Abstract
Objective:
To investigate the specificity of in vivo amyloid imaging with [11C]–Pittsburgh Compound B (PIB) in Parkinson disease dementia (PDD).
Methods:
We performed detailed neuropathologic examination for 3 individuals with PDD who had PIB PET imaging within 15 months of death.
Results:
We observed elevated cortical uptake of [11C]-PIB on in vivo PET imaging in 2 of the 3 cases. At autopsy, all 3 individuals had abundant cortical Lewy bodies (Braak PD stage 6), and were classified as low-probability Alzheimer disease (AD) based on NIA-Reagan criteria. The 2 PIB-positive individuals had abundant diffuse Aβ plaques but only sparse neuritic plaques and intermediate neurofibrillary tangle pathology. The PIB-negative individual had rare diffuse plaques, no neuritic plaques, and low neurofibrillary tangle burden.
Conclusions:
[11C]–Pittsburgh Compound B (PIB) PET is specific for fibrillar Aβ molecular pathology but not for pathologic diagnosis of comorbid Alzheimer disease in individuals with Parkinson disease dementia. The ability to specifically identify fibrillar Aβ amyloid in the setting of α-synucleinopathy makes [11C]-PIB PET a valuable tool for prospectively evaluating how the presence of Aβ amyloid influences the clinical course of dementia in patients with Lewy body disorders.
GLOSSARY
- AD
= Alzheimer disease;
- BP
= binding potentials;
- CDR
= Clinical Dementia Rating;
- DAT
= dementia of the Alzheimer type;
- DLB
= dementia with Lewy bodies;
- DV
= distribution volume;
- MMSE
= Mental State Examination;
- NPI-Q
= Neuropsychiatric Inventory Questionnaire;
- PDD
= Parkinson disease dementia;
- PIB
= Pittsburgh Compound B;
- UPDRS
= Unified Parkinson's Disease Rating Scale.
Individuals with Parkinson disease (PD) are nearly 6 times more likely to develop dementia than age-matched controls, and the majority of individuals with PD who survive more than 15 years after diagnosis will develop dementia.1,2 Clinicopathologic investigations have revealed heterogeneous histopathology, with Alzheimer disease (AD) pathology (amyloid plaques and neurofibrillary tangles) present in a subset of individuals with PD dementia (PDD).1 When present, AD pathology is typically found in conjunction with other neuropathologic changes, including limbic and cortical Lewy bodies and degeneration of subcortical monoaminergic and cholinergic pathways. The contribution of AD pathology to the pathogenesis of dementia in the setting of PD is thus uncertain. The presence of AD pathology has been postulated to influence clinical manifestations of dementia, for example masking features of dementia with Lewy bodies (DLB) such as hallucinations and fluctuations3,4 or influencing the timing of dementia onset in patients with Lewy body disorders.5
Antemortem evaluation of Aβ plaque burden by PET imaging using amyloid-specific radiotracers can potentially clarify the role of these lesions in the pathogenesis of Lewy body–associated dementias (PDD and DLB). The tracer N-methyl-[11C]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole (or [11C]-PIB for Pittsburgh Compound-B) has shown great promise for this purpose, demonstrating rapid diffusion across the blood–brain barrier, high affinity to a single binding site on synthetic Aβ (Kd = 4.7 nM), and high affinity to a single binding site in homogenates of frontal cortex from brains with AD (Kd = 1.4 nM).6,7 PET images of [11C]-PIB in individuals with dementia of the Alzheimer type (DAT) reveal widespread increased tracer uptake in neocortical regions, with relative sparing of the occipital and sensory/motor cortices and minimal uptake in the cerebellar cortex.8
In an ongoing cohort study, we have performed [11C]-PIB PET imaging in individuals with Lewy body disorders (including cognitively normal PD, PDD, and DLB) followed by longitudinal clinical and psychometric assessments. All enrolled participants have consented to postmortem neuropathologic evaluation. Our preliminary results, reported previously in abstract form, are consistent with findings of other investigators,9–11 with a subset of individuals with Lewy body disorders (∼20%) demonstrating elevated cortical [11C]-PIB uptake.12 We report here the postmortem neuropathologic findings for 3 individuals with PDD who had in vivo [11C]-PIB PET imaging and subsequently had autopsy.
METHODS
Standard protocol approvals, registrations, and patient consents.
