Abstract
Objective:
To investigate the presenting characteristics of new-onset afebrile seizures in infants (age 1–24 months) and the yield of neuroimaging.
Methods:
Prospective data were obtained from a standardized evaluation and management plan mandated by a critical care pathway. A total of 317 infants presented with new-onset afebrile seizures between 2001 and 2007. EEG was performed on 90.3%, head CT was obtained on 94%, and MRI was obtained on 57.4%.
Results:
We found half of the infants had partial features to their seizures, yet evidence for primary generalized seizures was rare. The majority had more than 1 seizure upon presentation. Seizures in this age group tended to be brief, with 44% lasting less than 1 minute. EEG abnormalities were found in half. One-third of CTs were abnormal, with 9% of all CTs requiring acute medical management. Over half of MRIs were abnormal, with cerebral dysgenesis being the most common abnormality (p < 0.05). One-third of normal CTs had a subsequent abnormal MRI—only 1 resulted in altered medical management.
Conclusions:
Infantile seizures are usually brief, but commonly recurrent, and strong consideration should be made for inpatient observation. Acute imaging with CT can alter management in a small but important number of infants. Due to the superior yield, strong consideration for MRI should be given for all infants, as primary generalized seizures are rare, and there is a high rate of cerebral dysgenesis.
GLOSSARY
- ED
= emergency department;
- ILAE
= International League Against Epilepsy;
- IRB
= Institutional Review Board.
Childhood seizures occur most commonly in infancy (1–24 months) with a decreasing incidence throughout the remainder of childhood.1 The American Academy of Neurology guidelines for the evaluation of the first nonfebrile seizure in a child2 recommend a routine EEG for all children and urgent neuroimaging for children with postictal focal neurologic deficits. Strong consideration is recommended for nonurgent neuroimaging in certain clinical circumstances, including cognitive or motor impairment of uncertain etiology, unexplained abnormalities on neurologic examination, abnormal EEGs not representing a benign syndrome, seizures of partial onset, or in children under the age of 1 year. Recommendations for imaging are hampered by a lack of prospectively obtained data. A call was made for further prospective data to be collected for new-onset seizures in children by age groupings, which is of special importance, as the characteristics of childhood seizures evolve in relation to age and maturity, from the neonatal period and infancy through adolescence.
Infants, in particular, have differing seizure characteristics in relation to older children.3–9 The literature for evaluating and characterizing infants with new-onset seizures is scarce. We describe the presenting characteristics of new-onset afebrile seizures in infants, and report on the yield of neuroimaging, to include CT and MRI, using data from a prospectively collected new-onset seizure database.
METHODS
Prospective data were obtained from a standardized evaluation and management plan mandated by a critical care pathway at Children's National Medical Center: patients presenting with either a first suspected or a newly recognized seizure are evaluated in the emergency department (ED), or less commonly as inpatients, with basic laboratory studies (complete blood count, serum electrolytes) and imaging with head CT. Patients are admitted to the Child Neurology service for at least 23 hours for observation or followed in consultation if on another service. Seizure education is provided and a strong effort made to obtain an EEG before discharge. Further laboratory testing, such as urine toxicology screens, CSF studies, screening for inborn errors of metabolism, and further imaging with MRI, are performed based upon clinician judgment. MRI was generally performed when focal findings were present, when CT was ambiguous, or to define abnormal findings on CT. The MRI examinations are read on a computerized workstation, and comparison to the CT scan is done at the time of MRI interpretation.
Infants between the chronologic ages of 1 and 24 months were included in the study. Infants were defined as 1 to 24 months as the literature supports the differing semiology in this age group compared to older children.3–9 Patients with a febrile illness and those with an infection of the CNS were excluded. Patients admitted for a suspicion of seizures, but discharged with a diagnosis of a “spell,” were also excluded.
