Abstract
Human immunodeficiency virus type 1 (HIV-1) establishes a persistent infection characterized by progressive depletion of CD4+ lymphocytes and immunosuppression. Although extensive research has examined the importance of apoptosis as a cause of cell death associated with HIV-1 infection, the role of autophagy has been largely ignored. Our laboratory has examined the autophagic process in HIV-1-infected cells. Following infection of human peripheral blood CD4+ T-cells or U937 cells with HIV-1 for 48 hours, the autophagy proteins Beclin 1 and LC3-II were found to be markedly decreased. Beclin 1 mRNA expression and autophagosomes were also reduced in HIV-1 infected cells. Thus, our data indicate that HIV-1 infection inhibits autophagy in infected cells in contrast to the previously described induction of autophagy by gp120 in uninfected bystander cells. It is likely that HIV-1 has evolved this mechanism as part of an elaborate attempt to evade the immune system while promoting its own replication. We believe that autophagy is an overlooked mechanism in HIV-1 pathogenesis and plays a particularly important role in the early cognitive impairment and dementia often associated with advanced AIDS. A model is presented that describes the potential role of autophagy in NeuroAIDS.
Keywords: autophagy, HIV-1, neuroAIDS, HIV-associated dementia, HIV pathogenesis, beclin 1, AIDS, HIV-related cognitive impairment
Human immunodeficiency virus type 1 (HIV-1) establishes a persistent infection characterized by progressive depletion of CD4+ lymphocytes and immunosuppression. Although extensive research has examined the importance of apoptosis as a cause of cell death associated with HIV-1 infection, the role of autophagy has been largely ignored. Similar to apoptotic programmed cell death (PCD), autophagy is an essential part of growth regulation and maintenance of homeostasis. Advances in the understanding of the role of autophagy in normal growth and development have led to a reclassification of apoptotic death as Type I PCD and autophagy as Type II PCD.1 However, whereas apoptosis directly leads to cell death, a primary function of autophagy is cell repair that prevents age-related diseases.
As a general rule of viral infection, the alterations of cellular processes induced by viral infection favor viral replication and spread. Recent data indicate that the exposure of T cells to HIV-1 gp120 can lead to bystander cell death through gp120/CXCR4 interactions.2,3 In contrast to the effect on bystander cells, we have found that in cells actively infected with HIV-1, autophagy is inhibited.4 Protein extracts of HIV-1 infected and uninfected CD4+ T-lymphocytes and U937 cells were semi-quantified by Western blot. The autophagy-related protein Beclin 1 and the 16 kDa microtubule-associated protein 1 light chain 3 (LC3) were quantified and validated using the intracellular protein GAPDH as an internal standard. Beclin 1 mRNA was quantified by real-time RT-PCR and autophagosomes were quantified by visualization under confocal microscopy following intracellular staining of the LC3 protein. Following infection of human peripheral blood CD4+ T-cells or U937 cells with HIV-1 for 48 hours, the autophagy proteins Beclin 1 and LC3-II were found to be markedly decreased. Beclin 1 mRNA expression and autophagosomes were also reduced in HIV-1 infected cells. The reduction of autophagic protein expression and autophagosomes in HIV-1 infected cells could be overcome by amino acid starvation or rapamycin. Thus, the initial cellular response to HIV-1 infection appears to be the inhibition of autophagy.
A role for autophagy in HIV-1 infection would not be unique for viral infections.5–7 Single stranded RNA viruses including poliovirus block the degradation of autophagosome membranes and use the membranes to anchor their RNA replication complexes.8–9 Viruses that do not use autophagosomal membranes for their replication appear to downregulate the formation of autophagosomes in order to enhance viral replication. HIV-1 would appear to fall into this latter category. Recently, Orvedahl et al., reported that HSV-1 ICP34.5 confers neurovirulence by antagonism of the autophagy function of Beclin.10 This escape mechanism appears to be critical for herpes simplex virus neurovirulence. Their findings suggest that inhibition of autophagy by herpesviruses may alter viral pathogenesis and lead to serious disease. Thus, there is considerable precedent for autophagy playing an important role in viral pathogenesis and in CNS disease.
