CL was also tested but the improvement of the overall fitting was minimal with a reduction of the model objective function value by 10 points. Based on the exploration of covariate-parameter relationships, CLCr was found to be important to clearance of lenalidomide. Renal functional status of patients were defined by use of either group 0, with normal CLCr > 80 mL/min (n=29), and 1 for mild renal impairment (RI) with 50 ≤ CLCr ≤ 80 mL/min (16). One patient with CLCr of 48 mL/min was included in the mild RI group. As shown in Figure 2, clearance in patients with mild RI (50 ≤ CLCr ≤ 80 mL/min) was significantly lower compared to those with normal renal function (CLCr > 80 mL/min). Therefore, clearance was estimated separately for groups of patients with respect to renal function, normal or mild RI, thereby resulting in reduction of objective function value of 16, which meets the pre-defined criteria. Although an attempt was made to model the effect of CLCr on CL in terms of a continuous variable, it appeared that CL did not increase constantly with increasing CLCr, but rather similar among individuals within the same group and thus the former model seemed to lead to better precision of parameter estimates.
Figure 2.
Comparison of lenalidomide clearance in patients with normal and mild impaired renal functions. The lines represent median value of each group
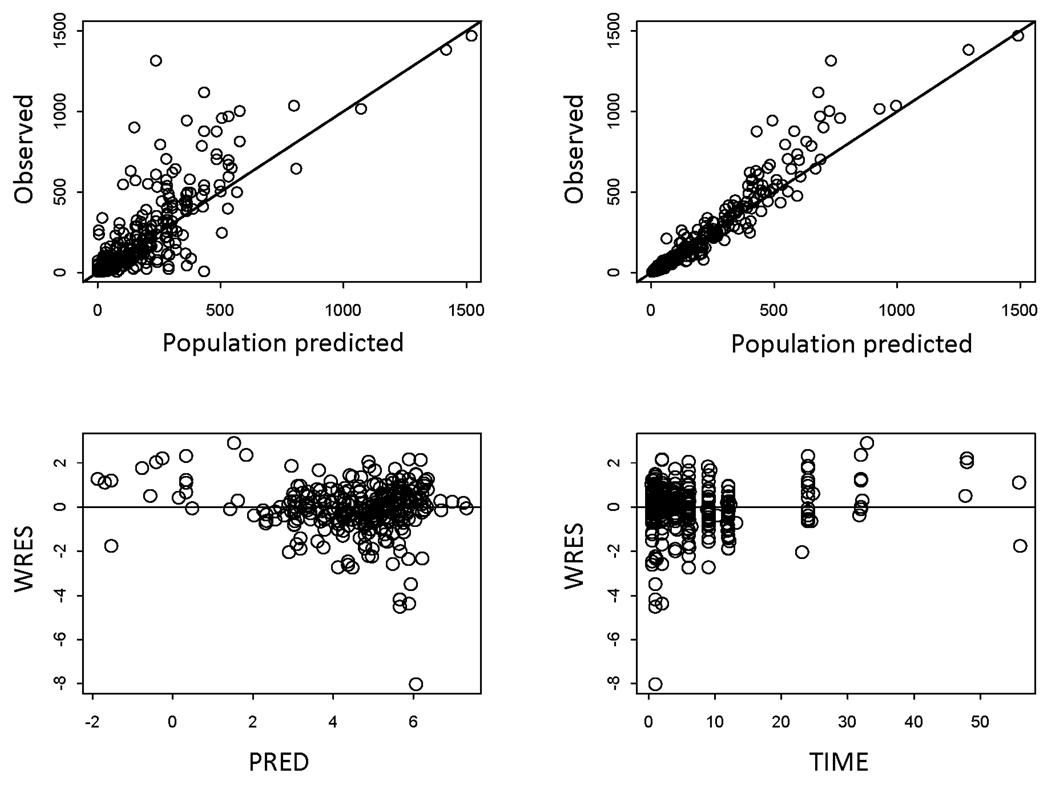
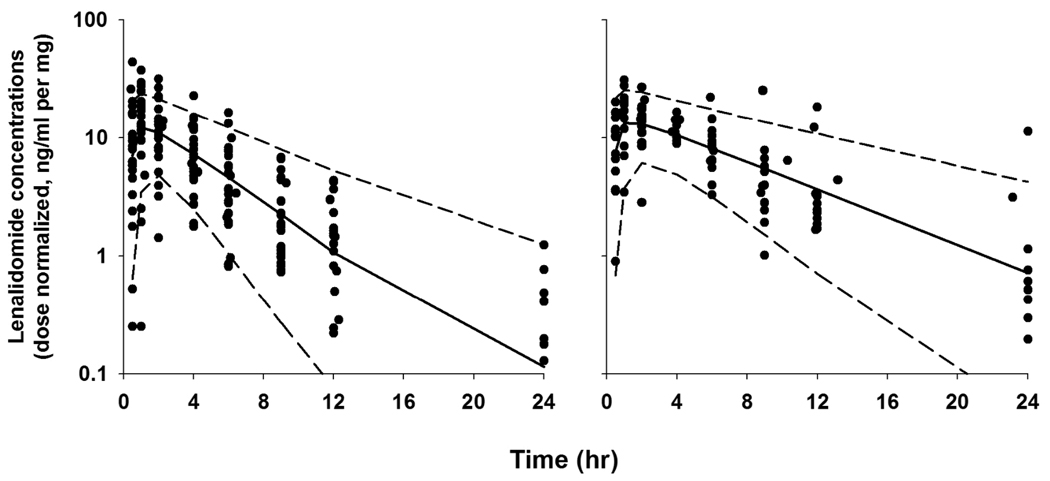
Table 6 summarizes the PK parameters estimated from the final PK model and 95% CI from the bootstrap analysis. The population mean clearance was 243 mL/min for patients with normal renal function whereas it reduced to approximately 50% in those with mild RI. This was consistent with the finding that renally impaired subjects had twice lenalidomide exposure compared to patients with normal renal function (dose-normalized AUC: 167 vs. 75 ng·h/ml per mg). Inclusion of CLCr reduced the interpatient variability on total lenalidomide clearance from 70.6% to 59.9%. The