Seizures with clear partial features by history, observation, examination, EEG, or imaging were classified as “partial.” Seizures without clear partial features were classified as tonic, clonic, tonic-clonic, myoclonic, atonic, or as infantile spasms based upon International League Against Epilepsy (ILAE) definitions.10 Seizure number and duration was determined based upon admission interview with parents and other witnesses, in addition to admission review of ambulance records, ED records, and inpatient records. Total duration included the longest estimated or documented continuous epileptic activity but could also include nearly continuous seizures at the discretion of the clinician. Uncertain duration was documented as “unknown.” Whether anticonvulsant medication was given prior to arrival in the ED was not a variable collected as part of the database, but prolonged seizures were often treated at the discretion of the paramedics and the ED physicians. When seizure frequency was too numerous for witnesses to quantify, a “multiple, difficult to quantify” designation is used. CT and MRI (1.5 T General Electric) findings were interpreted by a certified pediatric neuroradiologist. The standard MRI sequence employed for infant seizure evaluation included sagittal and axial T1, axial spin-echo proton density and T2, coronal T2, high-resolution coronal T2 of the mesial temporal lobe structures, axial magnetization transfer T1 for patients over 10 kilograms, axial diffusion, and optional axial or sagittal fast spin-echo T2, 2/0 mm. Brain MRI scans were routinely prescreened by the attending neuroradiologist and contrast administered as indicated (e.g., mass, inflammation). EEGs were performed using the standard 10–20 international system.
Data from a standardized form were stored in a password-protected Microsoft Office Access database. χ2 analyses were conducted to test for significant distributional differences.
Standard protocol approvals, registrations, and patient consents.
The Institutional Review Board (IRB) approved the protocol. An ethics review was included in the IRB review of the protocol. The work was conducted as part of quality assurance monitoring for the new-onset seizure clinical pathway. The IRB deemed the protocol met requirements for a limited data set and thus waived requirements for individual consent and assent.
RESULTS
Of 1,118 patients presenting with new-onset afebrile seizures between January 2001 and February 2007, 317 (28.3%) were age 1–24 months (30 had a history of prematurity; none with corrected gestational age <1 month). A total of 165 (52%) were boys and 152 (48%) were girls. This was a predominantly urban minority population: of those reporting race and ethnicity, 61% were African American, 14% Hispanic, 20% Caucasian, and 5% Asian. A total of 26% reported a family history of seizures. Of the 317 infants, 122 (38.5%) were between 1 and 6 months old; 87 (27.5%) were between 6 and 12 months; 108 (34%) were between 12 and 24 months. Fifteen patients had preexisting developmental delays of varying severity based on clinical assessment, and 5 preexisting cerebral palsy. CT was performed on 298 (94%), MRI on 182 (57.4%), and EEG on 286 (90.2%). Eleven infants had MRI only. An additional 141 infants were excluded from the study due to a concurrent febrile illness, and 110 infants presenting and discharged with “spells” (apnea, breath-holding, pallid syncope, reflux, benign sleep myoclonus, tremors) during this time period were excluded (in children with spells, EEG [n = 94] were normal, CT [n = 92] and MRI [n = 19] were normal or demonstrated incidental findings).
Seizure characterization.
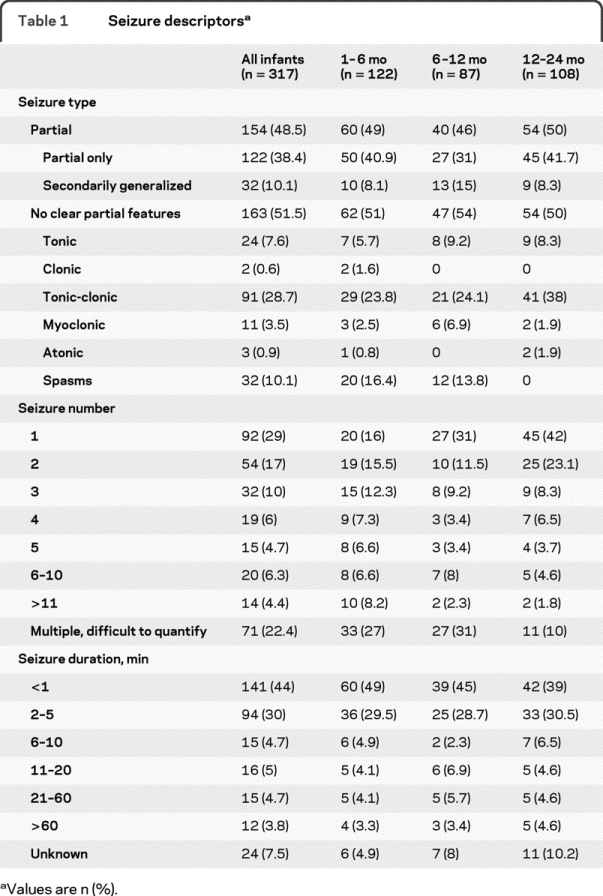
Nearly half the patient group had clear features of partial seizures (table 1). This was similar for all age subgroups (χ2 = 0.34, p = 0.84). Classification of seizures without partial features differed among age groups (χ2 = 33.10, p < 0.01). The majority were classified as tonic-clonic, especially prominent in the 12–24 month age group. Infantile spasms were the second most common seizure type in this category, comprising 25%–32% of those less than 1 year, but not observed in those older than 12 months. Among infants with seizures without partial features, only 6 had generalized epileptiform discharges on EEG.
