Abstract
Background:
Vitamin D deficiency has potential adverse effects on neurocognitive health and subcortical function. However, no studies have examined the association between vitamin D status, dementia, and cranial MRI indicators of cerebrovascular disease (CVD).
Methods:
Cross-sectional investigation of 25-hydroxyvitamin D [25(OH)D], dementia, and MRI measures of CVD in elders receiving home care (aged 65–99 years) from 2003 to 2007.
Results:
Among 318 participants, the mean age was 73.5 ± 8.1 years, 231 (72.6%) were women, and 109 (34.3%) were black. 25(OH)D concentrations were deficient (<10 ng/mL) in 14.5% and insufficient (10–20 ng/mL) in 44.3% of participants. There were 76 participants (23.9%) with dementia, 41 of which were classified as probable AD. Mean 25(OH)D concentrations were lower in subjects with dementia (16.8 vs 20.0 ng/mL, p < 0.01). There was a higher prevalence of dementia among participants with 25(OH)D insufficiency (≤20 ng/mL) (30.5% vs 14.5%, p < 0.01). 25(OH)D deficiency was associated with increased white matter hyperintensity volume (4.9 vs 2.9 mL, p < 0.01), grade (3.0 vs 2.2, p = 0.04), and prevalence of large vessel infarcts (10.1% vs 6.9%, p < 0.01). After adjustment for age, race, sex, body mass index, and education, 25(OH)D insufficiency (≤20 ng/mL) was associated with more than twice the odds of all-cause dementia (odds ratio [OR] = 2.3, 95% confidence interval [CI] 1.2–4.2), Alzheimer disease (OR = 2.5, 95% CI 1.1–6.1), and stroke (with and without dementia symptoms) (OR = 2.0, 95% CI 1.0–4.0).
Conclusions:
Vitamin D insufficiency and deficiency was associated with all-cause dementia, Alzheimer disease, stroke (with and without dementia symptoms), and MRI indicators of cerebrovascular disease. These findings suggest a potential vasculoprotective role of vitamin D.
GLOSSARY
- 25(OH)D
= 25-hydroxyvitamin D;
- AIREN
= Association Internationale pour la Recherché et l'Enseignement en Neurosciences;
- BMI
= body mass index;
- CI
= confidence interval;
- CVD
= cerebrovascular disease;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- FFQ
= food frequency questionnaire;
- FLAIR
= fluid-attenuation inversion recovery;
- GFR
= glomerular filtration rate;
- MPRAGE
= magnetization prepared rapid acquisition gradient echo;
- MTL
= medial temporal lobe;
- NAME
= Nutrition and Memory in Elders;
- NINDS
= National Institute of Neurological Disorders and Stroke;
- OR
= odds ratio;
- WMH
= white matter hyperintensities.
Cerebrovascular disease (CVD) and microvascular changes are etiologic hallmarks of vascular dementia1,2 and also may contribute to the worsening of Alzheimer disease (AD) symptoms.3 Medial temporal lobe, cortical atrophy, and white matter hyperintensities (WMH), also referred to as leukoaraiosis, have been reported in AD1 but do not occur exclusively in individuals with this diagnosis. Furthermore, studies have shown that MRI demonstration of CVD is associated with an increased risk of dementia and poorer cognition.4
There is evidence of neuroprotective functions of vitamin D and growing evidence of a potential role of vitamin D in cognitive function.5–7 Vitamin D may help to prevent neurodegenerative diseases of aging through protection against comorbidities such as cardiovascular and cerebrovascular disease,8,9 peripheral artery disease,10 oxidation and inflammation,11 and neuronal health.12,13 However, there is a paucity of data on the association between biomarkers of vitamin D, cognition, dementia, and MRI measures of CVD.
The objectives of the current study were to explore mechanisms through which vitamin D may be involved in neurocognitive function. Using a subset of the population previously studied, we examined associations between 25-hydroxyvitamin D [25(OH)D] and diagnoses of neurodegenerative diseases, MRI indicators for vascular brain pathology, and volumetric measures of brain structures in a racially diverse elderly population receiving home care in Boston, Massachusetts.
METHODS
Detailed methodology for the Nutrition and Memory in Elders (NAME) study have been described previously.14 Between 2003 and 2007, participants were recruited at 4 Boston-area home care agencies that provide services for home living. Individuals eligible for home services were at least 60 years old, had low income (determined by the Commonwealth of Massachusetts), had a diminished ability to perform activities of daily living, and had an unmet need in a critical area (food or personal care). Exclusion criteria included non–English fluency, severe auditory or visual impairment, mental retardation, and brain tumor. The institutional review board at Tufts Medical Center approved the protocol.
All 1,248 NAME study participants agreed to 3 home evaluations over 1 to 3 weeks: evaluation 1 included informed consent and neuropsychological testing; evaluation 2 included fasting phlebotomy; and evaluation 3 included medical history, medication ascertainment, and physical assessment. Subsequently, 365 participants underwent examination at Tufts Medical Center, including brain imaging within 1 month of the final home visit.
