Abstract
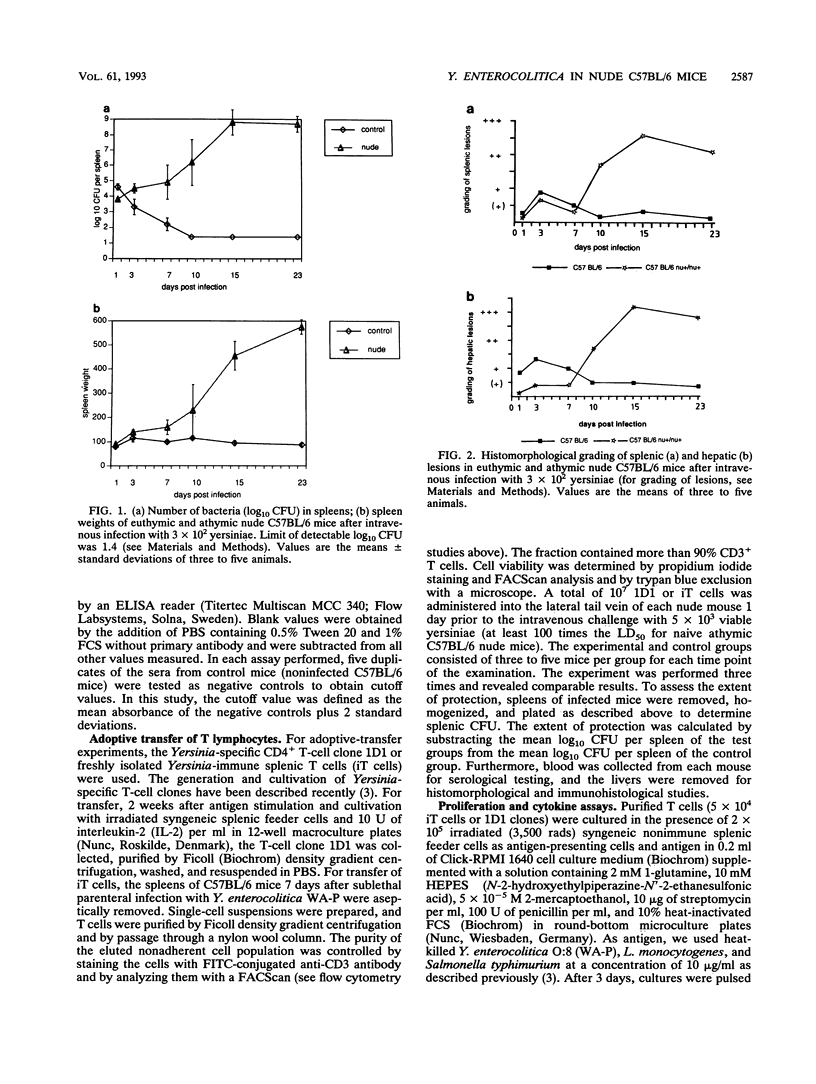
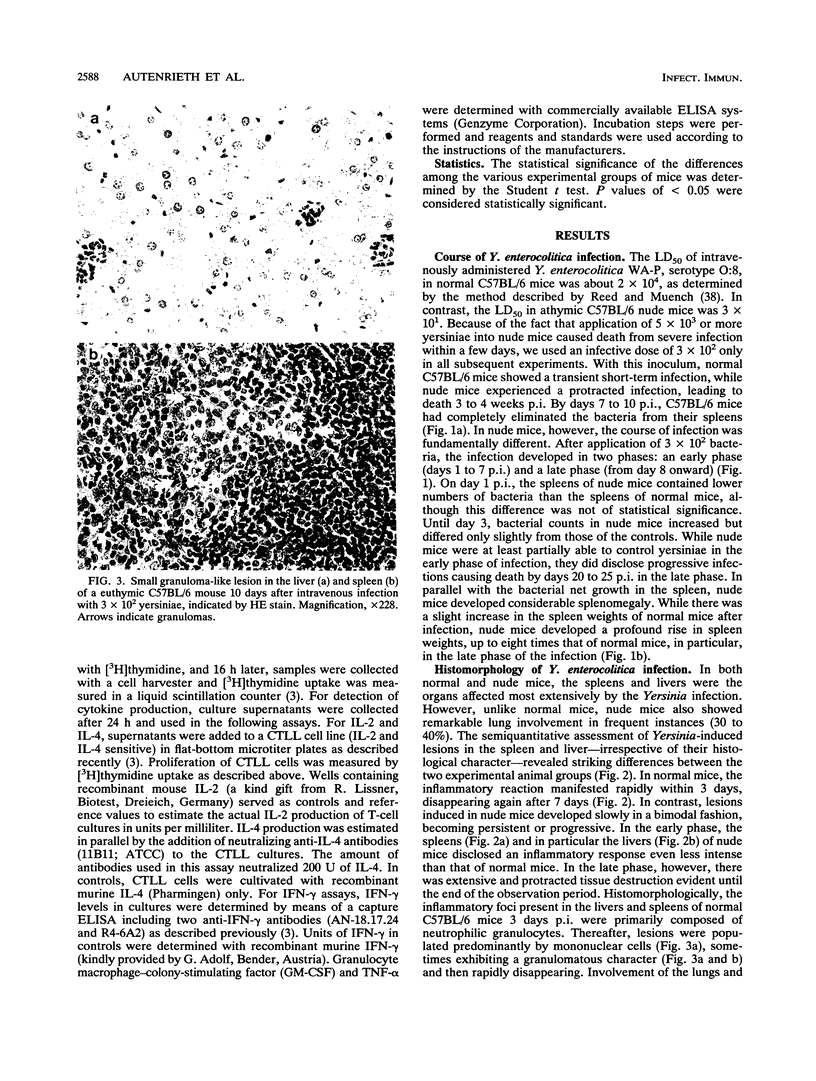
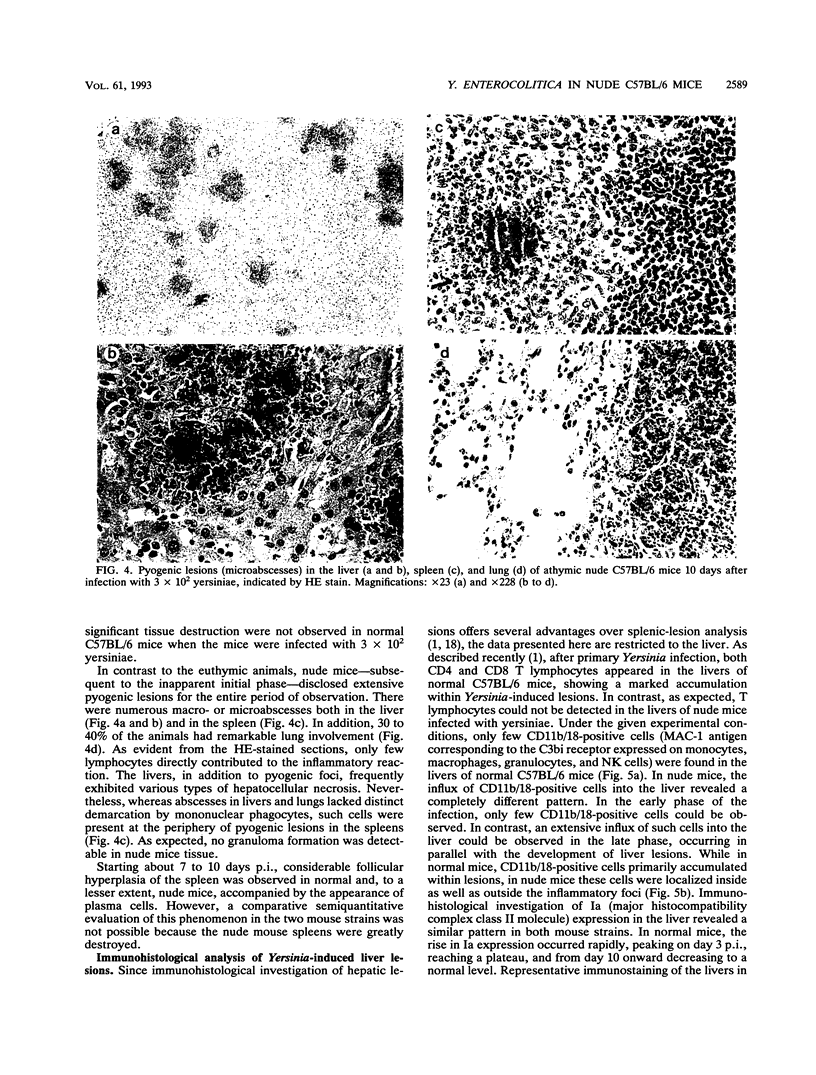
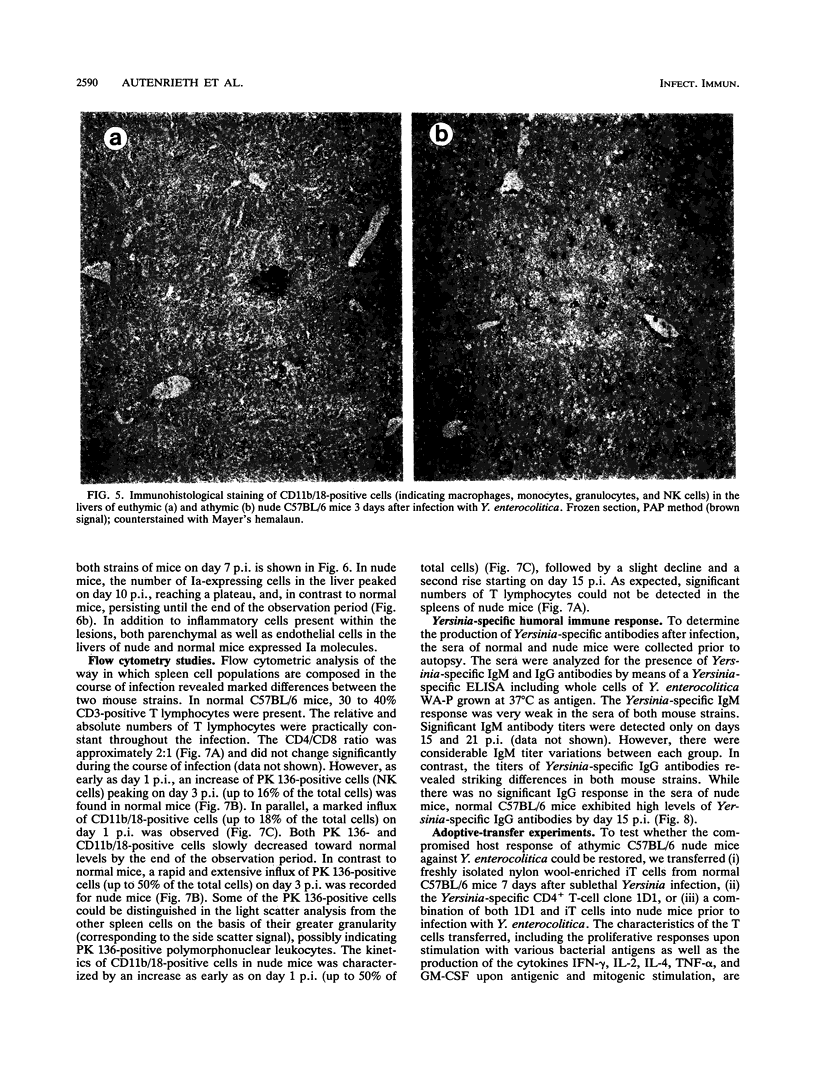
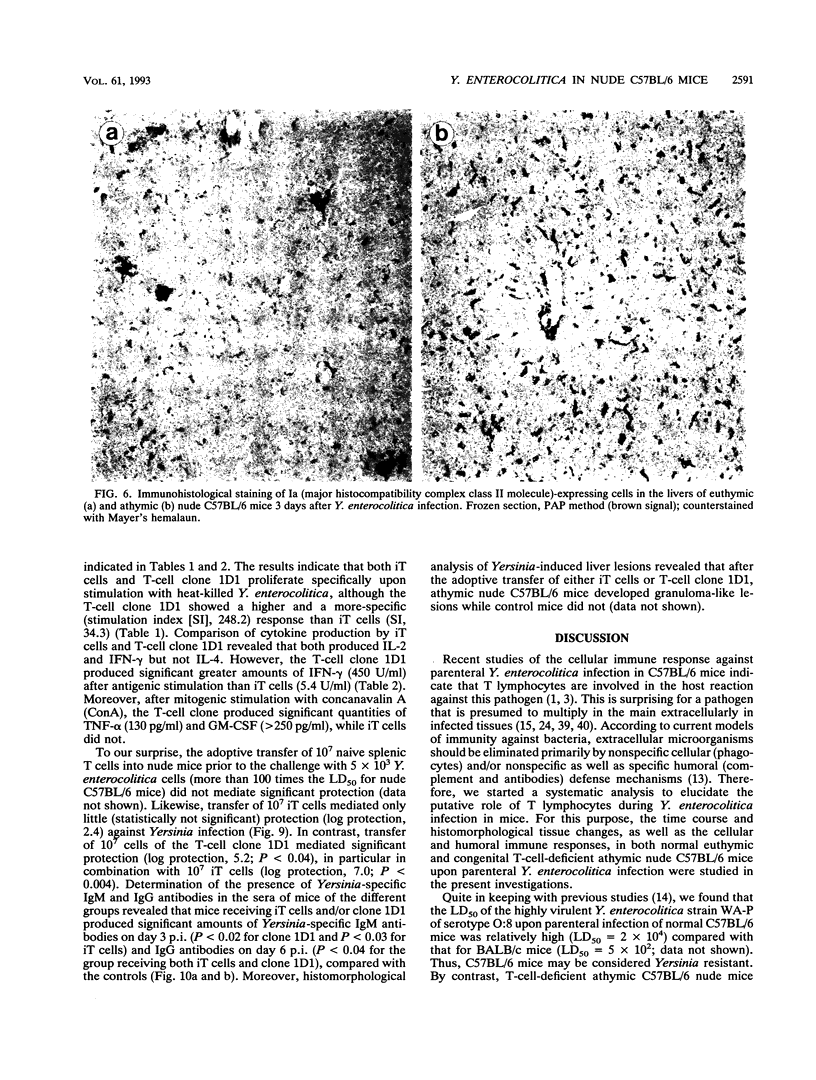
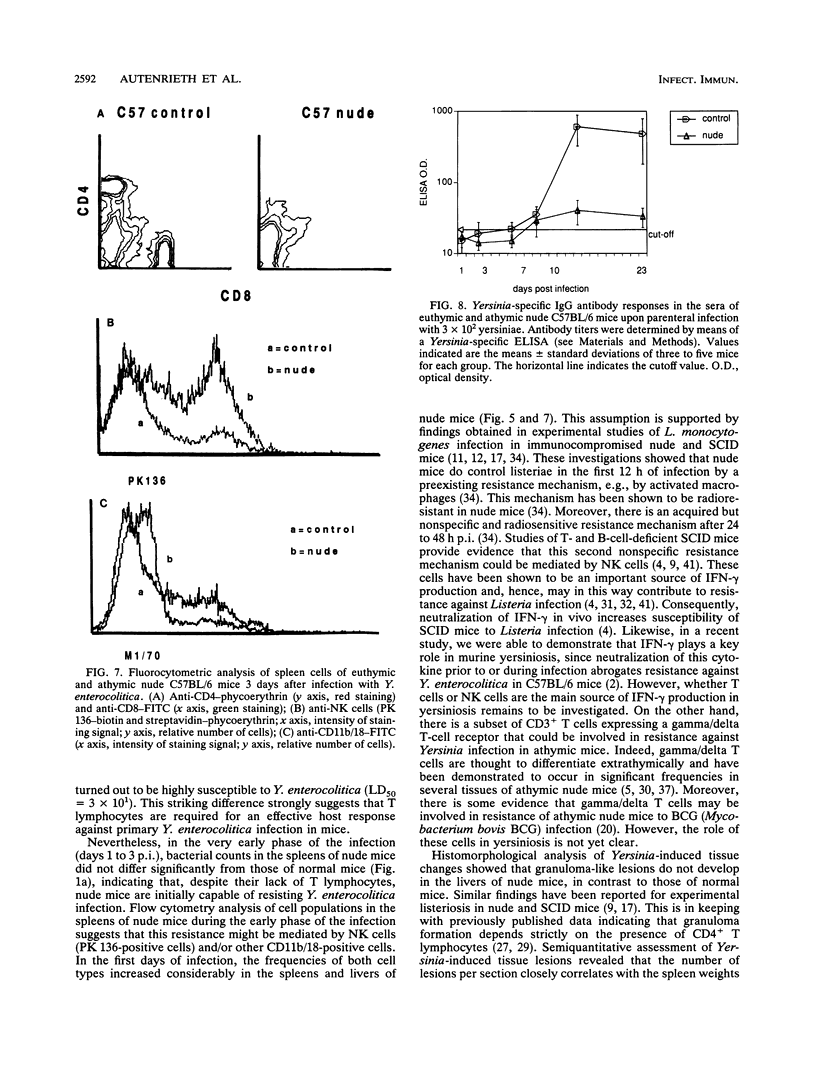
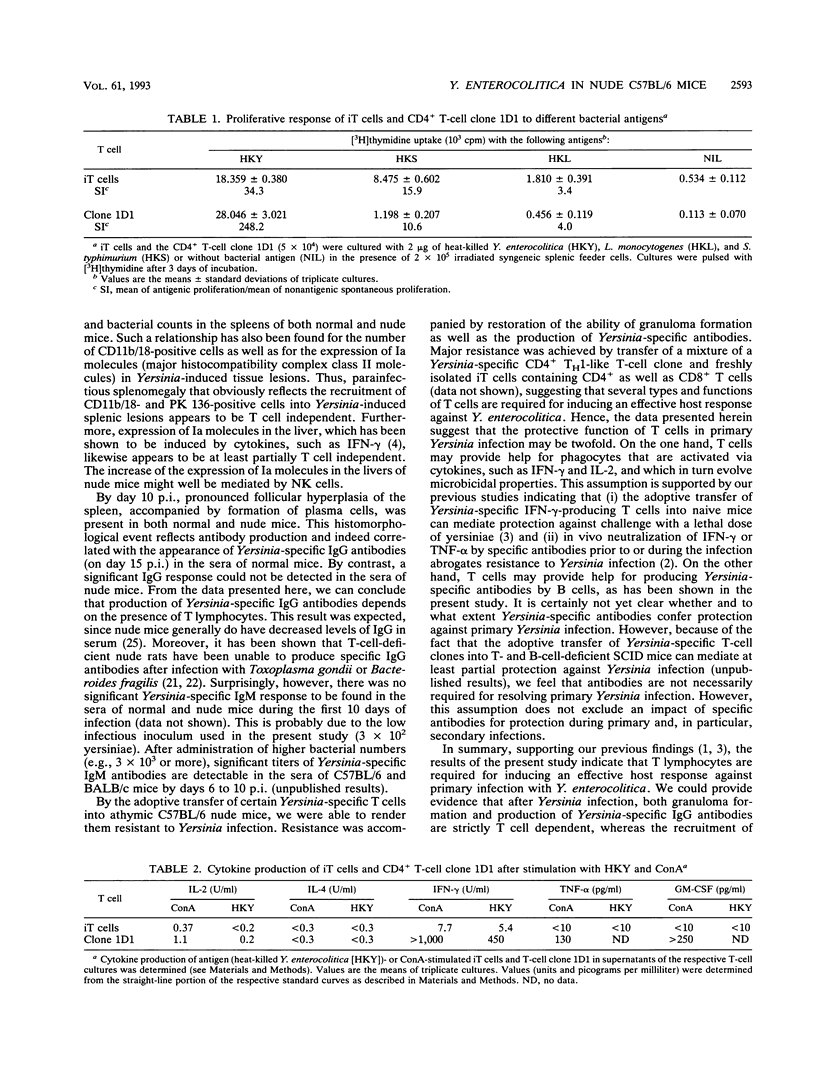
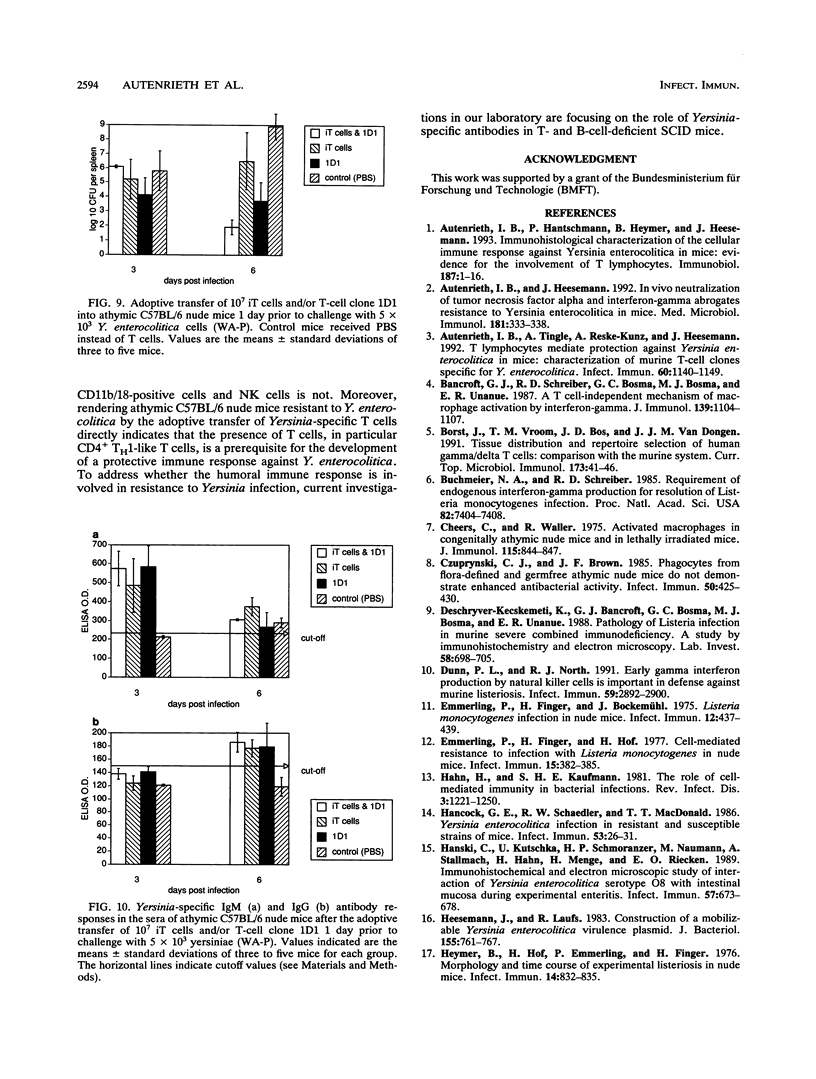
To elucidate the role of T lymphocytes in primary infection with Yersinia enterocolitica, we investigated the elimination rate of this pathogen, the histomorphology of tissue lesions, and the immune responses of athymic T-cell-deficient C57BL/6 nude mice and their euthymic littermates after parenteral infection with Y. enterocolitica of serotype O:8. While a low inoculum of 3 x 10(2) Y. enterocolitica cells (about 0.01 times the median lethal dose for normal C57BL/6 mice) was cleared by normal C57BL/6 mice within 7 to 10 days, athymic nude C57BL/6 mice developed progressive infections after this inoculum, leading to death on days 20 to 25 postinfection (p.i.). While normal C57BL/6 mice experienced short-term transient infections, nude mice exhibited a biphasic, progressive infectious process. Thus, in the early