Table 1 Seizure descriptors
Seizure number and duration upon presentation.
The majority of infants with a new onset of seizures presented with recurrent seizures; 71% presented with more than 1, and 38% presented with 5 or more seizures (table 1). Multiple seizures were seen more often in the 1–6 month age group (84% with more than one seizure), with decreasing incidence with increasing age (6–12 months, 69%; 12–24 months, 58%) (χ2 = 17.99, p < 0.01). The majority (74%) of new-onset seizures in infants lasted 5 minutes or less, with 44% lasting less than 1 minute (table 1). The incidence of seizures lasting more than 20 minutes was 8.5%.
CT findings.
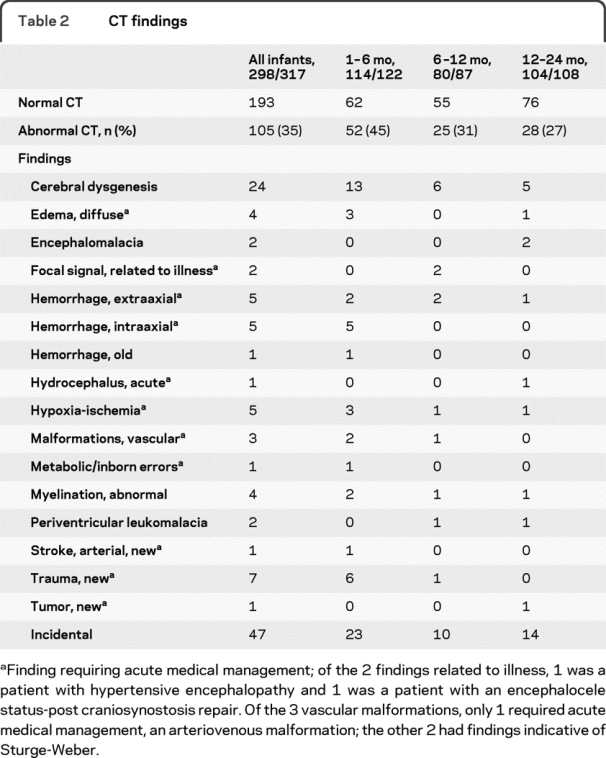
A total of 298 of 317 infants had CT scans (94%). A total of 105 CT scans had abnormal findings (35.2%), of which 47 were incidental (table 2). The yield of abnormalities on CT was highest in the youngest age groups, with decreasing incidence of abnormalities with increasing age (χ2 = 9.09, p < 0.05). For all infants, 27 CT scans (26% of abnormal CT scans, 9% of all CT scans) required acute medical management.
Table 2 CT findings
MRI findings.