All study procedures were approved by Washington University's Human Research Protection Office. Prior to enrollment, written informed consent was obtained for all participants. For individuals lacking capacity due to dementia, a surrogate decision-maker (spouse, first-degree family member, or health care proxy) provided informed consent and ongoing assent was obtained from the participant throughout the study procedures.
Subject selection and clinical assessment.
Individuals were recruited from the greater St. Louis area; there were no gender or race restrictions. Participants with clinically probable or definite idiopathic PD (modified United Kingdom PD Brain Bank criteria13) or DLB14 were screened via detailed clinical history (including review of motor, cognitive, and neuropsychiatric symptoms, comorbid medical conditions, and medications) and neurologic examination. Exclusionary criteria included other conditions that could contribute substantially to the subject's motor and/or cognitive impairment, including neurologic (e.g., Parkinson-plus disorders other than DLB), psychiatric (e.g., major affective disorders, unless cognitive impairment and mood symptoms were clearly temporally dissociated), or medical conditions (e.g., drug-induced or other delirium). From October 2006 to March 2009, we enrolled 10 healthy elderly control individuals and 40 individuals with iPD or DLB. Three of the enrolled participants subsequently died and donated their brains for the study.
All 3 of the deceased participants met clinical diagnostic criteria for PDD1; none had a known family history of dementia or PD. Individual histories are provided in appendix e-1 on the Neurology® Web site at www.neurology.org, and clinical data are summarized in table 1. Motor symptoms were assessed using the Unified Parkinson's Disease Rating Scale (UPDRS) subscale 3 (motor subscale) and Hoehn-Yahr staging while having benefit from medication (ON state). Levodopa equivalent daily dose was calculated using the following corrections: dose levodopa in sustained release form × 0.75; dose of levodopa taken with catechol-O-methyltransferase inhibitor × 1.3. None of the 3 participants reported here took a dopamine agonist or monoamine oxidase inhibitor at the time of the evaluation.
Table 1 Clinical data
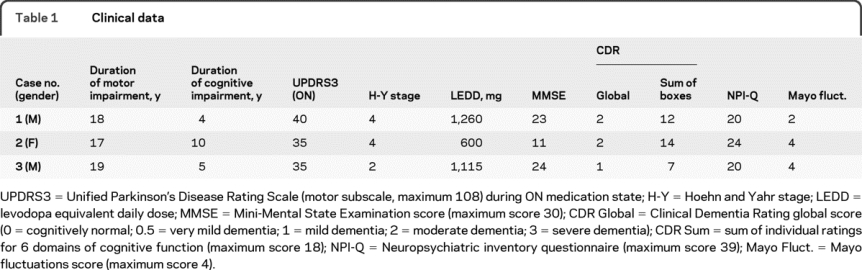
Severity of dementia was staged according to the Clinical Dementia Rating (CDR).15 Impairment of function in 6 domains (memory, orientation, judgment and problem solving, community affairs, home and hobby, and personal care) is rated on a 0–3 scale (0 = normal; 1 = mild; 2 = moderate; 3 = severe). Global (weighted average) and sum CDR ratings are presented. Other standardized assessments included Mini-Mental State Examination (MMSE16), Neuropsychiatric Inventory Questionnaire (NPI-Q17), and Mayo Fluctuations scale.18
In vivo amyloid imaging.
[11C]-PIB was synthesized according to published methods.19 PET imaging was performed using a Siemens 961 HR ECAT PET scanner (CTI, Knoxville, TN). Approximately 12 mCi of radiotracer (range, 10.4–14.5; specific activity ≥1,200 Ci/mmol) was injected via an antecubital vein, and a 60-minute, 3-dimensional (septa retracted) dynamic PET scan was collected. Images were reconstructed as 5-minute frames using scatter correction and a ramp filter. Frames were corrected for head motion using in-house software, and coregistered to the patient's T1-weighted magnetization-prepared rapid gradient echo magnetic resonance scan obtained the same day. For visual display, the data from 30 to 60 minutes after radiotracer injection were summed, Gaussian filtered (full width half-maximum = 6 mm), and normalized to average brainstem intensity values.