phase (days 1 to 7 p.i.), a rapid influx of CD11b/18-positive cells (Mac-1 antigen) and natural killer cells was evident in the spleens and livers of the nude mice. The late phase (from day 8 p.i. onward) was characterized by a rapid progression of the infection and a further influx of CD11b/18-positive cells into the liver accompanied by an increase in bacterial counts and development of tissue lesions particularly in the liver and spleen. In normal mice, granuloma-like lesions composed of CD11b/18-, CD4-, and CD8-positive cells could be observed. However, granulomata were not found in nude mice. Yersinia-specific immunoglobulin G antibodies appeared on day 15 p.i. in the sera of normal mice, while nude mice failed to develop significant antibody titers. Adoptive transfer of Yersinia-specific T cells into athymic nude mice mediated resistance to Y. enterocolitica infection and restored both the ability of granuloma formation and the production of specific antibodies. In summary, the data presented herein strongly suggest that T lymphocytes play an essential role in the defense of C57BL/6 mice against Y. enterocolitica.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autenrieth I. B., Hantschmann P., Heymer B., Heesemann J. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology. 1993 Jan;187(1-2):1–16. doi: 10.1016/S0171-2985(11)80241-X. [DOI] [PubMed] [Google Scholar]
- Autenrieth I. B., Heesemann J. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med Microbiol Immunol. 1992;181(6):333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- Autenrieth I. B., Tingle A., Reske-Kunz A., Heesemann J. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect Immun. 1992 Mar;60(3):1140–1149. doi: 10.1128/iai.60.3.1140-1149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Borst J., Vroom T. M., Bos J. D., Van Dongen J. J. Tissue distribution and repertoire selection of human gamma delta T cells: comparison with the murine system. Curr Top Microbiol Immunol. 1991;173:41–46. doi: 10.1007/978-3-642-76492-9_7. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Phagocytes from flora-defined and germfree athymic nude mice do not demonstrate enhanced antibacterial activity. Infect Immun. 