We believe that our findings have particular importance in the pathogenesis of HIV-related central nervous system (CNS) impairment. Significant brain involvement occurs in ~50% of persons with AIDS (HIV associated dementia [HAD]), but CNS abnormalities can also occur in ~30% of early, medically asymptomatic carriers.11 HIV-1 predominantly infects macrophages and microglial cells in the CNS. Although much research has focused on the identification of a “neurotropic” virus, there has been no consistent strain of HIV-1 identified as the cause of HAD. Increasing evidence suggests that the effects of HIV-1 on the CNS are altered by host genetics, and results from both a direct productive infection of microglial cells with accumulation of neurotoxic substances, and a non-permissive infection resulting in functional changes in key cells including neurons and astrocytes.
To assess the potential role of HIV-1 infection and its secreted proteins on CNS disease, we have assessed the effect of gp120 on autophagy of neuronal SK-N-SH cells in vitro with gp120 peptides. The autophagy proteins Beclin 1, LC3-II and ATG5 detected by Western blot were increased at day 1 and day 2 after treatment. Autophagosomes were also found to be increased (Zhou D and Spector SA, unpublished data). Thus, whereas we have observed that during permissive infection HIV-1 inhibits autophagy, the exposure of neuronal cells to gp120 in the absence of infection leads to enhanced expression of autophagy. Although these results will need to be confirmed, they are consistent with those recently published by Espert et al.,2 and suggest that HIV-1 may alter autophagic processes within the CNS that allow continued infection of macrophages and microglial cells through the downregulation of autophagy, whereas exposure of neurons to gp120 can result in increased autophagy and potentially disruption of neuronal cell function. Preliminary studies of brain samples of persons identified as having HIV-encephalitis (HIVE) and HIV-associated dementia suggest that autophagic markers are moderately increased compared to HIV-infected brains with no impairment (Zhou D, et al., unpublished data).
It is important to note that there is considerable interaction between autophagy and apoptosis. Cells can be killed by autophagy when apoptosis is inhibited, and apoptotic cell death can be prevented through autophagic cell signaling. Moreover, autophagy can contribute to apoptotic signals to promote cell killing and elimination of apoptotic cell debris.1 A major role of autophagy, however, is to promote neuron homeostasis and survival. The accumulation of autophagosomes within neurons reflects the failure of autophagy to rescue the cells from degeneration and death. Thus, autophagy when acting as a survival mechanism can potentially affect neuron function without resulting in cell death, analogous to what is observed with HAD.
In total, our recently published findings combined with additional unpublished data suggest a model of how autophagy contributes to the development of NeuroAIDS. In this model (Fig. 1), HIV-infection of macrophages/microglia leads to inhibition of autophagy that prevents viral elimination and promotes cell survival. With persistent productive infection, there is release of viral proteins, neurotoxins and cytokines that result in induction of autophagy. With moderate levels of virally induced toxins, induction of autophagy promotes cell survival but alters neuron function leading to progressive HAD/HIVE. With high levels of viral induced toxic products, there is high level induction of autophagy in neurons leading to PCD-I and PCD-II and advanced NeuroAIDS. Although this model is speculative, numerous examples exist for autophagy playing an important role in neurological disorders such as Alzheimer’s, Huntington’s, Parkinson’s, etc.12–16 Our initial data and those of others, lead us to believe that HIV-1 infection within the brain results in an alteration of neuronal autophagy leading to the cognitive impairment associated with NeuroAIDS, and provides new potential strategies for treatment of HIV-1 infection.
Figure 1.