between-patient variability for V/F was moderate (33%). The addition of absorption lag time (Tlag = 0.37 hr) was needed and improved the overall fittings. Absorption patterns were highly variable between patients as reflected in BSV of ka (146%) but no demographic characteristics were found to explain such variability. Typical diagnostic plots for the final model are presented in Figure 3. In some patients, Cmax was underestimated especially at high doses with a simple first-order absorption model with a Tlag, but exploration of other absorption models was limited due to lack of observations at early absorption phases. The performance of the final model was evaluated based on a visual predictive check (Figure 4). The observed data and model-predicted data were compared with respect to renal function after dose normalization due to a small number of patients in each dose group. The predictive performance of the final model was satisfactory as the central tendency and prediction intervals from simulations adequately reflected the observations.
Table 6.
Pharmacokinetic parameters estimated from the final population PK model using NONMEM.
| Parameter | Population Mean (95% CIa) |
Bootstrap Median (95% CIb) |
|---|---|---|
|
CL/F (ml/min) Normal Mild RI |
243 (193 – 297) 136 (97 – 175) |
243 (202 – 297) 136 (103 – 178) |
| V/F (L) | 57.8 (49.4 – 66.2) | 57.8 (51.3 – 66.7) |
| ka (1/hr) | 4.60 (0.66 – 8.54) | 4.67 (2.52 – 9.19) |
| Tlag (hr) | 0.37 (0.30 – 0.43) | 0.37 (0.25 – 0.43) |
| BSV of CL/F (CV%) | 59.9 (39 – 75.0) | 57.5 (43.6 – 74.1) |
| BSV of V/F (CV%) | 33.0 (1.0 – 48.0) | 31.5 (14.5 – 52.4) |
| BSV of ka (CV%) | 146 (95 – 184) | 143 (92 – 197) |
| BSV of Tlag (CV%) | 30.0 (2.2 – 47.9) | 29.1 (4.5 – 65.1) |
| Corr of CL/F and V/F | 0.81 (0.35 – 1.0) | 0.83 (0.53 – 1.0) |
| Residual error (CV%) | 34.0 (26.9 – 40.0) | 33.8 (27.4 – 40.9) |
CI, confidence interval; RI, renal impairment; BSV, between-subject variability; CV, coefficient of variation; Corr, correlation between individual estimates.