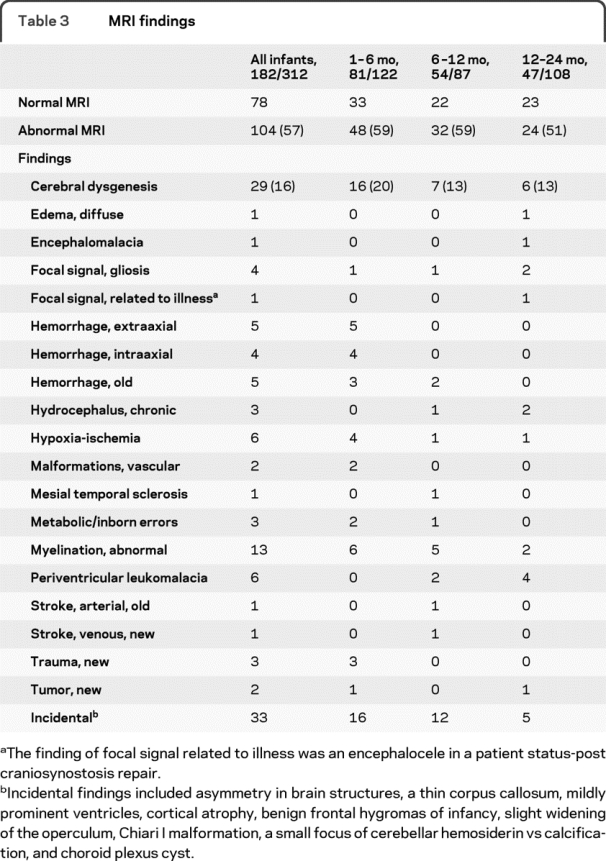
A total of 182 of 317 infants had MRI scans (57.4%). Younger infants were more likely to have an MRI (table 3). Overall, 104 MRI scans (57%) had 124 abnormal findings; 33/124 were incidental findings. A total of 28 of the 104 abnormal MRI scans had incidental findings only. The yield of abnormalities on MRI was comparable across age groups (χ2 = 1.81, p > 0.41). Cerebral dysgenesis was the most common MRI abnormality (16% of all MRI scans) (χ2 = 9.09, p < 0.05). The incidence of dysgenesis was highest in the youngest age group with 16 in the 1–6 month group (χ2 = 51.76, p < 0.05). Cerebral dysgenesis included Aicardi syndrome/dysgenesis of the corpus callosum, band heterotopia, cortical dysplasia to include 5 cases of focal cortical dysplasia, Dandy-Walker, lissencephaly, polymicrogyria, schizencephaly, subependymal heterotopias, and tuberous sclerosis.
Table 3 MRI findings
Negative CT, positive MRI.
Of the 193 normal CTs, 97 underwent subsequent MRI, of which 32 had an abnormal MRI (33%). Excluding those with only incidental findings, there were 16 MRIs with abnormal findings: cerebral dysgenesis, 4; periventricular leukomalacia, 4; abnormal myelination, 3; gliosis, 2; new hypoxia-ischemia, vascular malformations, and mesial temporal sclerosis 1 each. Only one of these resulted in altered acute medical management: in a 6-week-old with postoperative seizures, with initial normal head CT, MRI the next day revealed acute bilateral watershed infarctions.
Preexisting delays, cerebral palsy, and EEG findings compared to MRI.
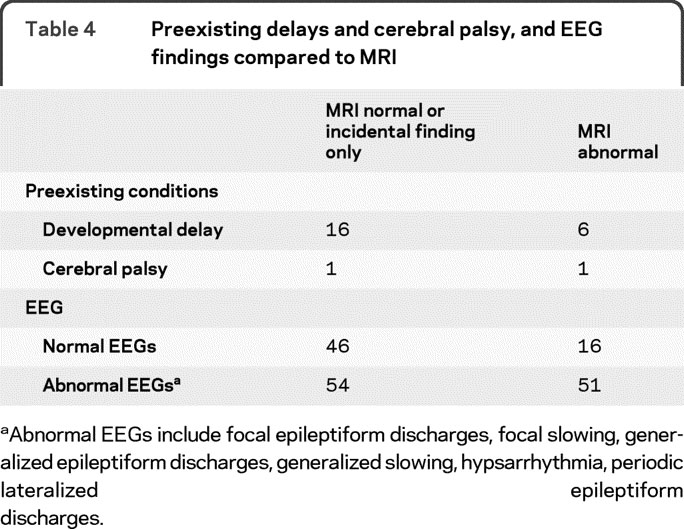
Of the 34 patients with preexisting developmental delays, 24 had brain MRI performed (table 4). There were 167 infants in whom both EEG and MRI were performed (table 4). Although patients with MRI abnormalities were more likely to have an abnormal EEG (76%; χ2 = 8.409, p < 0.01), 24% of patients with abnormal MRIs had a normal EEG. Concordance of location between those with both focal findings on EEG and an abnormal MRI is shown in table e-1 on the Neurology® Web site at www.neurology.org.