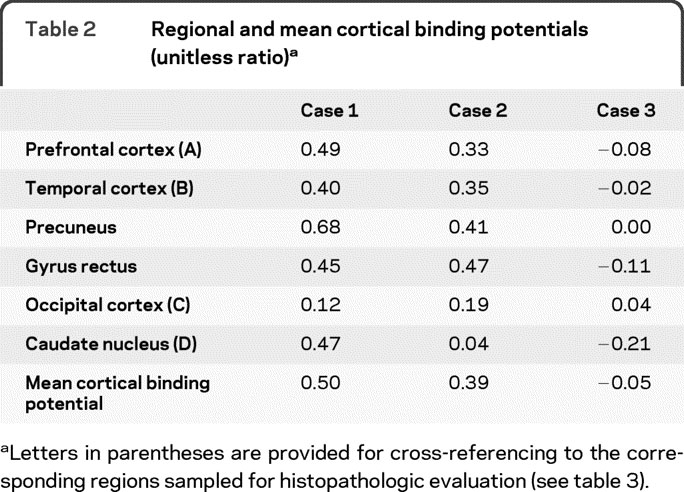
For quantitative analyses, 3-dimensional regions of interest (prefrontal cortex, gyrus rectus, lateral temporal cortex, precuneus, occipital lobe, caudate nucleus, brainstem, and cerebellum) were created for each subject based on their individual MRI scans, with boundaries defined as previously described.20 Time-activity curves were analyzed using Logan graphical analysis, with the cerebellum (which has minimal specific binding due to low Aβ plaque content) as the reference tissue input function.20,21 Binding potentials (BP) were calculated from the tracer distribution volume (DV, reflected in the slope of the Logan graphical analysis) as BP = DV − 1. Mean cortical binding potentials were calculated for each subject as the average of all cortical regions except occipital lobe (which typically has lower Aβ plaque burden, even in advanced AD). For comparison, published mean values of MCBP calculated using identical methods are 0.63 in DAT and 0.09 in age-matched controls20; values greater than 0.2 are associated with low CSF Aβ42 levels22 and are considered abnormally elevated.
Neuropathology.
Brains were fixed in 10% neutral buffered formalin for 2 weeks, paraffin wax-embedded, and sections cut at 6 μm. Blocks were taken from frontal, temporal, parietal, and occipital lobes, thalamus, striatum including the nucleus basalis of Meynert, amygdala, hippocampus, midbrain, pons, medulla oblongata, and the cervical spinal cord. Histologic stains included hematoxylin and eosin and modified Bielschowsky silver impregnation. Immunohistochemistry was performed using the following antibodies: Aβ (10D5, Elan Pharmaceuticals, San Francisco, CA), phosphorylated tau (PHF-1, supplied by Dr. Peter Davies, Albert Einstein Medical School, Bronx, NY), ubiquitin (Dako, Glostrup, Denmark), α-synuclein (LB-509, Zymed, CA), and TDP-43 (Proteintech, Inc., Chicago, IL). Amyloid and tau burden was scored semiquantitatively in each of the sampled regions (0 = none; 1 = few/mild; 2 = moderate; 3 = severe); α-synucleinopathy with Lewy bodies was rated according to the scheme proposed by McKeith et al.14 where 0 = none; 1 = <1 Lewy body per × 10 microscopic field; 2 = 1–3 Lewy bodies; 3 = 4–10 Lewy bodies; and 4 = >10 or numerous Lewy bodies.
RESULTS
In vivo amyloid imaging.
Images representing the distribution of [11C]-PIB PET activity from 30 to 60 minutes after tracer injection are shown in figure 1. Images from a 77-year-old female control participant without neurologic disease are displayed for comparison. Visual inspection revealed high signal in multiple cortical areas for 2 patients (case 1 and case 2), with relative sparing of primary sensorimotor and visual cortex. This pattern was highly similar to that previously described for patients clinically diagnosed with DAT.8,22,23 In contrast, case 3 and the control participant showed uptake predominantly in white matter regions. This likely reflects nonspecific retention, as a recent study showed that PIB binding to white matter homogenates is nonsaturable.24
Figure 1 [11C]-PIB PET images
(A) Case 1. (B) Case 2. Increased signal is evident in multiple cortical areas in these 2 individuals, including orbitofrontal and prefrontal cortex, precuneus, and temporal lobes. (C) Case 3. (D) Control participant. These 2 individuals have minimal PIB signal in cortical areas. PIB retention in white matter areas is likely due to nonspecific binding (see text).
