Abstract
During the aging process, mammals lose up to a third of their skeletal muscle mass and strength. Although the mechanisms underlying this loss are not entirely understood, we attempted to moderate the loss by increasing the regenerative capacity of muscle. This involved the injection of a recombinant adeno-associated virus directing overexpression of insulin-like growth factor I (IGF-I) in differentiated muscle fibers. We demonstrate that the IGF-I expression promotes an average increase of 15% in muscle mass and a 14% increase in strength in young adult mice, and remarkably, prevents aging-related muscle changes in old adult mice, resulting in a 27% increase in strength as compared with uninjected old muscles. Muscle mass and fiber type distributions were maintained at levels similar to those in young adults. We propose that these effects are primarily due to stimulation of muscle regeneration via the activation of satellite cells by IGF-I. This supports the hypothesis that the primary cause of aging-related impairment of muscle function is a cumulative failure to repair damage sustained during muscle utilization. Our results suggest that gene transfer of IGF-I into muscle could form the basis of a human gene therapy for preventing the loss of muscle function associated with aging and may be of benefit in diseases where the rate of damage to skeletal muscle is accelerated.
One of the primary consequences of aging, which leads to significantly impaired function in the elderly population, is the loss of skeletal muscle strength and mass (1). Both decrease up to one-third in humans between the ages of 30 and 80 years (2). In addition, loss of the fastest, most powerful muscle fiber types (type IIb fibers) has been documented (3). Similar age-related muscle alterations have been observed in rats and mice, indicating that the trend is maintained in other mammalian species (4, 5).
Insulin-like growth factor I (IGF-I) is critical in mediating the growth of muscle and other tissues (6). Systemic administration of IGF-I results in increased muscle protein content and reduced protein degradation (7). Moreover, overexpression of IGF-I has been correlated with muscle hypertrophy in transgenic mouse lines (8). With aging there is a decrease in the production and activity of the growth hormone/IGF-I axis that leads to an increase in catabolic processes (1), and is exhibited by the age-related loss of muscle mass and strength. The prevention of muscle mass loss has been achieved in healthy individuals by growth hormone administration, and it is associated with increases in IGF-I levels (9). However, these studies failed to show functional hypertrophy: no change in muscle strength was demonstrated. Thus although there are clear indications that local expression of IGF-I in skeletal muscle might maintain muscle mass in elderly individuals, it is unclear if muscle function can be preserved. To test whether IGF-I expression can prevent aging-related loss of muscle mass and function, we utilized virus-mediated gene transfer of an IGF-I cDNA regulated by a powerful muscle-specific promoter [the myosin light chain 1/3 promoter/enhancer (10)]. Recombinant AAV (rAAV) was chosen as the gene delivery vehicle for this study based on its ability to achieve persistent expression in differentiated muscle without an immune response (11, 12). Furthermore, it has been shown that rAAV can transduce mature myotubes or adult muscle fibers, but not satellite cells (11, 12). Based on the ability of IGF-I to activate satellite cells (muscle stem cells) in culture (13), we hypothesized that IGF-I overexpression and secretion by muscle fibers might activate surrounding satellite cells and promote an increase in muscle mass. Because the ultimate goal of any therapy would be to either restore or maintain force-generating capacity of skeletal muscle, we compared the functional properties of virus-injected and control muscles.
MATERIALS AND METHODS
Viral Construct.
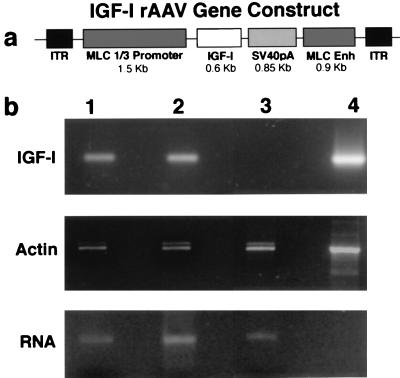
Fig. 1a shows a schematic of the IGF-I construct. An AAV transfer plasmid (pSUB201) was constructed that contained a myosin light chain 1/3 promoter/enhancer [which drives expression in differentiated fast-twitch muscle fibers (10)], the rat IGF-IA cDNA (14), simian virus 40 polyadenylation signal, and the inverted terminal repeats necessary for viral packaging. rAAV was prepared by University of North Carolina Gene Therapy Vector Core (Chapel Hill, NC), following published procedures (15).