Home data ascertainment.
Information related to demographics and medical history was obtained through interviewer-administered questionnaires. Interviewers examined medication, and participants were queried about medication use. Dietary intake was captured using a validated semiquantitative food frequency questionnaire (FFQ).15 Use of multivitamins and vitamin D supplements was verified and captured. The brand name and type of supplement were explored using nutrient data capture software and captured on the FFQ. Blood pressure was the average of 2 measurements after 5 minutes of rest in a seated position. Habitual physical activity was assessed using the Paffenbarger questionnaire.16 Additionally, subjects were asked to report typical activity level and the number of hours spent sleeping or lying down (nighttime sleep, naps, reclining), light activity (office work, light housework, driving, personal care, standing), and sitting activity (eating, reading, watching television).
Phlebotomy.
A fasting blood sample was obtained, divided into aliquots, centrifuged, and transported on ice. Serum chemistries were assessed immediately. Samples were stored at −70°C; these were later analyzed for circulating 25(OH)D concentration in batch from 50 μL of plasma using the DiaSorin 125I radioimmunoassay (DiaSorin, Inc., Stillwater, MN) after a single freeze-thaw cycle.17 The inter- and intra-assay coefficients of variation were 9.4% and 10.8%, and the assay reference range was 8–38 ng/mL; values outside of this range were reevaluated in confirmatory analyses.
Definitions.
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. In cases where standing height was unobtainable, height was estimated using knee height measures.18 Diabetes was defined by fasting serum glucose of ≥126 mg/dL or use of insulin or oral hypoglycemic agents, according to American Diabetes Association guidelines. Hypertension was defined as ≥140 or 90 mm Hg for systolic or diastolic blood pressure, or use of antihypertensive medication. Cerebrovascular disease was defined as periventricular hyperintensities, large and small vessel infarcts, and brain atrophy.
After indirect calibration of serum creatinine values, glomerular filtration rate (GFR) was estimated using the 4-variable Modification of Diet in Renal Disease Study equation re-expressed for isotope dilution mass spectroscopy standard.19 Subjects with estimated GFR <60 mL/minute per 1.73 m2 were classified with reduced kidney function (Kidney Disease Outcomes Quality Initiative clinical practice guidelines, 2002). Vitamin D categories were determined based on the distribution in this population and consistent with relevant reference populations and were defined as deficient (<10 ng/mL), insufficient (10–20 ng/mL), or sufficient (>20 ng/mL).20,21 Vitamin D “insufficiency” will be used to describe physiologic 25(OH)D concentrations ≤20 ng/mL for reference.
Neurologic examination.
A board-certified neurologist completed a neurologic history and physical examination. All subjects were scored on the NIH Stroke Scale. Subjects were classified with symptomatic CVD or evidence of peripheral neuropathy defined as involvement of small or large fiber–mediated sensation. Presence of stroke was classified according to the Trial of Org 10172 in Acute Stroke Treatment criteria.22
Dementia classification.
A consensus committee including a psychiatrist, neuropsychologist, neurologist, and neuroradiologist reviewed clinical data and assigned the following diagnoses: dementia (probable or possible AD, vascular, and other) or no diagnosable cognitive decline. AD was defined using National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria.23 Vascular dementia was defined using National Institute of Neurological Disorders and Stroke (NINDS)–Association Internationale pour la Recherché et l'Enseignement en Neurosciences (AIREN) criteria.24 Other dementias were classified according to DSM-IV criteria.25 For subjects determined to have both AD and CVD, the NINDS-AIREN guidelines were followed, and the subject was assigned to the Alzheimer's group.24
MRI.
Cranial MRI was performed with a 1.5-T Symphony superconducting magnet (Siemens, Iselin, NJ). The MRI protocol included 1) T1-weighted axial images, (2) intermediate and T2-weighted conventional spin-echo axial images, (3) fluid-attenuation inversion recovery (FLAIR) turbo spin-echo axial images, and (4) magnetization prepared rapid acquisition gradient echo (MPRAGE) coronal images.
High-resolution images were reviewed by primary and secondary trained analysts under the supervision of a neuroradiologist. Interrater reliability was r = 0.92 (p < 0.001). Analyze™ image analysis software (Biomedical Imaging Resource, 1986–2004) was used to quantify brain segmentation and region of interest analysis. Analysts were blinded to the subjects' nutritional status and study results.
Quantitative segmentation of CSF from brain was performed using the method described by DeCarli et al.26 Measurements of total intracranial volume, total brain volume, and CSF volume (subarachnoid and ventricular) were obtained. Medial temporal lobe (MTL) volumes were determined by outlining the hippocampus, amygdala, and parahippocampal gyrus on consecutive images of MPRAGE sequence. Brain and CSF volumes were corrected for intracranial volume, and MTL volumes were corrected for brain volume/intracranial volume.