Binding potentials for selected regions of interest and mean cortical binding potentials for the 3 individuals are presented in table 2. Cases 1 and 2 had elevated PIB binding potentials in multiple cortical regions, with relative sparing of the occipital cortex. Case 1 also had elevated binding in the caudate nucleus. In contrast, case 3 had binding potentials near zero for all regions.
Table 2 Regional and mean cortical binding potentials (unitless ratio)
Neuropathology.
Macroscopic findings included mild frontal, temporal, and parietal atrophy and dilatation of the lateral ventricles (less in case 3 than in cases 1 and 2), and normal hippocampal size in all 3 cases. As expected, there was pronounced loss of pigment in the substantia nigra (see representative images in figure e-1 on the Neurology® Web site at www.neurology.org).
Histologic findings for case 2 are illustrated in figure 2. In the substantia nigra, there was neuronal loss, extracellular pigment, gliosis, Lewy bodies, and Lewy neurites (figure 2, B and C). The parahippocampal gyrus contained abundant Lewy bodies (figure 2D) and moderate neurofibrillary tangle pathology (figure 2G). Extensive Aβ deposits in the frontal lobe were evident by immunohistochemistry (figure 2A); there was also cerebral amyloid angiopathy in leptomeningeal vessels.

Figure 2 Postmortem examination, microscopic (case 2)
(A) Aβ (10D5) immunohistochemistry. Low-power micrograph shows extensive Aβ deposits in the frontal lobe; there is also cerebral amyloid angiopathy in some leptomeningeal vessels. (B) Hematoxylin and eosin. In the substantia nigra, there is neuronal loss, extracellular pigment, gliosis, and a Lewy body (arrow) in a surviving pigmented neuron. (C–F) α-synuclein (LB-509) immunohistochemistry. (C) Lewy bodies and a Lewy neurite in the substantia nigra are more readily seen by α-synuclein immunohistochemistry. (D) Lewy bodies are present in the parahippocampal gyrus. (E) Lewy bodies are present in subfield CA4 of the hippocampus. (F) Dystrophic neurites are present in the CA1/CA2 subfields of the hippocampus. (G) Phosphorylated tau (PHF-1) immunohistochemistry. Neurofibrillary tangles and neuropil threads are present in the parahippocampal gyrus. (A, D–F bar = 500 μm; B, C, G, bar = 100 μm.)
Regional semiquantitative assessments of Aβ, tau, and α-synuclein burden for the 3 cases are presented in table 3. All 3 cases had abundant Lewy bodies in multiple neocortical and limbic regions. Cases 1 and 2 also had a high burden of Aβ plaque pathology (Braak amyloid stage C25), predominantly in the form of diffuse plaques. Although a few isocortical neurofibrillary tangles were seen in these 2 cases, they were very sparse and thus overall neurofibrillary tangle burden was rated as limbic stage (Braak NFT stage III26). In contrast, case 3 had minimal Aβ plaques (Braak amyloid stage A), and only sparse transentorhinal and entorhinal neurofibrillary tangles (Braak NFT stage I). Cerebral amyloid angiopathy was mild in case 1 and absent in the other 2 cases. Modest vascular pathology in the form of arteriolosclerosis was also a feature of all 3 brains (not shown). There were no infarcts in any of the 3 cases.