Figure 1.
(a) Schematic of the rAAV)/IGF-I gene construct. Rat IGF-I cDNA expression was driven by a fast muscle specific promoter/enhancer (myosin light chain 1/3) and stabilized by simian virus 40 polyadenylation sequence (SV40pA). The gene construct was inserted into an rAAV transfer plasmid (pSUB201) between the inverted terminal repeats (ITRs). (b) (Top) Persistence of IGF-I expression shown by reverse transcription–PCR. Lane 1, 27-month-old EDL, 9 months post-AAV injection; lane 2, 6 month old EDL, 4 months post-AAV injection; lane 3, uninjected control EDL; lane 4, positive control using plasmid shown in a. (Middle) Constitutive expression of β-cytoplasmic actin in EDL muscles. Lanes 1–3, as described above; Lane 4, positive control using template supplied with oligonucleotides (CLONTECH). (Bottom) Integrity of RNA isolated from EDL muscles. Equal volume RNA samples were run on a nondenaturing agarose gel (Invitrogen). Lanes 1–3, as described above.
Injections.
All experiments involving animals were approved by University of Pennsylvania’s Animal Care and Use Committee. Anesthetized C57BL/6 mice of 2, 18, and 24 months of age were injected with 100 μl of 10% glycerol/PBS containing ≈1010 rAAV particles into the interstitial space of the anterior muscle compartment of the right hind limb. Preliminary experiments with vital dye (0.1% Evans blue) injections into the same compartment showed that the injected solution was localized to the anterior compartmental muscles. Muscles within the anterior compartment were bathed in the virus solution for a sufficient time such that viral particles could be endocytosed into the adult muscle fibers. The anterior compartment contains the extensor digitorum longus (EDL), the muscle that was examined for the effects of viral transgene (IGF-I) expression. Once the mice regained consciousness they were returned to the animal facility until further study.
Mechanical Measurements.
Approximately 4–9 months after viral injection, mice were killed by cervical dislocation under anesthesia, and EDL muscles were removed for isolated muscle force measurements. The tendons were attached to a rigid post and to an isometric force transducer in a bath of Ringer’s solution gas-equilibrated with 95% O2/5% CO2. Optimum length of the muscle was determined by twitch force from supramaximal stimulation. Maximal tetanic force was determined by using a 120-Hz 500-msec pulse and was delivered via two parallel platinum plate electrodes. After force measurements were completed, the muscles were removed from the bath, blotted, and weighed (muscle mass measurements were based on wet weight). They were then pinned at optimum length, surrounded by embedding compound (TissueTek), and rapidly frozen in melting isopentane. Muscles were stored at −80°C for subsequent analysis.
Detection of Transgene Expression.
Reverse transcription–PCR was used to detect the presence of IGF-I transcripts in injected muscles. Total RNA isolated from frozen tissue (RNAqueous) was subjected to reverse transcription and PCR (Perkin–Elmer) using oligonucleotides specific for rat IGF-I (TGCTCACCTTTACCAGCTCGG, sense primer; GCCCGGATGGAACGAGCTGACT, antisense primer). Primers that amplified β cytoplasmic actin (CLONTECH) served as a positive control for the procedure.
Myosin Heavy Chain Composition.
Immunohistochemistry was used to determine neonatal and type IIb myosin heavy chain composition (16). Primary antibody dilutions were as follows: type I myosin (BA-F8), 1:50; type IIa myosin (SC-71), 1:10; type IIb myosin (BF-F3), 1:3; neonatal myosin (BF-34), 1:10. 7-Amino-4-methylcoumarin-3-acetic acid-conjugated, goat anti-mouse IgM antibodies and donkey anti-mouse IgG (heavy and light chains) (Jackson ImmunoResearch, West Grove, PA) were used as secondary antibodies. Microscopy was performed on a Leitz DMR microscope (Leica). Image acquisition and analysis was carried out using a MicroMAX digital camera system (Princeton Instruments, Princeton, NJ) and imaging software.
