Abstract
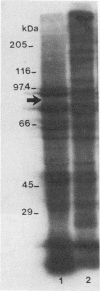
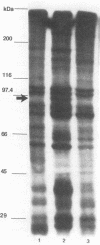
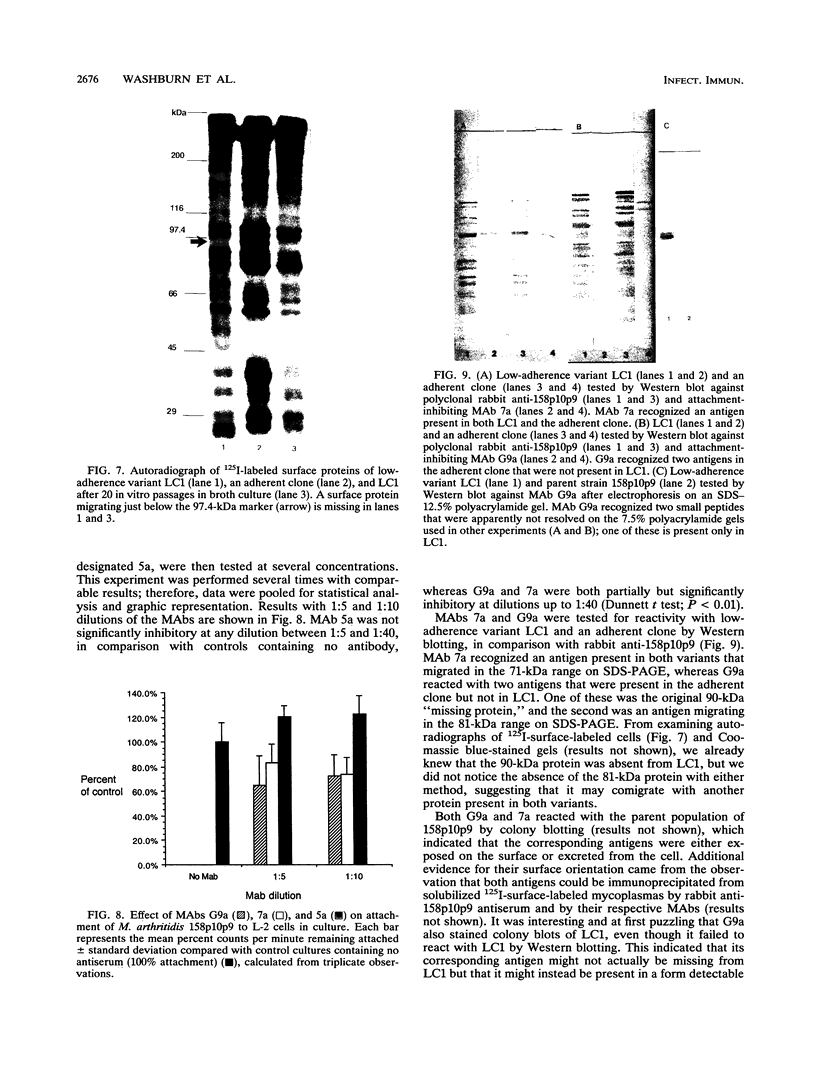
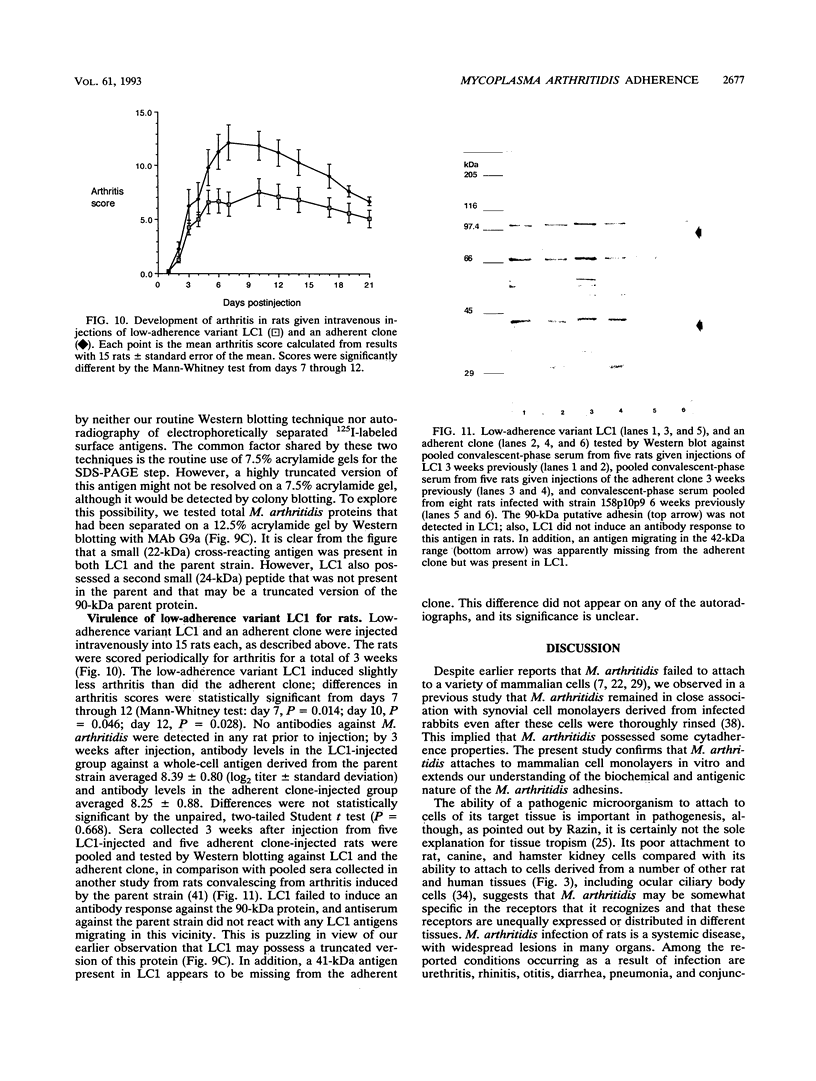
Although other investigators have reported that Mycoplasma arthritidis failed to attach to several types of mammalian cells in vitro, we showed that it attached well to rat synovial fibroblasts, lung cells, and skin cells but not to kidney cells, suggesting that receptor sites are unequally expressed or distributed among different rat tissues. M. arthritidis also attached poorly to canine kidney and to baby hamster kidney cells, although it did attach to human fetal lung and fetal amnion cells. Four M. arthritidis strains, two virulent and two avirulent, all attached equally well to rat lung cells. Attachment was inhibited by trypsinization, suggesting that the major adhesins are protein. Fab' fractions of rabbit antisera against four M. arthritidis strains partially inhibited adherence of both homologous and heterologous strains, although not to the same extent, indicating some degree of antigenic heterogeneity among their adhesins. A filter-cloned variant of M. arthritidis 158p10p9, designated LC1, attached poorly compared with the parent strain. Missing from this variant were two proteins migrating within the 81- to 90-kDa range by polyacrylamide gel electrophoresis; in their place was a 24-kDa antigen that may be a truncated version of one of these proteins. A monoclonal antibody that partially inhibited attachment recognized all these peptides by Western immunoblotting. An additional attachment-inhibiting monoclonal antibody recognized a 71-kDa antigen present in both low-adherence and fully adherent populations. The low-adherence variant LC1 induced slightly but significantly less arthritis in Lewis rats than did a fully adherent clone.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler D. K., Grabowski M. W., Barile M. F. Mycoplasma pneumoniae attachment: competitive inhibition by mycoplasmal binding component and by sialic acid-containing glycoconjugates. Infect Immun. 1982 Nov;38(2):598–603. doi: 10.1128/iai.38.2.598-603.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Cahill J. F., Wiley B. B., Ward J. R. Immunological responses of the rat to Mycoplasma arthritidis. J Bacteriol. 1969 Jun;98(3):930–937. doi: 10.1128/jb.98.3.930-937.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Miller M. L., Ward J. R. A comparative study on the virulence of Mycoplasma arthritidis and "Mycoplasma hominis, type 2" strains in rats. Proc Soc Exp Biol Med. 1967 Jan;124(1):103–107. doi: 10.3181/00379727-124-31676. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R. Interaction of Mycoplasma arthritidis and other mycoplasmas with murine peritoneal macrophages. Infect Immun. 1973 May;7(5):691–699. doi: 10.1128/iai.7.5.691-699.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallo S. F., Baseman J. B. Cross-hybridization between the cytadhesin genes of Mycoplasma pneumoniae and Mycoplasma genitalium and genomic DNA of Mycoplasma gallisepticum. Microb Pathog. 1990 May;8(5):371–375. doi: 10.1016/0882-4010(90)90096-9. [DOI] [PubMed] [Google Scholar]
- Forsyth M. H., Tourtellotte M. E., Geary S. J. Localization of an immunodominant 64 kDa lipoprotein (LP 64) in the membrane of Mycoplasma gallisepticum and its role in cytadherence. Mol Microbiol. 1992 Aug;6(15):2099–2106. doi: 10.1111/j.1365-2958.1992.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Golightly-Rowland L., Cole B. C., Ward J. R., Wiley B. B. Effect of Animal Passage on Arthritogenic and Biological Properties of Mycoplasma arthritidis. Infect Immun. 1970 Jun;1(6):538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWELL E. V., JONES R. S. Factors influencing pathogenicity of Mycoplasma arthritidis (PPLO). Proc Soc Exp Biol Med. 1963 Jan;112:69–72. doi: 10.3181/00379727-112-27952. [DOI] [PubMed] [Google Scholar]
- Hannan P. C., Hughes B. O. Reproducible polyarthritis in rats caused by Mycoplasma arthritidis. Ann Rheum Dis. 1971 May;30(3):316–321. doi: 10.1136/ard.30.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanns W., Schulz L. C., Kirchhoff H., Heitmann J. Studies of polyarthritis caused by mycoplasma arthritidis in rats. III. Histopathological findings. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 May;254(3):423–434. [PubMed] [Google Scholar]
- Hill A., Dagnall G. J. Experimental polyarthritis in rats produced by Mycoplasma arthritidis. J Comp Pathol. 1975 Jan;85(1):45–52. doi: 10.1016/0021-9975(75)90083-3. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Pnini S., Banai M., Baseman J. B., Cassell G. H., Bredt W. Attachment of mycoplasmas to erythrocytes: a model to study mycoplasma attachment to the epithelium of the host respiratory tract. Isr J Med Sci. 1981 Jul;17(7):589–592. [PubMed] [Google Scholar]
- Kirchhoff H., Heitmann J., Ammar A., Hermanns W., Schulz L. C. Studies of polyarthritis caused by Mycoplasma arthritidis in rats. I. Detection of the persisting Mycoplasma antigen by the enzyme immune assay (EIA) and conventional culture technique. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Mar;254(1):129–138. [PubMed] [Google Scholar]