Table 3 Regional molecular pathology
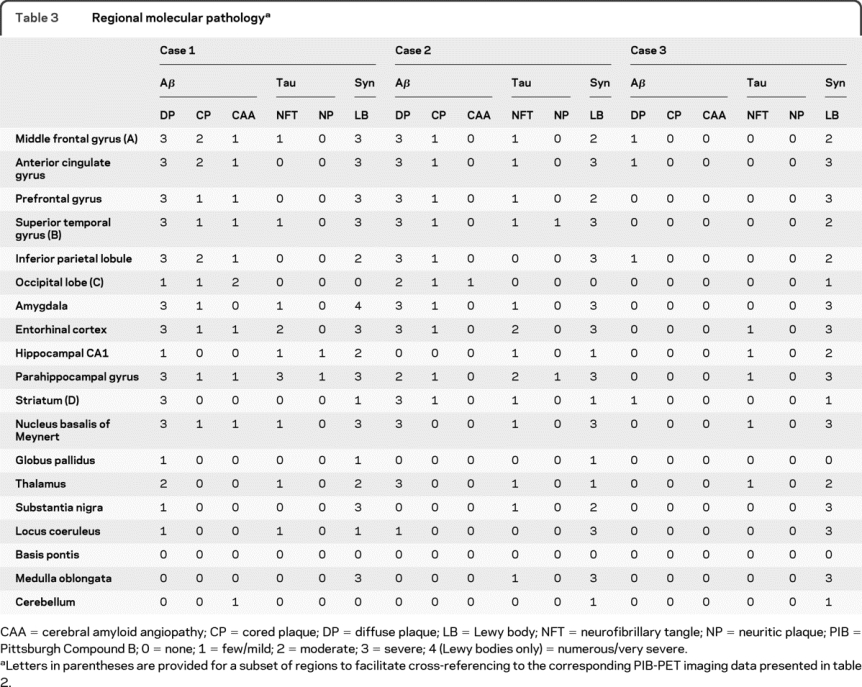
The presence of Lewy bodies in brainstem, limbic, and neocortical areas was consistent with PD stage 6 (range: 0–6) in all 3 cases.27 The density and distribution of these lesions was also sufficient to meet “high probability” criteria for dementia with Lewy bodies14; because the parkinsonism preceded the cognitive changes by more than 1 year, the entity which describes these clinicopathologic features is called PDD rather than DLB.1 In cases 1 and 2, the numerous neocortical Aβ plaques were sufficient for neuropathologic diagnosis of AD by Khachaturian criteria,28 but the modest numbers of neuritic plaques and neocortical tangles were only sufficient to fulfill the criteria for “possible” AD according to Consortium to Establish a Registry for Alzheimer's Disease criteria,29 and there was only a “low likelihood” that the cognitive changes are caused by AD according to the NIA-Reagan Institute criteria.30 No other neurodegenerative diseases were identified in any of the three cases.
DISCUSSION
The absence of elevated cortical [11C]-PIB binding in an individual with PDD who had abundant cortical Lewy bodies and minimal cortical amyloid plaque pathology underscores the specificity of this tracer for fibrillar Aβ amyloid in in vivo imaging. Previous in vitro studies have demonstrated that PIB does not demonstrate specific binding to Lewy bodies (an amyloid composed of fibrillar α-synuclein31) in cortex homogenates from patients with Lewy body dementia lacking Aβ plaque, or to synthetic α-synuclein aggregates.32 Our postmortem evaluation of individuals who underwent amyloid imaging strongly supports the notion that PIB PET is specific for Aβ amyloid plaque pathology in vivo in patients with Lewy body disorders. The burden of cortical Lewy bodies in case 3 was comparable to that in the other 2 cases, yet cortical retention of PIB was not elevated in this individual. Only the 2 cases with high levels of cortical Aβ detected by immunohistochemistry had elevated cortical retention of PIB on in vivo imaging. Our methodology did not permit detailed quantitative correlations between regional PIB uptake and Aβ lesion burden on histopathology, as previously reported for a case of AD.23 Nonetheless, all measured cortical regions with an elevated PIB binding potential (>0.2) in our study had severe (grade 3) Aβ plaque burden.
An unexpected finding in our study was that the 2 PIB-positive PDD cases had low probability AD by NIA-Reagan criteria. Evolving criteria for neuropathologic diagnosis of AD account for some of the conflicting results regarding the role of AD pathology in the pathogenesis of PDD.1,33 Consortium to Establish a Registry for Alzheimer's Disease and NIA-Reagan criteria emphasize neuritic rather than diffuse Aβ plaques as the major correlate of dementia in AD. However, diffuse plaques may be the predominant pathology in the earliest, mild stages of AD, and may indicate preclinical AD in cognitively normal individuals.20,34 The PIB-positive individuals in this study had more advanced dementia (CDR stage 2), a stage at which neuritic plaques are nearly universally present in patients with DAT.35 Thus in the setting of PD, [11C]-PIB PET may have insufficient specificity for antemortem diagnosis of comorbid AD due to the inability to distinguish between diffuse and neuritic Aβ plaques.