(Estimate) ± 1.96×(Standard error of the estimate).
The 2.5th and 97.5th values of the ranked bootstrap parameter estimate.
Figure 3.
Top panel: Plots of population and individual model predicted concentrations of lenalidomide against the observed concentrations. Bottom panel: Weighted residuals versus population predicted concentrations and weighted residuals versus time from the final model. The open circles are the individual data.
Figure 4.
Visual predictive check for the final pharmacokinetic model. Symbols are observed plasma concentrations of lenalidomide from patients with normal renal function (left panel) and mild RI (right panel). The solid line and broken lines indicate the median and 90% prediction intervals from model-derived simulations. The observations and model-predictions were normalized relative to their dose.
Molecular markers
Serum cytokine tests which were performed to determine clinical concentrations of IFN-γ, IL-2, IL-4, IL-10, IL-12p70, IL-13, and TNF-α indicated that the concentrations were mostly below the limit of quantification, and hence not evaluable.
Discussion
Lenalidomide is a lead compound of the IMiDs™ immunomodulatory drugs. It exhibits both immunomodulating and antiangiogenic properties in various in vitro and in vivo assays (8, 22, 23). Clinically, it has been shown to be well tolerated and effective in a variety of cancers. The FDA has approved oral doses of 5 and 10 mg/day for treatment of low risk 5q- MDS and 25 mg/ day for refractory multiple myeloma. Other phase I/II studies have also recently shown efficacy in other tumor types, including renal cell carcinoma and melanoma (24, 25). Despite lack of clear activity, it has shown overall tolerability and safety in primary gliomas (26) in a heavily pre-treated population.
Thalidomide, its parent compound, has been shown to have modest efficacy in patients with prostate cancer (3, 5). It is conceivable that lenalidomide would exhibit similar potency, and perhaps better tolerability in this patient population. Since the approved doses of lenalidomide has been in two hematologic malignancies, dosing information and toxicity may not be particularly relevant in patients with refractory solid tumors, with most patients presenting with adequate bone marrow reserve despite advanced stages of disease. Furthermore, in vitro studies using lenalidomide suggests that tumor growth effects were observed at concentrations higher than what is achieved in the clinics, although good immunomodulatory effects have been observed at similar clinical concentrations as has been indicated by activity of lenalidomide in patients with multiple myeloma and MDS (27, 28). These may partly explain why angiogenic effects of lenalidomide monotherapy have not yet been fully realized in solid tumors, since these are considered highly angiogenic. Therefore, the results of this phase I single-center, open-label trial provides information necessary to proceed with further phase Ib or phase II testing in patients with solid tumors. The initial continuous dosing was amended after observation of two grade 3 non-hematologic adverse events. Although these may not have been true DLTs due to lenalidomide, protocol mandated these to be documented DLTs. Simultaneous phase I studies at the time have explored feasibility of intermittent dosing, allowing for adoption of an intermittent dosing scheme which was well tolerated by most patients.
Following oral administration, lenalidomide was rapidly absorbed and its absorption rate was highly variable between patients. It exhibits different PK characteristics from thalidomide which is depicted by a flip-flop kinetics due to solubility/dissolution-rate limited slow absorption (29). The disposition of lenalidomide was characterized by a monoexponential linear PK over a wide range of dosages (5 – 40 mg). Majority of lenalidomide is known to be renally eliminated unchanged (21). As consistent with the previous findings (21, 30), clearance of lenalidomide was significantly affected by renal function defined by CLCr. In this study, clearance for patients with mild RI was about 50% of that for individuals with normal renal function, resulting in greater lenalidomide exposure in the renally impaired subjects. Similarly, it has been reported that in patients with multiple myeloma lenalidomide exposure in those with mild RI is 56% greater than those with normal function (20). Lenalidomide PK was not influenced by body weight or BSA. The lack of a clinically relevant effect of size measures on lenalidomide PK supports the use of flat dosing regimen rather than BSA-adjusted dosing strategy.