WMH, indicators of vascular pathology, were defined as hyperintense changes on intermediate-intensity/FLAIR and T2-weighted images with no corresponding T1 abnormality. To reliably assess WMH, both qualitative and quantitative methods were used. For qualitative analyses, a neuroradiologist (R.A.B.) compared the findings of WMH on spin-density–weighted axial images to a set of scans that demonstrate successively increasing changes, from barely detectable (grade 0–1) to extensive and confluent (grade 9).27 Quantitative methods involved histogram analysis procedures described by De Carli et al.26
Brain infarcts were measured to quantify subclinical and clinical CVD. Large vessel infarcts were defined as cortical infarcts of any size and subcortical infarcts >1.5 cm in maximum dimension, whereas small vessel infarcts were defined as subcortical infarcts 3 mm to 1.5 cm. To qualify for an infarct, the lesion had increased signal on T2 and FLAIR images. For subcortical infarcts in white matter, additional low signal on T1-weighted images was required to be considered as infarct.27
Statistical analysis.
There were 365 participants who consented to MRI. Subjects were excluded for the following reasons: missing 25(OH)D concentrations (n = 26), ineligible because of presence of metal or withdrew consent (n = 10), or movement artifact (n = 11). Of the remaining eligible subjects, consensus diagnosis was available for all 318 subjects, and complete MRI data were available for 305 subjects. For the analyses, participants were categorized as black (n = 109) or nonblack (n = 209).
All analyses were completed using SAS (version 9.1, ©2007, SAS Institute, Inc., Cary, NC). Variable distributions were evaluated, and those not normally distributed (specifically WMH volume) were log-transformed when appropriate. Initial comparisons of demographic and descriptor variables stratified by race were performed using t tests and χ2 tests, as appropriate, because of the known differences in vitamin D status by race. Group means were adjusted and compared with analysis of covariance, and interactions were investigated by including interaction terms in the models. Univariate and multiple regression models were constructed to examine associations of plasma 25(OH)D and volumetric measures of brain structures.
Logistic regression was used to examine associations with all-cause dementia, AD, and stroke (with and without dementia) with 25(OH)D concentration. Covariates were selected a priori, based on clinical factors and confounding effects: age, BMI, plasma homocysteine, and physical activity were included as continuous; and sex, race, education, ApoE allele status (presence or absence of any E4 allele), diabetes, hypertension, home care center, and multivitamin use were entered as dichotomous variables. Other nutritional covariates possibly associated with dementia, such as ω-3 fatty acids and B vitamins, were also explored.
RESULTS
Baseline characteristics for the participants in this subset (n = 318) were similar to the full cohort (n = 1,080; data not shown). The mean age was 73.5 ± 8.1 years, and the majority of participants were women (73%) (table 1). Nonblack participants were significantly older than black participants, had lower BMI, and were more likely to have at least a high school education (each p < 0.01). Hypertension, diabetes, and reduced kidney function were prevalent, at 84, 31, and 28% of participants. More than 50% of the population used multivitamins, and approximately 21% used vitamin D supplements.
Table 1 Descriptive characteristics of elderly adults receiving home care services in Boston, Massachusetts
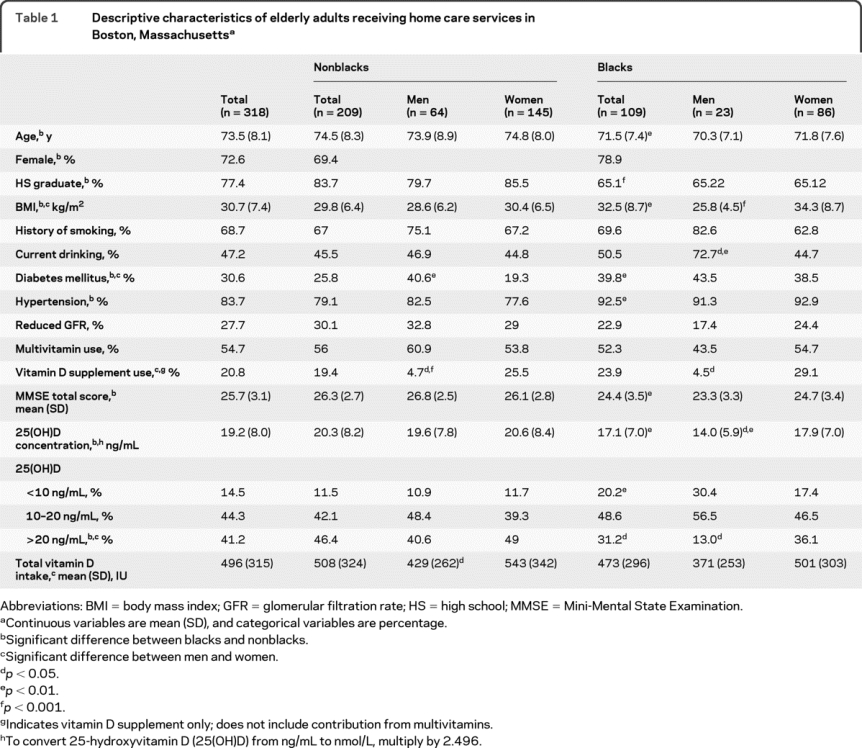
Vitamin D insufficiency (≤20 ng/mL) and deficiency were prevalent (44.3% and 15%). Blacks were more likely than nonblacks to have 25(OH)D concentrations ≤20 ng/mL (69% vs 54%, p < 0.01) and were twice as likely to have 25(OH)D concentrations <10 ng/mL (20% vs 12%, p < 0.01). 25(OH)D concentrations did not vary significantly by season in either race or in the full population (December through February: 17 ng/mL; March through May:18 ng/mL; June through August: 20 ng/mL; September through November: 19 ng/mL).