1985 Nov;50(2):425–430. doi: 10.1128/iai.50.2.425-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschryver-Kecskemeti K., Bancroft G. J., Bosma G. C., Bosma M. J., Unanue E. R. Pathology of Listeria infection in murine severe combined immunodeficiency. A study by immunohistochemistry and electron microscopy. Lab Invest. 1988 Jun;58(6):698–705. [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Hof H. Cell-mediated resistance to infection with Listeria monocytogenes in nude mice. Infect Immun. 1977 Feb;15(2):382–385. doi: 10.1128/iai.15.2.382-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hancock G. E., Schaedler R. W., MacDonald T. T. Yersinia enterocolitica infection in resistant and susceptible strains of mice. Infect Immun. 1986 Jul;53(1):26–31. doi: 10.1128/iai.53.1.26-31.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski C., Kutschka U., Schmoranzer H. P., Naumann M., Stallmach A., Hahn H., Menge H., Riecken E. O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989 Mar;57(3):673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Laufs R. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J Bacteriol. 1983 Aug;155(2):761–767. doi: 10.1128/jb.155.2.761-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymer B., Hof H., Emmerling P., Finger H. Morphology and time course of experimental listeriosis in nude mice. Infect Immun. 1976 Sep;14(3):832–835. doi: 10.1128/iai.14.3.832-835.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A. A., North R. J. Evidence for an alpha/beta T cell-independent mechanism of resistance to mycobacteria. Bacillus-Calmette-Guerin causes progressive infection in severe combined immunodeficient mice, but not in nude mice or in mice depleted of CD4+ and CD8+ T cells. J Exp Med. 1992 Aug 1;176(2):581–586. doi: 10.1084/jem.176.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. L. SCID mouse models of acute and relapsing chronic Toxoplasma gondii infections. Infect Immun. 1992 Sep;60(9):3719–3724. doi: 10.1128/iai.60.9.3719-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Leary R. M., Ward D. C., Harmon C. C., Michalek S. M. Humoral response to Porphyromonas (Bacteroides) gingivalis in rats: time course and T-cell dependence. Infect Immun. 1992 Sep;60(9):3579–3585. doi: 10.1128/iai.60.9.3579-3585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono D. H., Ogasawara M., Yu D. T. An animal model to study serum immunoglobulin response to infection by Yersinia enterocolitica. Clin Exp Rheumatol. 1985 Apr-Jun;3(2):117–121. [PubMed] [Google Scholar]