RESULTS AND DISCUSSION
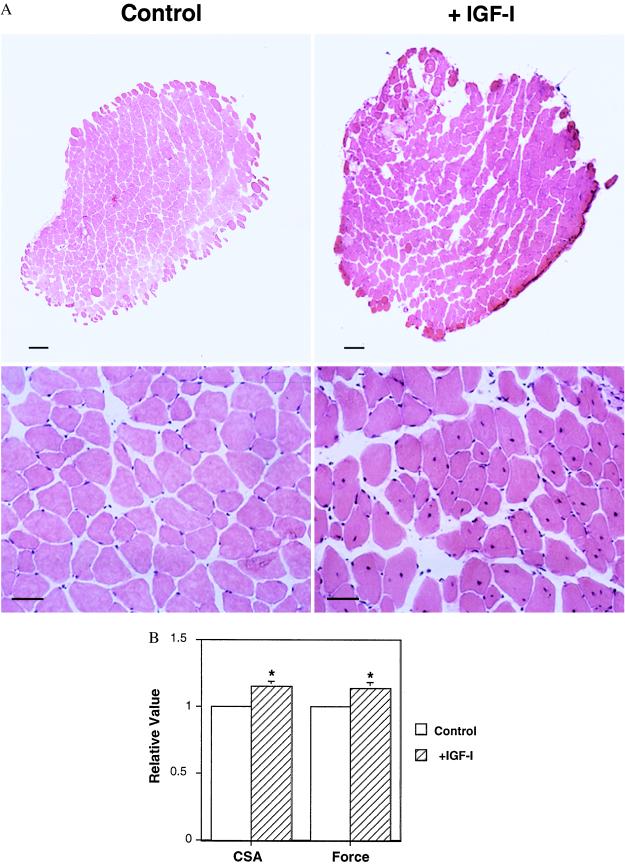
Fig. 1b illustrates expression of the myosin light chain-driven IGF-I mRNA resulting from direct muscle injection of the recombinant AAV shown schematically in Fig. 1a. The expression persisted for as long a period as we examined; for mice examined at 27 months of age, after injection at 18 months of age, this represented 9 months of continued expression. The effects of this IGF-I expression on muscle morphology can be seen in Fig. 2 in hematoxylin/eosin-stained muscle cross sections (17). In young adult mice (examined at 6 months of age after injection at 2 months of age), average mass of the EDL muscle increased by 15%. Central nuclei were visible in the muscle fibers, indicative of ongoing regeneration (18). The presence of neonatal myosin provided further evidence of regeneration, because satellite cells recapitulate the progression through the series of developmental myosin heavy chain isoforms when they are activated and fuse with existing muscle fibers (19). In the case of the old mice examined at 27 months of age, after injection at either 18 or 24 months of age, the muscle mass increased 19% compared with age-matched control muscles. Thus the normal decrease of muscle mass associated with aging (15% in mice) was prevented by viral administration of IGF-I (Fig. 3). As in the young animals, central nuclei were evident 4 months after viral injection in the old animals. However, no central nuclei were observed at 9 months after injection, even though IGF-I expression was maintained (Fig. 1). This suggests that the muscles eventually reach a new steady state where regeneration is no longer evident. In both young and old animals when central nuclei are observed, they are in normal sized or larger fibers that also contain peripheral nuclei (see Fig. 2A Lower Right). Thus it appears that the satellite cells fuse with existing fibers, rather than forming new fibers.
Figure 2.
(A) Effect of IGF-I expression in EDL muscles of young adult (6 month) mice. Cross-sections stained with hematoxylin/eosin of control and AAV-injected EDL muscles from young adult mouse, 4 months post-AAV injection. AAV-injected EDLs (Upper Right) displayed significant increases in cross-sectional area compared with contralateral control EDLs (Upper Left). Muscle fiber regeneration was evident in injected EDLs (Lower Left) by the presence of central nuclei (arrow marks a central nucleus), which were absent in control sections (Lower Right). [Bars = 100 μm (Upper); 25 μm (Lower).] (B) Effect of IGF-I expression on muscle mass and force generation. The uninjected leg of each animal served as the control (defined as a value of 1) for the injected leg (expressed relative to the control value). Thus muscle mass (wet weight) and isometric tetanic force of AAV-injected muscles were expressed relative to the same measurements in the contralateral control muscle from the same animal. The average relative value (n = 5) for the injected limbs is plotted (±SEM). Asterisks denote a P < 0.05 for paired comparisons (Student’s t tests) between AAV-injected and control muscles.