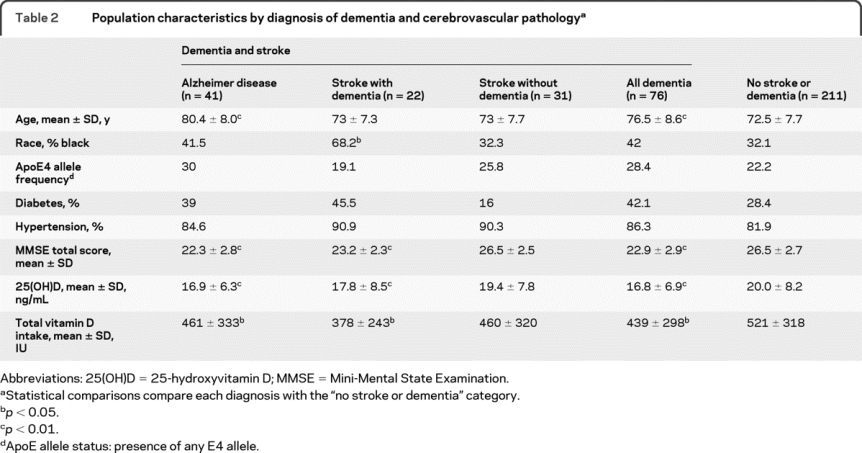
There were 76 participants with dementia; 41 were clinically classified with AD, 21 were classified with vascular dementia, 31 were classified with stroke without symptoms of dementia, and 14 were classified with other dementias (this category included: Lewy body dementia, alcohol related dementia, Parkinson disease, and dementia secondary to severe head trauma). Of the 41 participants with AD, 35 (85.4%) had evidence of vascular pathology (WMH grade >1). Participants with dementia were older and had lower vitamin D intakes and 25(OH)D concentrations than those without dementia (table 2).
Table 2 Population characteristics by diagnosis of dementia and cerebrovascular pathology
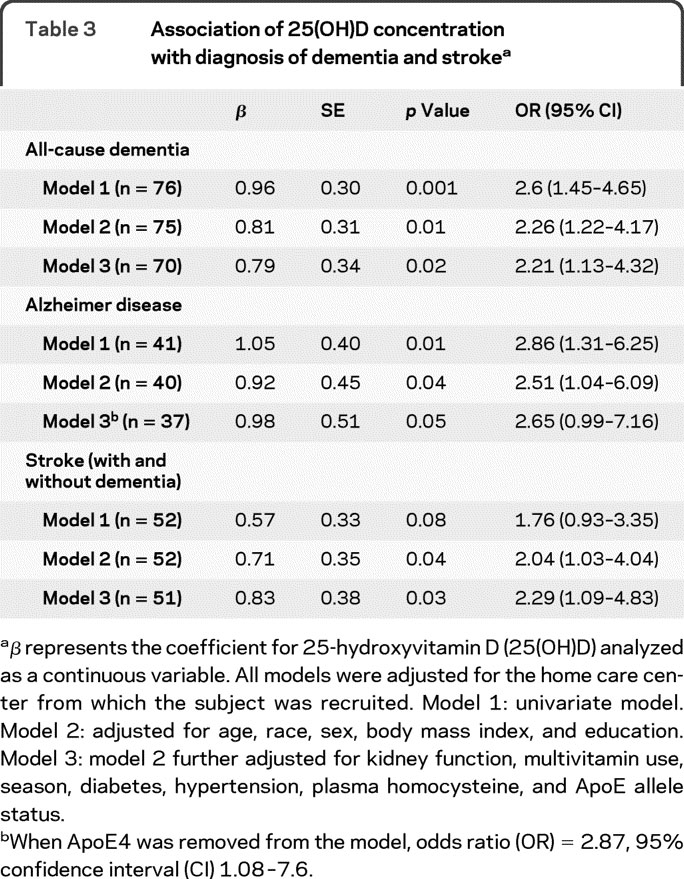
25(OH)D insufficiency (≤20 ng/mL) was associated with more than twice the risk of all-cause dementia (AD, stroke with dementia, and other; odds ratio [OR] = 2.6, 95% confidence interval [CI] 1.5–4.7) after adjustment for age, race, sex, BMI, education, kidney function, multivitamin use, season, diabetes, hypertension, physical activity, plasma homocysteine, and ApoE4 allele status (table 3, model 3). Similarly, 25(OH)D insufficiency was associated with more than twice the risk of AD in univariate models (OR = 2.9, 95% CI 1.3–6.3) after adjustment for age, race, sex, BMI, and education (OR = 2.5, 95% CI 1.04–6.1). In fully adjusted models, the association was attenuated (OR = 2.7, 95% CI 0.99–7.2); however, after ApoE4 allele status was removed from the model, the association was significant (OR = 2.9, 95% CI 1.1–7.6). The association between 25(OH)D insufficiency and AD in fully adjusted models was also attenuated after addition of plasma vitamin B6 and dietary intakes of ω-3 fatty acids (data not shown).