Since Lewy body pathology was advanced in all cases, regardless of Aβ burden, α-synucleinopathy was likely central to the pathogenesis of PDD in these individuals. However, since Aβ and α-synuclein pathologies may interact synergistically,36 it is possible that the presence of diffuse Aβ plaque influences the evolution of dementia in individuals with PD, even in the absence of neurofibrillary tangle pathology indicative of comorbid AD. Diffuse Aβ plaques are typically abundant in cases with cortical Lewy bodies37,38 and Lewy body counts are highly correlated with amyloid plaque counts in PD and PDD.33,39 However, the timing of deposition of amyloid, Lewy bodies, and neurofibrillary tangles relative to clinical manifestations of dementia can only be inferred from postmortem studies. Serial imaging to measure the rate of amyloid deposition40 in individuals with and without Lewy body disorders (subsequently confirmed by autopsy) can directly test whether α-synucleinopathy exacerbates amyloidosis and accelerates cognitive decline.5 Likewise, prospective longitudinal follow-up of PIB-positive and PIB-negative individuals with PD will be valuable for ascertaining whether comorbid Aβ pathology influences the timing of onset, rate of progression, or spectrum of clinical manifestations in PDD. This information will be crucial for developing targeted therapies that can slow or prevent the onset of dementia in patients with PD.
ACKNOWLEDGMENT
The authors thank the clinical, radiology, pathology, and technical staff for making information and tissue samples available for this study and the families of patients for participation. The authors thank the members of the Alzheimer Disease Research Center at Washington University for help with training in Clinical Dementia Rating.
DISCLOSURE
This study was funded by NIH grants P01-AG03991, P50-AG05681, U01-AG16976, K01-HD048437, R01-S041509, 5 T32-N2007205, UL1 RR024992 (Clinical Science Translational Award at Washington University), P30NS05710 (Neuroscience Blueprint Grant at Washington University), American Parkinson Disease Association (APDA) Center for Advanced PD Research at Washington University; Greater St. Louis Chapter of the APDA; and the Barnes-Jewish Hospital Foundation (the Elliot H. Stein Family Fund and the Jack Buck Fund for PD Research). Dr. Burack receives salary and research support from the American Academy of Neurology (Clinical Research Training Fellowship), Medtronic, Inc. (training fellowship), and foundation grants through the University of Rochester; and during the study enrollment period her spouse held stock in Amgen, Medtronic, Inc., Novartis, Pfizer Inc., Wyeth, and AstraZeneca. J. Hartlein and H.P. Flores report no disclosures. L. Taylor-Reinwald receives research support as study coordinator/research assistant from the NIH/NIA [P01-AG03991 and P50-AG05681]. Dr. Perlmutter serves on scientific advisory boards for the American Parkinson Disease Association and the Dystonia Medical Research Foundation; serves on the editorial boards of Neurology®; has received honoraria from Ceregene, the Parkinson Disease Study Group (grant reviews), the NIH (review activities), and for speaking and educational activities not sponsored by industry; and has received/receives research support from Medtronic, Inc. (partial fellowship support), the NIH [RO1 1RO1NS41509 (PI), RO1 NS050425 (PI), RO1 NS058714 (PI), P30 NS057105 (Co-I Core B), NCRR RR024992 (Project PI), R01ES013743 (Co-I), R01 NS039821 (Co-I), R01NS058797 (Co-I), and CO6 RR020092 (Program Director)], the Huntington Disease Society of America, HiQ Foundation, Bander Medical Business Ethics Foundation at Washington University, McDonnell Center for Higher Brain Function, the Michael J. Fox Foundation, Greater St. Louis Chapter of the American Parkinson Disease Association, American Parkinson Disease Association, APDA Advanced PD Research Center at Washington University, and the Barnes-Jewish Hospital Foundation. Dr. Cairns serves on the editorial boards of Acta Neuropathologica and Brain Pathology and receives research support from the NIH [P01-AG03991 (Neuropathology Core PI) and P50-AG05681 (Neuropathology Core PI)], and the Charles and Joanne Knight Alzheimer Research Initiative.
Supplementary Material
Address correspondence and reprint requests to Dr. Michelle A. Burack, Department of Neurology and Pediatrics, 601 Elmwood Avenue, Box 673, University of Rochester Medical Center, Rochester, NY 14642 michelle_burack@urmc.rochester.edu
Supplemental data at www.neurology.org
Disclosure: Author disclosures are provided at the end of the article.
Received June 18, 2009. Accepted in final form October 12, 2009.