- Kotani H., McGarrity G. J. Identification of mycoplasma colonies by immunobinding. J Clin Microbiol. 1986 Apr;23(4):783–785. doi: 10.1128/jcm.23.4.783-785.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Wilson R. M., Baseman J. B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect Immun. 1982 Mar;35(3):809–817. doi: 10.1128/iai.35.3.809-817.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Haemadsorption and haemagglutination by mycoplasmas. J Gen Microbiol. 1968 Mar;50(3):465–478. doi: 10.1099/00221287-50-3-465. [DOI] [PubMed] [Google Scholar]
- Razin S., Jacobs E. Mycoplasma adhesion. J Gen Microbiol. 1992 Mar;138(3):407–422. doi: 10.1099/00221287-138-3-407. [DOI] [PubMed] [Google Scholar]
- Razin S. Molecular biology and genetics of mycoplasmas (Mollicutes). Microbiol Rev. 1985 Dec;49(4):419–455. doi: 10.1128/mr.49.4.419-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. Phenotypic switching in mycoplasmas: phase variation of diverse surface lipoproteins. Science. 1990 Jan 19;247(4940):315–318. doi: 10.1126/science.1688663. [DOI] [PubMed] [Google Scholar]
- Schütze E., Laber G., Walzl H. Klinische und histopathologische Befunde bei der Mykoplasma-Polyarthritis der Ratte. III. Der Krankheitsablauf in der 7.-30. und 54.-61. Woche. Zentralbl Bakteriol Orig A. 1976 Jan;234(1):91–104. [PubMed] [Google Scholar]
- Simberkoff M. S., Elsbach P. The interaction in vitro between polymorphonuclear leukocytes and mycoplasma. J Exp Med. 1971 Dec 1;134(6):1417–1430. doi: 10.1084/jem.134.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerson N. L., James W. D., Walls B. E., Chanock R. M. Growth of Mycoplasma pneumoniae on a glass surface. Ann N Y Acad Sci. 1967 Jul 28;143(1):384–389. doi: 10.1111/j.1749-6632.1967.tb27680.x. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Furr P. M., Davies H. A., Manchee R. J., Mouches C., Bove J. M. Mycoplasmal adherence with particular reference to the pathogenicity of Mycoplasma pulmonis. Isr J Med Sci. 1981 Jul;17(7):599–603. [PubMed] [Google Scholar]
- Taylor-Robinson D., Manchee R. J. Adherence of mycoplasmas to glass and plastic. J Bacteriol. 1967 Nov;94(5):1781–1782. doi: 10.1128/jb.94.5.1781-1782.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirkill C. E., Roth A. M., Munn R. J., Lee P., Tyler N. K. Interactions of cultured rat synovial and ocular ciliary body cells with two strains of Mycoplasma arthritidis. In Vitro Cell Dev Biol. 1990 Feb;26(2):140–146. doi: 10.1007/BF02624104. [DOI] [PubMed] [Google Scholar]