Table 3 Association of 25(OH)D concentration with diagnosis of dementia and stroke
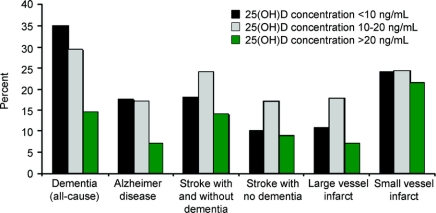
25(OH)D deficiency was associated with a higher prevalence of stroke (with and without dementia) and AD (18.4% vs 13.9%, p < 0.001 and 17.4% vs 6.9%, p < 0.01; table 4; figure). In fully adjusted logistic regression models, where stroke with and without dementia were combined (n = 52), 25(OH)D insufficiency was associated with twice the risk of stroke (OR = 2.3, 95% CI 1.1–4.8; table 3, model 3). However, when subjects with stroke were stratified by the presence (n = 21) or absence of dementia (n = 31), 25(OH)D status was not associated with prevalence of stroke (data not shown).
Table 4 Adjusted mean brain volumetric measures and consensus diagnoses according to 25(OH)D concentrations and intakes
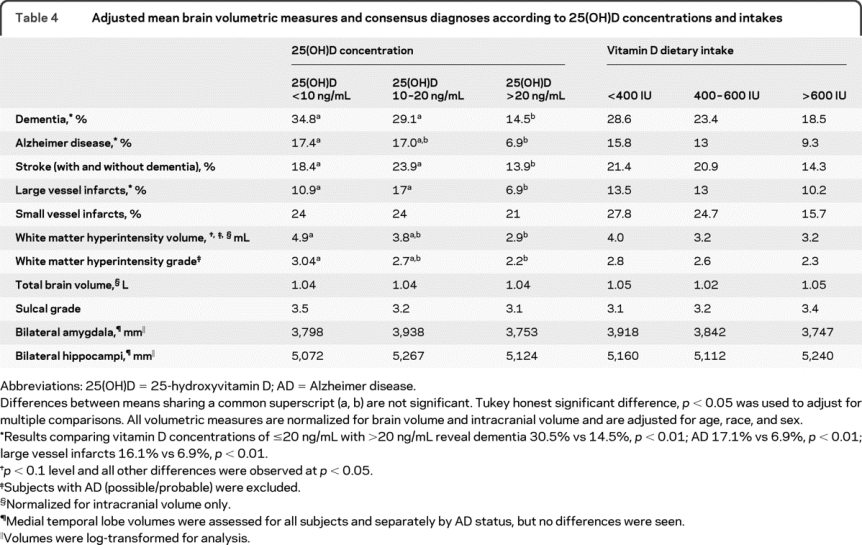
Figure 25-Hydroxyvitamin D status and prevalence of dementia and stroke
WMH volume was inversely associated with 25(OH)D concentrations (data not shown), and 25(OH)D deficiency was associated with higher geometric mean WMH volume (4.9 vs 2.9 mL, p = 0.004), WMH grade (3.2 vs 2.2, p = 0.02), and prevalence of large vessel infarcts (16.1% vs 6.9%, p < 0.01) (table 4; figure). This trend was evident in both dichotomous (25(OH)D ≤20 and >20 ng/mL) and categorical (25(OH)D <10, 10–20, and >20 ng/mL) comparisons (table 4).
