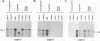Abstract
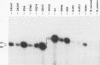
A comparison of the osp operon in 24 Lyme disease isolates, including representatives from each of the three established species, Borrelia burgdorferi, Borrelia garinii, and group VS461, was conducted. Several properties were assessed to determine whether the variability observed in this operon was reflective of the species of the isolate. At the transcriptional level, start site and Northern (RNA) blot analyses were conducted. B. garinii and VS461 group isolates were found to possess an untranslated leader sequence 6 nucleotides longer than that observed in B. burgdorferi isolates. By Northern blot analyses all Lyme disease isolates, except the B. garinii isolate VS102, were found to produce a polycistronic full-length ospAB message. Isolate VS102 produced a truncated message lacking the ospB portion of the transcript. Southern blot analyses suggest that the deletion occurred at the DNA level and was not due to a posttranscriptional event. Analysis of the outer surface proteins by two-dimensional gel electrophoresis demonstrated that the OspB isoelectric points were variable, with the OspB of B. garinii isolates exhibiting a pronounced acidic shift. The reactivity of different isolates to OspA and -B monoclonal antibodies and to a hyperimmune anti-ospAB serum was also variable. The results presented here demonstrate genotypic and phenotypic heterogeneity in the osp operon at both the inter- and intraspecies levels. The results have implications concerning the use of the osp genes or their gene products in the development of a Lyme disease vaccine, as diagnostic markers of Lyme disease, and in subtyping of Lyme disease isolates.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam T., Gassmann G. S., Rasiah C., Göbel U. B. Phenotypic and genotypic analysis of Borrelia burgdorferi isolates from various sources. Infect Immun. 1991 Aug;59(8):2579–2585. doi: 10.1128/iai.59.8.2579-2585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J. C., Assous M., Grimont P. A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Boerlin P., Peter O., Bretz A. G., Postic D., Baranton G., Piffaretti J. C. Population genetic analysis of Borrelia burgdorferi isolates by multilocus enzyme electrophoresis. Infect Immun. 1992 Apr;60(4):1677–1683. doi: 10.1128/iai.60.4.1677-1683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward D. W., Schwan T. G., Garon C. F. Immune capture and detection of Borrelia burgdorferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. J Clin Microbiol. 1991 Jun;29(6):1162–1170. doi: 10.1128/jcm.29.6.1162-1170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiffert H., Ohlenbusch A., Fehling W., Lotter H., Thomssen R. Nucleotide sequence of the ospAB operon of a Borrelia burgdorferi strain expressing OspA but not OspB. Infect Immun. 1992 May;60(5):1864–1868. doi: 10.1128/iai.60.5.1864-1868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice from Lyme borreliosis by oral vaccination with Escherichia coli expressing OspA. J Infect Dis. 1991 Dec;164(6):1224–1227. doi: 10.1093/infdis/164.6.1224. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Persing D. H., Sun X., Kantor F. S., Flavell R. A. Borrelia burgdorferi strain 25015: characterization of outer surface protein A and vaccination against infection. J Immunol. 1992 Apr 1;148(7):2256–2260. [PubMed] [Google Scholar]
- Hughes C. A., Kodner C. B., Johnson R. C. DNA analysis of Borrelia burgdorferi NCH-1, the first northcentral U.S. human Lyme disease isolate. J Clin Microbiol. 1992 Mar;30(3):698–703. doi: 10.1128/jcm.30.3.698-703.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M., Noppa L., Barbour A. G., Bergström S. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origins. Infect Immun. 1992 May;60(5):1845–1853. doi: 10.1128/iai.60.5.1845-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T. L., Halperin J. J., Whitman M. PCR detection of Borrelia burgdorferi DNA in cerebrospinal fluid of Lyme neuroborreliosis patients. Neurology. 1992 Jan;42(1):32–42. doi: 10.1212/wnl.42.1.32. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Pascocello J. A. Antigenic characteristics of Borrelia burgdorferi isolates from ixodid ticks in California. J Clin Microbiol. 