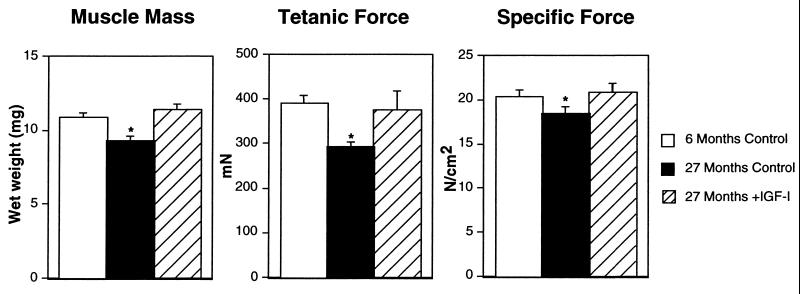
Figure 3.
Effect of IGF-I expression in EDL muscles of old (27 month) mice. (Left) Average (±SEM) muscle mass (n = 7); (Center) average (±SEM) tetanic force (n = 7); (Right) average (±SEM) specific force (n = 7). For determination of force per cross-sectional area (specific force), cross-sectional area was estimated using muscle mass and optimum muscle length (5). Asterisks denote a P < 0.05 for comparisons (Student’s t tests) to young adult control muscles.
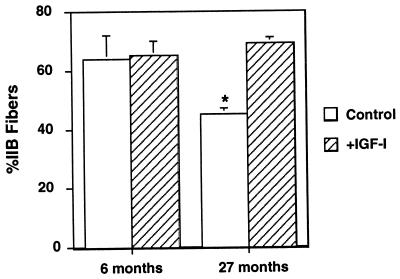
From a functional standpoint, the effects of the viral IGF-I expression were dramatic in extent. In the young experimental animals, the increase in muscle mass and cross-sectional area translated into increased force production (isometric force increased by 14%), without any changes in fiber number (control, 726 ± 54; +IGF-I, 785 ± 96), fiber types (Fig. 4) or other contractile parameters. Thus IGF-I expression induced hypertrophy of individual fibers with concomitant increases in force. In the old animals, the results of viral IGF-I expression were even more remarkable. Increases in force generation observed in experimental muscles was greater than the increase in cross-sectional area (27% vs. 15%, respectively). This is due to the fact that reduction in the specific force (force per cross-sectional area), which is typical of aging skeletal muscle, has been prevented by IGF-I expression (Fig. 3). Furthermore, viral IGF-I expression completely prevented the significant loss of the fastest, most powerful fiber types (IIb fibers) seen in the uninjected control old EDL muscles (Fig. 4). Thus, two important hallmarks of aging muscle were prevented by viral IGF-I administration.
Figure 4.
Maintenance of IIb fibers in muscle of old (27 month) mice by IGF-I expression. The loss of IIb fibers normally associated with aging was prevented by IGF-I expression in 27-month-old mice (n = 7). No effect on fiber type distribution by IGF-I expression was observed in muscles of 6-month-old mice (n = 5). Asterisks denote a P < 0.05 for comparisons (Student’s t tests) to young adult control muscles. Fiber types were determined as described by Schiaffino and Salviati (16).
The effects of the IGF-I production were local. Average circulating IGF-I levels were not elevated (248 ± 19.5 ng/ml plasma for an uninjected mouse; 217 ± 46.5 ng/ml plasma for an AAV/IGF-I-injected mouse), and the muscles of contralateral limbs of animals receiving single limb injections were indistinguishable from muscles of uninjected animals. This is an important advantage of the AAV delivery system, because elevation of IGF-I levels in the blood could lead to undesirable effects in other tissues (20). The mechanism by which IGF-I is restricted is unclear; it could be that the muscle extracellular matrix traps all secreted IGF-I, or that IGF-I is incapable of gaining access to the blood if secreted from muscle. On the other hand, past failures of increased circulating levels of IGF-I to markedly improve muscle function (9) may have resulted either from an inability to sufficiently elevate local concentrations of IGF-I in muscle, or from a failure of the circulating IGF-I to gain access to and activate satellite cells.