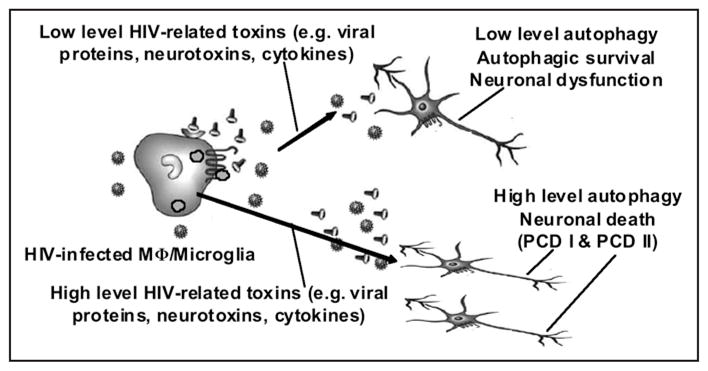
Model of autophagy in NeuroAIDS. HIV-infection of macrophages (Mφ)/microglia leads to inhibition of autophagy that prevents viral elimination and promotes cell survival. With persistent productive infection, there is release of viral proteins, neurotoxins and cytokines that results in induction of autophagy in bystander (uninfected) neurons. With moderate quantities of virally related toxins, the levels of autophagy that are induced lead to sustained neuron survival but alter neuron function leading to cognitive impairment. With high levels of virally-induced toxic products, neurons markedly increase levels of autophagy associated with progressive CNS impairment with development of HIV-associated dementia. Continued exposure to high levels of neurotoxins results in excessively high levels of autophagy in neurons leading to programmed cell death type 1 (apoptosis) and type II (autophagy), and advanced NeuroAIDS (illustration adapted from17).
Acknowledgments
Supported in part by Grants from the National Institute of Allergy and Infectious Diseases (AI-68632, AI-39004 and AI-36214) {Virology Core, University of California, San Diego, Center for AIDS Research}.
Footnotes
Addendum to: Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. Aids 2008; 22:695–9.
References
- 1.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 2.Espert L, Denizot M, Grimaldi M, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161–72. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espert L, Denizot M, Grimaldi M, et al. Autophagy and CD4+ T lymphocyte destruction by HIV-1. Autophagy. 2007;3:32–4. doi: 10.4161/auto.3275. [DOI] [PubMed] [Google Scholar]
- 4.Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. Aids. 2008;22:695–9. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120:159–62. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munz C. Viral evasion of autophagy. Cell Host & Microbe. 2007;1:9–11. doi: 10.1016/j.chom.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Jackson WT, Giddings TH, Jr, Taylor MP, et al. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orvedahl A, Alexander D, Talloczy Z, et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host & Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Heaton RK, Grant I, Butters N, et al. The HNRC 500—neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–51. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 12.He C, Klionsky DJ. Autophagy and neurodegeneration. ACS Chem Biol. 2006;1:211–3. doi: 10.1021/cb600182h. [DOI] [PubMed] [Google Scholar]
- 13.Jellinger KA, Stadelmann C. Mechanisms of cell death in neurodegenerative disorders. J Neural Transm Suppl. 2000;59:95–114. doi: 10.1007/978-3-7091-6781-6_13. [DOI] [PubMed] [Google Scholar]
- 14.Jellinger KA, Stadelmann C. Problems of cell death in neurodegeneration and Alzheimer’s Disease. J Alzheimers Dis. 2001;3:31–40. doi: 10.3233/jad-2001-3106. [DOI] [PubMed] [Google Scholar]
- 15.Ravikumar B, Acevedo Arozena A, Imarisio S, et al. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet. 2005;37:771–6. doi: 10.1038/ng1591. [DOI] [PubMed] [Google Scholar]
- 16.Rubinsztein DC, Huntington JA. Paradoxical aggregation versus oligomerisation properties of mutant and wild-type huntingtin fragments. Exp Neurol. 2006;199:243–4. doi: 10.1016/j.expneurol.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA. 1999;96:8212–6. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]