1989 Oct;27(10):2344–2349. doi: 10.1128/jcm.27.10.2344-2349.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFebvre R. B., Perng G. C., Johnson R. C. Characterization of Borrelia burgdorferi isolates by restriction endonuclease analysis and DNA hybridization. J Clin Microbiol. 1989 Apr;27(4):636–639. doi: 10.1128/jcm.27.4.636-639.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy D. C., Nauman R. K., Paxton H. Detection of Borrelia burgdorferi using the polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1089–1093. doi: 10.1128/jcm.28.6.1089-1093.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Garon C. F. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J Clin Microbiol. 1992 Nov;30(11):2830–2834. doi: 10.1128/jcm.30.11.2830-2834.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Garon C. F. Phylogenetic analysis of the genus Borrelia: a comparison of North American and European isolates of Borrelia burgdorferi. J Bacteriol. 1992 Jan;174(1):241–244. doi: 10.1128/jb.174.1.241-244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Lubke L., Hauglum W., Garon C. F. Species-specific identification of and distinction between Borrelia burgdorferi genomic groups by using 16S rRNA-directed oligonucleotide probes. J Clin Microbiol. 1992 Mar;30(3):628–632. doi: 10.1128/jcm.30.3.628-632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Samuels D. S., Garon C. F. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993 Feb;175(4):926–932. doi: 10.1128/jb.175.4.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers W., Meis J., Rosa P., Claas E., Nohlmans L., Koopman R., Horrevorts A., Galama J. Amplification of Borrelia burgdorferi DNA in skin biopsies from patients with Lyme disease. J Clin Microbiol. 1991 Nov;29(11):2401–2406. doi: 10.1128/jcm.29.11.2401-2406.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. L., Young K. K., Barbour A. G. Detection of Borrelia burgdorferi DNA by the polymerase chain reaction. Mol Cell Probes. 1990 Feb;4(1):73–79. doi: 10.1016/0890-8508(90)90041-w. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Telford S. R., 3rd, Rys P. N., Dodge D. E., White T. J., Malawista S. E., Spielman A. Detection of Borrelia burgdorferi DNA in museum specimens of Ixodes dammini ticks. Science. 1990 Sep 21;249(4975):1420–1423. doi: 10.1126/science.2402635. [DOI] [PubMed] [Google Scholar]
- Postic D., Edlinger C., Richaud C., Grimont F., Dufresne Y., Perolat P., Baranton G., Grimont P. A. Two genomic species in Borrelia burgdorferi. Res Microbiol. 1990 May;141(4):465–475. doi: 10.1016/0923-2508(90)90072-x. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Hogan D., Schwan T. G. Polymerase chain reaction analyses identify two distinct classes of Borrelia burgdorferi. J Clin Microbiol. 1991 Mar;29(3):524–532. doi: 10.1128/jcm.29.3.524-532.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T., Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992 Oct;6(20):3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Simpson W. J. Factors influencing the antigenic reactivity of Borrelia burgdorferi, the Lyme disease spirochete. Scand J Infect Dis Suppl. 1991;77:94–101. [PubMed] [Google Scholar]
- Stålhammar-Carlemalm M., Jenny E., Gern L., Aeschlimann A., Meyer J. Plasmid analysis and restriction fragment length polymorphisms of chromosomal DNA allow a distinction between Borrelia burgdorferi strains. Zentralbl Bakteriol. 1990 Oct;274(1):28–39. doi: 10.1016/s0934-8840(11)80972-2. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., Pretzman C., Postic D., Saint Girons I., Baranton G., McClelland M. Genomic fingerprinting by arbitrarily primed polymerase chain reaction resolves Borrelia burgdorferi into three distinct phyletic groups. Int J Syst Bacteriol. 1992 Jul;42(3):370–377. doi: 10.1099/00207713-42-3-370. [DOI] [PubMed] [Google Scholar]
- Wilske B., Anderson J. F., Baranton G., Barbour A. G., Hovind-Hougen K., Johnson R. C., Preac-Mursic V. Taxonomy of Borrelia spp. Scand J Infect Dis Suppl. 1991;77:108–129. [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Busch K. V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]