In contrast, we hypothesize that the mechanism by which the virus-mediated muscle fiber secretion of IGF-I prevents aging-related loss of function is, at least in part (beyond its general anabolic effects), through the activation of satellite cells that leads to increased rate of muscle repair. Although further experiments will be required to validate this hypothesis, the following observations form the basis for our proposal. During muscle regeneration, IGF-I levels have been shown to increase (21). Additionally, it is known that IGF-I activates satellite cells in culture, increasing the rate of cellular proliferation and formation of myotubes, ultimately resulting in hypertrophied myotubes (13). Muscles in which satellite cells have been destroyed by irradiation fail to hypertrophy in response to chronic overload, suggesting that activation of satellite cells is essential for normal hypertrophy (22). IGF-I-induced satellite cell activation in our experimental muscles is supported by the appearance of central nuclei and developmental myosin expression 4 months after viral infection. Furthermore, the DNA content of the muscles (23) increased in excess of the increase in muscle mass (28% increase in DNA content vs. 15% increase in mass). The outcome is preservation of both muscle strength and mass, and prevention of loss of the fastest fiber types (IIb). Based on these results, we further hypothesize that the primary cause of the aging-related loss in strength that precedes, and is in excess of, the loss of muscle mass, is a failure to activate satellite cells to repair cumulative injury that results from normal muscle use.
The prevention of type IIb fiber loss is intriguing, as it has been proposed that the loss of those fibers during senescence results from the death of the motor neurons that innervate them (24). However, it is unclear if the motor neuron loss is a primary event or a secondary consequence of the inability of fast motor neurons to compete with slow motor neurons for innervation of regenerating fibers (25). We cannot discern between an indirect mechanism of fast (IIb) motor neuron preservation via the stabilization of their targets, the fast IIb fibers, or a direct action of IGF-I on the motor neurons to enhance their survival (26). Either mechanism could contribute to the preservation of fiber type distribution in aging muscle that we have observed.
Previously, we have suggested that failure to adequately repair muscle damage resulting from increased susceptibility to contraction-induced injury underlies the loss of muscle function in muscular dystrophies (27). It is noteworthy that aging-related changes in skeletal muscle mirror the early functional changes that are seen in muscular dystrophy, albeit on a much slower time scale. Just as in the muscles of old mice, the muscles of the mouse model of Duchenne muscular dystrophy (the mdx mouse) show both decreased force per cross-sectional area and preferential loss of IIb fibers (28). These considerations suggest that IGF-I expression in dystrophic muscle (perhaps in milder dystrophies than Duchenne), although not addressing the primary defect that results in increased susceptibility to injury, could increase the rate of regeneration and thereby tend to preserve muscle function.
Functional deficits associated with the aging process likely derive from different sources depending upon the cell type and tissue in question. Certainly the age-dependent loss of function in postmitotic cells is fundamentally different in nature from the aging problems associated with replicating cell populations. In the case of skeletal muscle, where normal maintenance of function involves repeated cycles of injury and satellite cell-mediated repair, loss of function can come from either increased susceptibility to injury of the muscle fibers or decreased ability to repair the fibers (i.e., failure to activate satellite cells) or a combination of both. The results of this study demonstrate that IGF-I overexpression in muscle can preserve the morphological and functional characteristics of the skeletal muscles of old mice such that they are equivalent to those of young adult muscles. An important component of the mechanism by which this is achieved appears to be enhanced muscle regeneration through the activation of satellite cells by IGF-I secreted from the infected muscle fibers. This approach may form the basis for gene therapy for both aging-related loss of muscle function and impairments associated with muscle disease. However, this work raises a number of ethical considerations about the use of IGF-I in gene therapy, as its beneficial effects could be used in humans for athletic or cosmetic purposes, rather than for disease treatment.
Acknowledgments
H.L.S. dedicates this work to the memory of his grandmother, Mattie Theo Richardson, whose life was diminished and ultimately shortened by lack of sufficient muscle strength to stand and walk. This work was supported by grants from the National Institutes of Health to H.L.S. (P01-AR/NS43648) and to N.R. (P01-AG13329), and from the Muscular Dystrophy Association.