Although clinical efficacy was not the primary endpoint of the study, stable disease was documented in about a third of the patients, the majority of who had prostate cancer. This study demonstrates the feasibility and favorable toxicity profile in this heavily pre-treated patient population, even with administration of multiple doses up to 40 mg/day. However, dose escalations beyond 40 mg/day was not performed based on limited clinical benefits observed on re-staging scans, with increased toxicity frequency at higher dose levels. Furthermore, based on existing data, dose escalations beyond 40 mg have been associated with life-threatening neutropenia (26). To date, this is the first clinical study exploring the feasibility of multiple and higher dosing schedules, especially in prostate cancer. Other tumor types exhibiting stable disease included one patient each with cholangiocarcinoma, ACC, and renal cell cancer (RCC). The patient who had been on-study the longest (duration of 11 months, 14 cycles) was the patient with RCC, who despite undergoing dose reduction twice (due to fatigue then neutropenia), had stable metastatic lesions involving the renal, pancreatic, and lung regions.
We attempted to measure potential biomarkers demonstrating angiogenesis or immunomodulatory effects of lenalidomide. However, as with other studies using thalidomide, we were not able to demonstrate any changes in these markers since they were below the quantifiable limits of detection.
In summary, lenalidomide is fairly well tolerated in refractory solid tumors and shows potential activity in a select group of cancers, most notably, prostate cancer. Further phase II trials using this agent are warranted in refractory solid tumors to better define efficacy data.
Table 4.
Treatment-related adverse events for intermittent dose (n=35)
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Blood/Bone Marrow | ||||
| Anemia | 1 | 1 | 1 | |
| Leukopenia | 1 | 1 | ||
| Neutropenia | 1 | 2 | 2 | 3 |
| Hemolysis | 1 | 1 | ||
| Thrombocytopenia | 3 | 2 | ||
| Cardiovascular | ||||
| Supraventricular arrythmia | 1 | |||
| Edema | 4 | |||
| Constitutional symptoms | ||||
| Fatigue | 14 | |||
| Fever | 4 | |||
| Insomnia | 5 | |||
| Dermatology/skin | ||||
| Rash/desquamation | 7 | 3 | ||
| Pruritus | 4 | 1 | ||
| Gastrointestinal | ||||
| Anorexia | 3 | 1 | ||
| Constipation | 4 | 5 | ||
| Diarrhea | 5 | 1 | ||
| Dysgeusia | 3 | |||
| Nausea | 9 | 1 | ||
| Infection | 1 | 2 | ||
| Neurology | ||||
| Dizziness | 8 | |||
| Pain | ||||
| Myalgia | 5 | 1 | ||
| Pulmonary | ||||
| Pleural effusion | 2 | 1 |
Acknowledgments
We would like to thank the nursing staff of NCI, data manager (Ms. Cynthia Graves), the research nurses (Lea Latham, R.N.), and the fellows of the Medical Oncology Branch at NCI for their care of our patients. Most importantly we acknowledge the participation of all patients with cancer who enroll in investigational trials.
This work was supported by the Intramural Research Program of the National Cancer Institute. This is a US Government work. There are no restrictions on its use. The views expressed within this paper do not necessarily reflect those of the US Government.