Consistent with the literature, hippocampal volume was smaller in subjects with AD (5,173 vs 4,744 mm3, p = 0.002), but there was no difference in hippocampal volume in subjects with cerebrovascular disease, with and without dementia. Additionally, there was no association between vitamin D and volumetric measures of the amygdala, hippocampi, total brain volume, cortical atrophy, or sulcal grade after adjustment for intracranial volume and covariates such as age, race, sex, and education. Although vitamin D concentration ≤20 ng/mL was associated with increased hippocampal volume in unadjusted models (5,124 vs 5,027 mm3), the association was attenuated after adjustment for age, race, and sex and was no longer evident after adjustment for intracranial volume (data not shown).
DISCUSSION
In these elderly individuals, low 25(OH)D was associated with all-cause dementia; further associations between 25(OH)D and WMH support the hypothesis that vascular factors may mediate the association between vitamin D deficiency and cognitive function. We previously reported a significant association between 25(OH)D concentration and executive function and global cognition.5 In this analysis in a subset of the population, we identified associations between 25(OH)D concentrations and diagnoses of AD and stroke (with and without symptoms of dementia). Furthermore, we saw associations between 25(OH)D and specific indicators of vascular pathology on MRI. Lower 25(OH)D was associated with the presence of large vessel infarcts and WMH volume and severity. Together, these findings suggest an association between vitamin D and subcortical function consistent with recent vasculoprotective evidence for this hormone.9,10,28
Dementia is oftentimes multifactorial, and AD and vascular dementia share etiologic features. We observed associations between 25(OH)D, AD, and stroke with and without dementia symptoms. Dementia symptomatology secondary to stroke is related to the location of the stroke and is a symptom of vascular impairment. We explored the independent associations between 25(OH)D and the prevalence of stroke when stratified by the presence (n = 31) or absence (n = 21) of dementia symptoms and found no association.
Our data also showed an independent association between lower circulating 25(OH)D and MRI indicators of cerebrovascular disease. 25(OH)D was inversely associated with WMH volume and severity. The presence of WMH and large volume infarcts are common findings in the elderly and are consistent with vascular comorbidities, such as systolic blood pressure and vascular disease.29 WMH are also associated with brain atrophy, impaired cerebral vascular function, and poorer frontal lobe cognition.29 Our findings showed that vitamin D deficiency was associated with a higher prevalence of WMH volume and severity and corresponded to an association between circulating 25(OH)D and vascular tissue health. The findings are consistent with recent reports that low vitamin D was associated with incident cardiovascular disease,9 stroke,28 and peripheral arterial disease.10 Furthermore, exploratory analyses revealed that WMH grade was associated with poorer performance on Trails B, a test of executive functioning. These findings are consistent with reports from the Rotterdam study, which found that WMH were associated with processing speed but not memory.30
In addition to vasculoprotective mechanisms, vitamin D deficiency has been associated with morphologic brain changes,31 motor impairments,32 and memory and learning impairments33 in animal models. We did not observe associations between vitamin D and volumetric measures of brain structures of the hippocampus and amygdala after adjustment for relevant covariates.
Although causal mechanisms cannot be inferred from these cross-sectional data, it is important to consider mechanism in which vitamin D may be neuroprotective. Vitamin D inhibits nitric oxide synthase,34 up-regulates enzymes in glutathione and neurotrophin synthesis,11,13 and regulates neuronal calcium.12 Other functional attributes of 25(OH)D have been shown to attenuate ischemic disease in animal models35 and protect neuronal integrity.11 Also, therapeutic intervention with vitamin D reduced cardiovascular comorbidities such as hypertension36 and cardiac hypertrophy. This evidence underscores a potential neurovascular benefit of vitamin D.
Vitamin D inadequacy in elders is well documented. Studies in community-dwelling elders have revealed that 73% of blacks and 35% of whites have low vitamin D concentrations (<50 nmol/L) and 21% of blacks and 11% of whites have very low serum vitamin D levels (<25 nmol/L) during the wintertime,21 and these rates are higher in inactive elders.37 Homebound elders are at increased risk of inadequacy because of limited sun exposure, decreased 7-dehydrocholesterol in the skin, physical impairment, high BMI, and inadequate nutrition. Although increased sun exposure is controversial, dietary modifications may be beneficial. Dietary intakes of at least 800 IU are thought necessary to achieve adequate 25(OH)D concentrations of 75 nmol/L,38 and less than 7% of our population had concentrations in this range. Additionally, our data showed that fewer than 27% of subjects were achieving adequate intakes (ages 50–70 years: 400 IU, ages >70 years: 600 IU). Of non–supplement users, fewer than 15% had intakes greater than 400 IU, 8% had intakes greater than 600 IU/day, and only 3% had intakes of 800 IU or more.
Approximately 30% of free-living elders with mild cognitive impairment are likely to develop dementia and require institutionalization. If the associations seen here are confirmed, maintenance of healthy vitamin D status could prove a cost-effective contribution toward preservation of cognitive dysfunction and support lengthened independent living for the elderly.