- Lian C. J., Hwang W. S., Pai C. H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987 May;55(5):1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati A. L., Jacobson E. B. Serum immunoglobulin levels in nude mice. Eur J Immunol. 1972 Oct;2(5):473–474. doi: 10.1002/eji.1830020518. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Niedobitek G., Stein H., Hahn H. Acquired resistance to Listeria monocytogenes is mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J Exp Med. 1989 Aug 1;170(2):589–594. doi: 10.1084/jem.170.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E. T cell subsets in granulomatous inflammation and immunity to L. Monocytogenes and B. abortus. Behring Inst Mitt. 1991 Feb;(88):99–111. [PubMed] [Google Scholar]
- Miller R. G., Benveniste P., Reimann J. Preferential development of gamma/delta-bearing T cells in athymic nude bone marrow. Curr Top Microbiol Immunol. 1991;173:29–32. doi: 10.1007/978-3-642-76492-9_5. [DOI] [PubMed] [Google Scholar]
- Nakane A., Numata A., Chen Y., Minagawa T. Endogenous gamma interferon-independent host resistance against Listeria monocytogenes infection in CD4+ T cell- and asialo GM1+ cell-depleted mice. Infect Immun. 1991 Oct;59(10):3439–3445. doi: 10.1128/iai.59.10.3439-3445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Numata A., Minagawa T. Endogenous tumor necrosis factor, interleukin-6, and gamma interferon levels during Listeria monocytogenes infection in mice. Infect Immun. 1992 Feb;60(2):523–528. doi: 10.1128/iai.60.2.523-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Anomalous high native resistance to athymic mice to bacterial pathogens. Infect Immun. 1977 Dec;18(3):636–645. doi: 10.1128/iai.18.3.636-645.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T., Abo T., Seki S., Kobata T., Yagita H., Okumura K., Kumagai K. Predominant appearance of gamma/delta T lymphocytes in the liver of mice after birth. Eur J Immunol. 1991 Jul;21(7):1733–1740. doi: 10.1002/eji.1830210722. [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M., Richard S., Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990 Mar;58(3):841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]