Footnotes
Authors’ Disclosure of Potential Conflicts of Interest
No disclosures
References
- 1.Folkman J. The vascularization of tumors. Sci Am. 1976;234(5):58–64. 70–73. doi: 10.1038/scientificamerican0576-58. [DOI] [PubMed] [Google Scholar]
- 2.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91(9):4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004;22(13):2532–2539. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 4.Fine HA, Figg WD, Jaeckle K, et al. Phase II trial of the antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas. J Clin Oncol. 2000;18(4):708–715. doi: 10.1200/JCO.2000.18.4.708. [DOI] [PubMed] [Google Scholar]
- 5.Figg WD, Dahut W, Duray P, et al. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin Cancer Res. 2001;7(7):1888–1893. [PubMed] [Google Scholar]
- 6.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 7.Tohnya TM, Hwang K, Lepper ER, et al. Determination of CC-5013, an analogue of thalidomide, in human plasma by liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;811(2):135–141. doi: 10.1016/j.jchromb.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Dredge K, Marriott JB, Macdonald CD, et al. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer. 2002;87(10):1166–1172. doi: 10.1038/sj.bjc.6600607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corral LG, Muller GW, Moreira AL, et al. Selection of novel analogs of thalidomide with enhanced tumor necrosis factor alpha inhibitory activity. Mol Med. 1996;2(4):506–515. [PMC free article] [PubMed] [Google Scholar]
- 10.Muller GW, Chen R, Huang SY, et al. Amino-substituted thalidomide analogs: potent inhibitors of TNF-alpha production. Bioorg Med Chem Lett. 1999;9(11):1625–1630. doi: 10.1016/s0960-894x(99)00250-4. [DOI] [PubMed] [Google Scholar]
- 11.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100(9):3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 12.Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol. 2008;26(9):1544–1552. doi: 10.1200/JCO.2007.14.5367. [DOI] [PubMed] [Google Scholar]
- 13.Teo SK, Sabourin PJ, O'Brien K, Kook KA, Thomas SD. Metabolism of thalidomide in human microsomes, cloned human cytochrome P-450 isozymes, and Hansen's disease patients. Journal of biochemical and molecular toxicology. 2000;14(3):140–147. doi: 10.1002/(sici)1099-0461(2000)14:3<140::aid-jbt3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Celgene Corporation, Inc. CC5013 Investigator's Brochure. 2000. [Google Scholar]
- 15.NONMEM users guides. Ellicott City, Maryland: Icon Development Solutions; 1989–2006. [Google Scholar]
- 16.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 17.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 18.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108(10):3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine HA, Kim L, Albert PS, et al. A Phase I Trial of Lenalidomide in Patients with Recurrent Primary Central Nervous System Tumors. Clin Cancer Res. 2007;13(23):7101–7106. doi: 10.1158/1078-0432.CCR-07-1546. [DOI] [PubMed] [Google Scholar]
- 20.Revlimid (lenalidomide) [package insert] Summit, NJ: Celgene Corporation; 2007. [Google Scholar]
- 21.Chen N, Lau H, Kong L, et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol. 2007;47(12):1466–1475. doi: 10.1177/0091270007309563. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 23.Dredge K, Horsfall R, Robinson SP, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69(1–2):56–63. doi: 10.1016/j.mvr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Bartlett JB, Michael A, Clarke IA, et al. Phase I study to determine the safety, tolerability and immunostimulatory activity of thalidomide analogue CC-5013 in patients with metastatic malignant melanoma and other advanced cancers. Br J Cancer. 2004;90(5):955–961. doi: 10.1038/sj.bjc.6601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choueiri TK, Dreicer R, Rini BI, et al. Phase II study of lenalidomide in patients with metastatic renal cell carcinoma. Cancer. 2006;107(11):2609–2616. doi: 10.1002/cncr.22290. [DOI] [PubMed] [Google Scholar]
- 26.Fine HA, Kim L, Albert PS, et al. A phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Clin Cancer Res. 2007;13(23):7101–7106. doi: 10.1158/1078-0432.CCR-07-1546. [DOI] [PubMed] [Google Scholar]
- 27.Barlogie B. Thalidomide and CC-5013 in multiple myeloma: the University of Arkansas experience. Semin Hematol. 2003;40 4 Suppl 4:33–38. doi: 10.1053/j.seminhematol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Wu A, Scheffler MR. Multiple-dose pharmacokinetics and safety of CC-5013 in 15 multiple myeloma patients. J Clin Oncol (Meeting Abstracts) 2004;22(14suppl) 2056-. [Google Scholar]
- 29.Teo SK, Scheffler MR, Kook KA, et al. Thalidomide dose proportionality assessment following single doses to healthy subjects. J Clin Pharmacol. 2001;41(6):662–667. doi: 10.1177/00912700122010555. [DOI] [PubMed] [Google Scholar]
- 30.Knop S, Einsele H, Bargou R, Cosgrove D, List A. Adjusted dose lenalidomide is safe and effective in patients with deletion (5q) myelodysplastic syndrome and severe renal impairment. Leuk Lymphoma. 2008;49(2):346–349. doi: 10.1080/10428190701799027. [DOI] [PubMed] [Google Scholar]