This study is cross-sectional and limited in ability to determine a temporal association between vitamin D, volumetric brain measures, and diagnoses of neurodegenerative disease. Furthermore, it must be considered in this cross-sectional study that the low vitamin D status may be a consequence of limited sun exposure due to cognitive or physical impairments. The participants in this study were recruited from community social service groups and are therefore not generalizable to a community-dwelling population. Therefore, we cautiously interpret the associations revealed herein. Although the extent to which vitamin D deficiency and cognitive function are related remains unclear, the biologic plausibility of this relationship is increasingly supported.
AUTHOR CONTRIBUTIONS
Statistical analysis was completed by J.S. Buell and reviewed by G.E. Dallal and K.L. Tucker.
ACKNOWLEDGMENT
The authors thank the NAME study team, John Griffith, PhD, and Lori Lyn Price, MS, and the Tufts Medical Center Clinical Research Center for their support and guidance in the data management of this study.
DISCLOSURE
Dr. Buell, Dr. Qiu, Dr. Rosenberg, and Dr. Bhadelia report no disclosures. Dr. Dawson-Hughes serves/has served on scientific advisory boards or as a consultant for Cytochroma Scientific, Danone, Johnson & Johnson, Eli Lilly and Co., Maxesence, Merck & Co. Inc., Quest Diagnostics, Inc., Tethys Bioscience, Unipath Ltd., and Wyeth Pharmaceuticals, Co.; served as an Associate Editor of the Journal of Bone and Mineral Research and on the editorial boards of The American Journal of Medicine and Bone; receives royalties from publishing Nutrition and Bone Health and Up-to-Date; has received research support from Unilever, the NIH [RO1AR052322-01A1 (PI), RO1 AR051361-01A1 (Coinvestigator), RO1 DK076092-01 (Coinvestigator), RO1 AG02707087-01A1 (Coinvestigator), ROI DK79003 (Coinvestigator), R21 DK78867 (Coinvestigator), and R21 AT003714-01 (Coinvestigator)]; and serves as a trustee of the National Osteoporosis Foundation and the International Osteoporosis Foundation. Dr. Scott has received honoraria from Kraft Foods Global Brands LLC for participation in a workshop. Dr. Weiner serves as Deputy Editor of the American Journal of Kidney Diseases; and receives research support from Covidien, Amgen, and the NIH [NIDDK K23DK071636 (PI)]. Dr. Dallal receives research support from the NIH [R01 AR052322-0121 (Coinvestigator) and NIDDK 1R01 DK69341 (Coinvestigator)], the National Dairy Council [GRANT (Coinvestigator)], and the USDA [58–1950-7-707/8–816 (PI)]; and has provided expert testimony subject to a confidentiality agreement. Dr. Bergethon receives royalties from publishing The Physical Basis of Biochemistry (Springer-Verlag, 1998); receives honoraria for non–industry-sponsored activities; and receives research support from the NIH [NINDS R01NS059933 (PI), NIDDK R13DK069541 (PI), NIGMS RR007591 (Co-PI), and NIA R01AG021790 (Co-PI)] and the Department of Defense [CIMIT (PI)]. Dr. Folstein receives royalties from publishing MiniMental (PAR Publishing, 2001). Dr. Patz has received nonindustry funding for travel; has applied for US Patent 4,558,943 (1985), US Patent 4,558,943 (1986), US Patent 4,558,943 (1988), US patent 5,365,172 (1994), and US Patent 5,572,132 (1996); has received royalties from publishing (Springer-Verlag, 2009); has received research support as a subcontract PI from Xemed, LLC [NIH SBIR R42CA124242-01 and NIH SBIR R41 HL083545–01], from the NIH [RO1 HL073632 (PI), EY13825 (Coinvestigator), and 5R01AG021790 (Coinvestigator)], from the Department of Radiology, Brigham and Women's Hospital for the Center for Pulmonary Functional Imaging (Scientific Director), the Partners Radiology Research Committee (Co-PI), and the FAMRI [Clinical Innovator Award (PI)]. Dr. Tucker serves as Associate Editor of the Journal of Nutrition and on the editorial board of the British Journal of Nutrition; has received non–industry-sponsored speaker honoraria; serves as a consultant for Emisphere Technologies; and receives research support from Kraft Foods, the USDA [CRIS 1950-5153–007-045 (PI)], and the NIH [R01AG027087A (PI), P01 AG023394 (PI), R01 AR41398 (Coinvestigator), HL54776 (Coinvestigator), R01 AR053205 (Coinvestigator), R01 DK064902 (Coinvestigator), and R01 AG21790 (Coinvestigator)].
Address correspondence and reprint requests to Dr. Katherine L. Tucker, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington St., Boston, MA 02111 katherine.tucker@tufts.edu
Editorial, page 13
See also pages 27 and 33
e-Pub ahead of print on November 25, 2009, at www.neurology.org.