ABBREVIATIONS
- IGF-I
insulin-like growth factor I
- AAV
adeno-associated virus
- rAAV
recombinant AAV
- EDL
extensor digitorum longus
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Lamberts S W J, van den Beld A W, van der Lely A-J. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 2.Tzankoff S P, Norris A H. J Appl Physiol. 1977;43:1001–1006. doi: 10.1152/jappl.1977.43.6.1001. [DOI] [PubMed] [Google Scholar]
- 3.Grimby G, Danneskiold-Samsoe B, Hvid K, Saltin B. Acta Physiol Scand. 1982;115:125–134. doi: 10.1111/j.1748-1716.1982.tb07054.x. [DOI] [PubMed] [Google Scholar]
- 4.Larrson L, Edstrom L. J Neurol Sci. 1986;76:69–89. doi: 10.1016/0022-510x(86)90143-7. [DOI] [PubMed] [Google Scholar]
- 5.Brooks S V, Faulkner J A. J Physiol (London) 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Florini J R, Ewton D Z, Coolican S A. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 7.Zdanowicz M M, Moyse J, Wingertzahn M A, O’Conner M, Teichberg S, Slonim A E. Endocrinology. 1995;136:4880–4886. doi: 10.1210/endo.136.11.7588220. [DOI] [PubMed] [Google Scholar]
- 8.Coleman M E, DeMayo F, Yin K C, Lee H M, Geske R, Montgomery C, Schwartz R J. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- 9.Papadakis M A, Grady D, Black D, Tierney M J, Gooding G A W, Schambelan M, Grunfeld C. Ann Intern Med. 1996;124:708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Donoghue M, Ernst H, Wentworth B, Nadal-Ginard B, Rosenthal N. Genes Dev. 1988;2:1779–1790. doi: 10.1101/gad.2.12b.1779. [DOI] [PubMed] [Google Scholar]
- 11.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 12.Clark K R, Sferra T J, Johnson P R. Hum Gene Ther. 1997;8:659–669. doi: 10.1089/hum.1997.8.6-659. [DOI] [PubMed] [Google Scholar]
- 13.Allen R E, Boxhorn L K. J Cell Physiol. 1989;138:311–314. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- 14.Shimatsu A, Rotwein P. J Biol Chem. 1987;262:7894–7900. [PubMed] [Google Scholar]
- 15.Snyder R O, Xiao X, Samulski R J. In: Current Protocols in Human Genetics. Dracopoli N C, Haines J L, Korf B R, Moir D T, Morton C C, Seidman C E, Seidman J G, Smith D R, editors. New York: Wiley; 1996. pp. 12.1.1–12.1.24. [Google Scholar]
- 16.Schiaffino S, Salviati G. In: Methods in Cell Biology. Emerson C P, Sweeney H L, editors. New York: Academic; 1997. pp. 349–367. [DOI] [PubMed] [Google Scholar]
- 17.Carson F L. Histotechnology. Chicago: Am. Soc. Clin. Pathol.; 1997. [Google Scholar]
- 18.Carlson B M, Faulkner J A. Med Sci Sports Exercise. 1983;15:187–98. [PubMed] [Google Scholar]
- 19.Sartore S, Gorza L, Schiaffino S. Nature (London) 1982;298:294–296. doi: 10.1038/298294a0. [DOI] [PubMed] [Google Scholar]
- 20.Stewart C E, Rotwein P. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 21.Krishan K, Dhoot G K. J Muscle Res Cell Motil. 1996;17:513–521. doi: 10.1007/BF00124351. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblatt J D, Parry D J. J Appl Physiol. 1992;73:253–257. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- 23.Labarca C, Paigen K. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 24.Einsiedel L J, Luff A R. J Neurol Sci. 1992;112:170–177. doi: 10.1016/0022-510x(92)90147-d. [DOI] [PubMed] [Google Scholar]
- 25.Desypris G, Parry D J. Am J Physiol. 1990;258:C62–C70. doi: 10.1152/ajpcell.1990.258.1.C62. [DOI] [PubMed] [Google Scholar]
- 26.Caroni P, Schneider C. J Neurosci. 1994;14(5):3378–3388. doi: 10.1523/JNEUROSCI.14-05-03378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrof B J, Shrager J B, Stedman H H, Kelley A M, Sweeney H L. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrof B J, Stedman H H, Shrager J B, Eby J, Sweeney H L, Kelley A M. Am J Physiol. 1993;265:C834–C841. doi: 10.1152/ajpcell.1993.265.3.C834. [DOI] [PubMed] [Google Scholar]