REFERENCES
- 1.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 2.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney Multicenter Study of Parkinson's Disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844. [DOI] [PubMed] [Google Scholar]
- 3.Merdes AR, Hansen LA, Jeste DV, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 2003;60:1586–1590. [DOI] [PubMed] [Google Scholar]
- 4.Del Ser T, Hachinski V, Merskey H, Munoz DG. Clinical and pathologic features of two groups of patients with dementia with Lewy bodies: effect of coexisting Alzheimer-type lesion load. Alzheimer Dis Assoc Disord 2001;15:31–44. [DOI] [PubMed] [Google Scholar]
- 5.Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 2006;67:1931–1934. [DOI] [PubMed] [Google Scholar]
- 6.Klunk WE, Wang Y, Huang GF, et al. The binding of 2-(4′-methylaminophenyl) benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J Neurosci 2003;23:2086–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacskai BJ, Hickey GA, Skoch J, et al. Four-dimensional multiphoton imaging of brain entry, amyloid binding, and clearance of an amyloid-beta ligand in transgenic mice. Proc Natl Acad Sci USA 2003;100:12462–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004;55:306–319. [DOI] [PubMed] [Google Scholar]
- 9.Maetzler W, Reimold M, Liepelt I, et al. [11C]PIB binding in Parkinson's disease dementia. Neuroimage 2008;39:1027–1033. [DOI] [PubMed] [Google Scholar]
- 10.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology 2008;71:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edison P, Rowe CC, Rinne JO, et al. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry 2008;79:1331–1338. [DOI] [PubMed] [Google Scholar]
- 12.Burack MA, Campbell MC, Foster ER, et al. Relationship of cortical Pittsburgh compound B (PIB) binding and clinical features in Parkinson disease dementia. Mov Disord 2008;21. [Google Scholar]
- 13.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson's disease. Am J Med Genet 1999;88:539–543. [PubMed] [Google Scholar]
- 14.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 17.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000;12: 233–239. [DOI] [PubMed] [Google Scholar]
- 18.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology 2004;62:181–187. [DOI] [PubMed] [Google Scholar]
- 19.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem 2003;46:2740–2754. [DOI] [PubMed] [Google Scholar]
- 20.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–452. [DOI] [PubMed] [Google Scholar]
- 21.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996;16:834–840. [DOI] [PubMed] [Google Scholar]
- 22.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006;59:512–519. [DOI] [PubMed] [Google Scholar]
- 23.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain 2008;131:1630–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fodero-Tavoletti MT, Rowe CC, McLean CA, et al. Characterization of PiB binding to white matter in Alzheimer disease and other dementias. J Nucl Med 2009;50:198–204. [DOI] [PubMed] [Google Scholar]
- 25.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 28.Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol 1985;42:1097–1105. [DOI] [PubMed] [Google Scholar]
- 29.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 30.The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging 1997;18:S1–S2. [PubMed] [Google Scholar]
- 31.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature 1997;388:839–840. [DOI] [PubMed] [Google Scholar]
- 32.Fodero-Tavoletti MT, Smith DP, McLean CA, et al. In vitro characterization of Pittsburgh compound-B binding to Lewy bodies. J Neurosci 2007;27:10365–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol 2002;59:102–112. [DOI] [PubMed] [Google Scholar]
- 34.Morris JC, Storandt M, McKeel DW Jr, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer's disease. Neurology 1996;46:707–719. [DOI] [PubMed] [Google Scholar]
- 35.Berg L, McKeel DW Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 1998;55:326–335. [DOI] [PubMed] [Google Scholar]
- 36.Masliah E, Rockenstein E, Veinbergs I, et al. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci USA 2001;98:12245–12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lippa CF, Smith TW, Swearer JM. Alzheimer's disease and Lewy body disease: a comparative clinicopathological study. Ann Neurol 1994;35:81–88. [DOI] [PubMed] [Google Scholar]
- 38.Dickson DW, Crystal H, Mattiace LA, et al. Diffuse Lewy body disease: light and electron microscopic immunocytochemistry of senile plaques. Acta Neuropathol 1989;78:572–584. [DOI] [PubMed] [Google Scholar]
- 39.Lashley T, Holton JL, Gray E, et al. Cortical alpha-synuclein load is associated with amyloid-beta plaque burden in a subset of Parkinson's disease patients. Acta Neuropathol 2008;115:417–425. [DOI] [PubMed] [Google Scholar]
- 40.Jack CR, Jr., Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 2009;132:1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



