Abstract
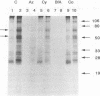
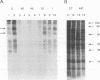
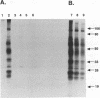
Substantial evidence indicates that molecules released by infectious organisms affect virulence and influence immunity to infection. The characterization of extracellular molecules and their mechanism of release is therefore critical to understanding host-parasite relationships. The cestode parasite Mesocestoides corti is known to release at the larval stage several molecules including the heat shock proteins hsp70 and hsp60. In this report, it is shown that several molecules released by M. corti, including 70- and 60-kDa proteins, are induced by a temperature shift from room temperature to 37 degrees C. Such a shift is comparable to the thermal stress of parasites transmitted from insect vector to vertebrate host. By drug inhibition studies and Western blot (immunoblot) analyses, it is shown that M. corti hsp70 and hsp60 as well as other released molecules are actively exported. The active release of stress proteins by parasites has not been described and may play a critical role in the disease process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Smith I., Grange J. M., Ratliff T. L., Steele J., Rook G. A. The secreted antigens of Mycobacterium tuberculosis and their relationship to those recognized by the available antibodies. J Gen Microbiol. 1988 Feb;134(2):531–538. doi: 10.1099/00221287-134-2-531. [DOI] [PubMed] [Google Scholar]
- Abraham K. M., Teale J. M. Isotype restriction during infection of mice with the cestode Mesocestoides corti: role of immune suppression. J Immunol. 1987 Mar 15;138(6):1699–1704. [PubMed] [Google Scholar]
- Abraham K. M., Teale J. M. The contribution of parasite-specific T cells to isotype restriction in Mesocestoides corti-infected mice. J Immunol. 1987 Oct 15;139(8):2530–2537. [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bentzon M. W., Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991 Apr;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshir F., Flint J. E., Richman S. J., Reese R. T. A 75 kd merozoite surface protein of Plasmodium falciparum which is related to the 70 kd heat-shock proteins. EMBO J. 1987 Feb;6(2):493–499. doi: 10.1002/j.1460-2075.1987.tb04780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman D. D., Mika-Grieve M., Grieve R. B. Circulating excretory-secretory antigen levels and specific antibody responses in mice infected with Toxocara canis. Am J Trop Med Hyg. 1987 Jan;36(1):75–82. doi: 10.4269/ajtmh.1987.36.75. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Joseph M., Torpier G. Effector mechanisms of immunity to schistosomes and their regulation. Immunol Rev. 1982;61:41–66. doi: 10.1111/j.1600-065x.1982.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Estes D. M., Teale J. M. Biochemical and functional analysis of extracellular stress proteins of Mesocestoides corti. J Immunol. 1991 Dec 1;147(11):3926–3934. [PubMed] [Google Scholar]
- Fisch P., Malkovsky M., Kovats S., Sturm E., Braakman E., Klein B. S., Voss S. D., Morrissey L. W., DeMars R., Welch W. J. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science. 1990 Nov 30;250(4985):1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- Fu C., Carter C. E. Detection of a circulating antigen in human schistosomiasis japonica using a monoclonal antibody. Am J Trop Med Hyg. 1990 Apr;42(4):347–351. doi: 10.4269/ajtmh.1990.42.347. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Bailey L. C., Bacon P. A. In vitro responses to a 65-kilodalton mycobacterial protein by synovial T cells from inflammatory arthritis patients. J Immunol. 1989 Oct 15;143(8):2494–2500. [PubMed] [Google Scholar]
- Hunziker W., Whitney J. A., Mellman I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell. 1991 Nov 1;67(3):617–627. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuuchi L., Buckley K. M., Lowe A. W., Kelly R. B. Targeting of secretory vesicles to cytoplasmic domains in AtT-20 and PC-12 cells. J Cell Biol. 1988 Feb;106(2):239–251. doi: 10.1083/jcb.106.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. K., Falkinham J. O., 3rd Superoxide dismutase activity of Mycobacterium avium, M. intracellulare, and M. scrofulaceum. Infect Immun. 1986 Sep;53(3):631–635. doi: 10.1128/iai.53.3.631-635.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerrow J. H., Brindley P., Brown M., Gam A. A., Staunton C., Neva F. A. Strongyloides stercoralis: identification of a protease that facilitates penetration of skin by the infective larvae. Exp Parasitol. 1990 Feb;70(2):134–143. doi: 10.1016/0014-4894(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Michiels T., Cornelis G. Nucleotide sequence and transcription analysis of yop51 from Yersinia enterocolitica W22703. Microb Pathog. 1988 Dec;5(6):449–459. doi: 10.1016/0882-4010(88)90006-x. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Marchalonis J. J., Smith P. M., Nicholas W. L., Warner N. L. Studies on immune responses to larval cestodes in mice. Immunoglobulins associated with the larvae of Mesocestoides corti. Aust J Exp Biol Med Sci. 1977 Apr;55(2):187–211. doi: 10.1038/icb.1977.14. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Tagaya M., Amherdt M., Perrelet A., Donaldson J. G., Lippincott-Schwartz J., Klausner R. D., Rothman J. E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991 Mar 22;64(6):1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Miller E. S., Roberts A. D., Furney S. K., Griffin J. P., Dobos K. M., Chi D., Rivoire B., Brennan P. J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992 Jan 1;148(1):189–196. [PubMed] [Google Scholar]
- Persson R., Ahlström E., Fries E. Differential arrest of secretory protein transport in cultured rat hepatocytes by azide treatment. J Cell Biol. 1988 Dec;107(6 Pt 2):2503–2510. doi: 10.1083/jcb.107.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla B. S. Heat shock proteins in host-parasite interactions. Immunol Today. 1991 Mar;12(3):A38–A41. doi: 10.1016/S0167-5699(05)80011-8. [DOI] [PubMed] [Google Scholar]
- Raptis A., Knipling L., Wolff J. Dissociation of catalytic and invasive activities of Bordetella pertussis adenylate cyclase. Infect Immun. 1989 Jun;57(6):1725–1730. doi: 10.1128/iai.57.6.1725-1730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G. J., Lacey E. Colchicine binding in the free-living nematode Caenorhabditis elegans. Biochim Biophys Acta. 1989 Dec 8;993(2-3):233–239. doi: 10.1016/0304-4165(89)90170-0. [DOI] [PubMed] [Google Scholar]
- Russo P., Kalkkinen N., Sareneva H., Paakkola J., Makarow M. A heat shock gene from Saccharomyces cerevisiae encoding a secretory glycoprotein. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3671–3675. doi: 10.1073/pnas.89.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Giannini S. H., Cantor C. R. Heat shock genes: regulatory role for differentiation in parasitic protozoa. Science. 1985 Jun 21;228(4706):1443–1446. doi: 10.1126/science.4012301. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Nagai S., Terasaka K., Yamada T. Immunogenic protein MPB57 from Mycobacterium bovis BCG: molecular cloning, nucleotide sequence and expression. FEBS Lett. 1988 Nov 21;240(1-2):115–117. doi: 10.1016/0014-5793(88)80350-8. [DOI] [PubMed] [Google Scholar]
- Young R. A. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]