- Thirkill C. E., Song D. Y., Gregerson D. S. Application of monoclonal antibodies to detect intraocular mycoplasma antigens in Mycoplasma arthritidis-infected Sprague-Dawley rats. Infect Immun. 1983 Apr;40(1):389–397. doi: 10.1128/iai.40.1.389-397.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD J. R., JONES R. S. The pathogenesis of mycoplasma (PPLO) arthritis in rats. Arthritis Rheum. 1962 Apr;5:163–175. doi: 10.1002/art.1780050205. [DOI] [PubMed] [Google Scholar]
- Walzi H., Schütze E., Laber G. Klinische und histopathologische Befunde bei der Mykoplasma-Polyarthritis der Ratte. II. Der Krankheitsablauf in der 2. bis 6. Woche. Zentralbl Bakteriol Orig A. 1975;231(1-3):229–242. [PubMed] [Google Scholar]
- Washburn L. R., Cole B. C., Gelman M. I., Ward J. R. Chronic arthritis of rabbits induced by mycoplasmas. I. Clinical microbiologic, and histologic features. Arthritis Rheum. 1980 Jul;23(7):825–836. doi: 10.1002/art.1780230709. [DOI] [PubMed] [Google Scholar]
- Washburn L. R., Hirsch S. Comparison of four Mycoplasma arthritidis strains by enzyme immunoassay, metabolism inhibition, one- and two-dimensional electrophoresis, and immunoblotting. J Clin Microbiol. 1990 Sep;28(9):1974–1981. doi: 10.1128/jcm.28.9.1974-1981.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn L. R., Hirsch S., McKenzie M., Voelker L. L. Vaccination of Lewis rats against Mycoplasma arthritidis-induced arthritis. Am J Vet Res. 1992 Jan;53(1):52–58. [PubMed] [Google Scholar]
- Washburn L. R., Ramsay J. R., Andrews M. B. Recognition of Mycoplasma arthritidis membrane antigens by rats and rabbits: comparison by immunoblotting and radioimmunoprecipitation. Vet Microbiol. 1988 May;17(1):45–57. doi: 10.1016/0378-1135(88)90078-8. [DOI] [PubMed] [Google Scholar]
- Washburn L. R., Ramsay J. R. Experimental induction of arthritis in LEW rats and antibody response to four Mycoplasma arthritidis strains. Vet Microbiol. 1989 Nov;21(1):41–55. doi: 10.1016/0378-1135(89)90017-5. [DOI] [PubMed] [Google Scholar]
- Washburn L. R., Ramsay J. R., Roberts L. K. Characterization of the metabolism inhibition antigen of Mycoplasma arthritidis. Infect Immun. 1985 Aug;49(2):357–364. doi: 10.1128/iai.49.2.357-364.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn L. R., Somerson N. L. Mycoplasma growth inhibition by arginine. J Clin Microbiol. 1977 Mar;5(3):378–380. doi: 10.1128/jcm.5.3.378-380.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]