Study funding: Supported in part by NIH grants AG21790-01 and K23 DK71636, the Tufts Medical Center Clinical Research Center, and USDA ARS agreement 58-1950-7-707.
Disclosure: Author disclosures are provided at the end of the article.
Received December 1, 2008. Accepted in final form July 16, 2009.
REFERENCES
- 1.Roman GC. Stroke, cognitive decline and vascular dementia: the silent epidemic of the 21st century. Neuroepidemiology 2003;22:161–164. [DOI] [PubMed] [Google Scholar]
- 2.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003;34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 3.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: The Nun Study. JAMA 1997;277:813–817. [PubMed] [Google Scholar]
- 4.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 5.Buell JS, Scott TM, Dawson-Hughes B, et al. Vitamin D is associated with cognitive function in elders receiving home health services. J Gerontol A Biol Sci Med Sci 2009;64:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys Highlight Issue: Vitamin D 2007;460:202–205. [DOI] [PubMed]
- 7.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry 2006;14:1033–1040. [DOI] [PubMed] [Google Scholar]
- 8.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002;110:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 2008;51:1073–1079. [DOI] [PubMed] [Google Scholar]
- 10.Melamed ML, Muntner P, Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol 2008;28:1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcion E, Sindji L, Leblondel G, Brachet P, Darcy F. 1,25-dihydroxyvitamin D3 regulates the synthesis of gamma-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J Neurochem 1999;73:859–866. [DOI] [PubMed] [Google Scholar]
- 12.Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 2001;21:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neveu I, Naveilhan P, Baudet C, Brachet P, Metsis M. 1,25-dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. Neuroreport 1994;6:124–126. [DOI] [PubMed] [Google Scholar]
- 14.Scott TM, Peter I, Tucker KL, et al. The Nutrition, Aging, and Memory in Elders (NAME) study: design and methods for a study of micronutrients and cognitive function in a homebound elderly population. Int J Geriatr Psychiatry 2006;21:519–528. [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc 1987;87:43–47. [PubMed] [Google Scholar]
- 16.Paffenbarger RS Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol 1978;108:161–175. [DOI] [PubMed] [Google Scholar]
- 17.DiaSorin. Catalog 68100E. Stillwater, MN: DiaSorin; 2001. [Google Scholar]
- 18.Chumlea WC, Roche AF, Steinbaugh ML. Estimating stature from knee height for persons 60 to 90 years of age. J Am Geriatr Soc 1985;33:116–120. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 20.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med 2002;112:659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab 2000;85:4125–4130. [DOI] [PubMed] [Google Scholar]
- 22.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 24.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 26.DeCarli C, Maisog J, Murphy D, Teichberg D, Rapoport S, Horowitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr 1992;16:274–284. [DOI] [PubMed] [Google Scholar]
- 27.Bryan RN, Wells SW, Miller TJ, et al. Infarctlike lesions in the brain: prevalence and anatomic characteristics at MR imaging of the elderly: data from the Cardiovascular Health Study. Radiology 1997;202:47–54. [DOI] [PubMed] [Google Scholar]
- 28.Pilz S, Dobnig H, Fischer JE, et al. Low Vitamin D levels predict stroke in patients referred to coronary angiography. Stroke 2008;39:2611–2613. [DOI] [PubMed] [Google Scholar]
- 29.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995;45:2077–2084. [DOI] [PubMed] [Google Scholar]
- 30.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000;47:145–151. [DOI] [PubMed] [Google Scholar]
- 31.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience 2003;118:641–653. [DOI] [PubMed] [Google Scholar]
- 32.Burne TH, Becker A, Brown J, Eyles DW, Mackay-Sim A, McGrath JJ. Transient prenatal Vitamin D deficiency is associated with hyperlocomotion in adult rats. Behav Brain Res 2004;154:549–555. [DOI] [PubMed] [Google Scholar]
- 33.Becker A, Eyles DW, McGrath JJ, Grecksch G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res 2005;161:306–312. [DOI] [PubMed] [Google Scholar]
- 34.Garcion E, Nataf S, Berod A, Darcy F, Brachet P. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res 1997;45:255–267. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Chiang YH, Su TP, et al. Vitamin D(3) attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology 2000;39:873–880. [DOI] [PubMed] [Google Scholar]
- 36.Forman JP, Bischoff-Ferrari HA, Willett WC, Stampfer MJ, Curhan GC. Vitamin D intake and risk of incident hypertension: results from three large prospective cohort studies. Hypertension 2005;46:676–682. [DOI] [PubMed] [Google Scholar]
- 37.Bischoff H, Stahelin HB, Vogt P, et al. Immobility as a major cause of bone remodeling in residents of a long-stay geriatric ward. Calcif Tissue Int 1999;64:485–489. [DOI] [PubMed] [Google Scholar]
- 38.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005;16:713–716. [DOI] [PubMed] [Google Scholar]