Table 4 Preexisting delays and cerebral palsy, and EEG findings compared to MRI
DISCUSSION
We found that a substantial proportion of new-onset seizures present by the age of 2 years. While new-onset seizures in infants are usually brief, they are commonly recurrent. Clear partial features are seen in half of new-onset seizures, but primary generalized seizures are uncommon in this age group. Acute imaging alters immediate management in a small but significant number of patients. Not surprisingly, MRI has superior yield compared to CT, but rarely alters acute medical management. Cerebral dysgenesis is the most common structural and etiologic abnormality found in children younger than 2. The younger the patient the more likely imaging will identify an abnormality, with cerebral dysgenesis being more common in the youngest patients.
The proportion of children with new-onset seizures who are younger than 2 (28%) is comparable to previous epidemiologic studies of seizures and epilepsy in children younger than 3 (22%, 24%).1,11–13 Electrographic evidence for primary generalized seizures among infants was rare (<2%). Previous reports in young children (<3 years) find 8%–9%; however, sleep-deprived EEGs were not obtained on all children in our study.12,14 Clear partial features were identified in nearly half of our patients. Seizure categorization was based upon parental and witness description of seizures and interictal EEG, in addition to neuroimaging. Without both ictal EEG and video to review it may be difficult to classify infantile seizures accurately into ILAE categories.3 Ictal video EEG shows primary generalized tonic-clonic seizures in infants with epilepsy to be uncommon.15
Few studies comment on the duration or number of seizures upon presentation in infants. Infants appear less likely to have prolonged seizures than older children and adolescents.16 In contrast, the seizure recurrence rate during admission was considerably higher than previous reports of 20% in children of all ages.17 The high risk of recurrence supports policies for prolonged observation either in the ED or on inpatient wards in this population.
We found EEG abnormalities in half of our patients, also comparable with prior studies.12,18–19 MRI abnormalities are usually associated with an abnormal EEG,19,20 but one quarter of infants with abnormal MRIs had a normal EEG.
There are also few published prospective studies of imaging in children with new-onset seizures, and none focus specifically on infants. Comparison with other prior studies is problematic as they 1) employ different definitions for significance in imaging findings; 2) include mixed patient populations—e.g., patients with complex febrile seizures, provoked seizures, and epilepsy; 3) varying ages. There are few structural imaging studies with control populations. In the NIH Clinical Center study of 1,000 normal volunteers aged 3–83 years, 15% had incidental findings that did not require further follow-up or evaluation, 1% had findings requiring urgent evaluation, and 1.8% had findings requiring routine evaluation.21 However, there were no infants in this study, and there may be selection bias for “volunteers” who seek brain MRI.
In 5 Class II studies, rates of significant abnormalities on head CT altering acute management after a new-onset seizure in children range from 3% to 8%, and the rate for all abnormalities 7%–21%.22–26 In 3 Class II studies in children with new-onset seizures and epilepsy, abnormalities found on CT range from 12% to 33%; these studies do not comment on findings that require urgent management.27–29 The inclusion of neonates27 increases the incidence of abnormalities.
MRI is known to confer superior yield to CT in the evaluation of adults with chronic epilepsy30 and magnetic resonance appears to hold superior yield in children.18,20 Children with partial epilepsies in childhood are more likely to have MRI abnormalities than those with generalized epilepsy.31 In our study, the classification of partial vs nonpartial as a predictive factor of abnormal imaging was not analyzed because neuroimaging was one of the criteria used by the clinician to classify seizures. Our findings that 57% of infants studied with MRI had abnormalities are higher than previous reports that examined children of all ages. Three Class II studies looking at new-onset seizures in children using either MRI alone or MRI and CT describe abnormalities in 14%–32%18,19,32 and for newly diagnosed epilepsy in children a rate of 12.7% for findings of etiologic relevance.20 In these studies, however, few of the patients were younger than 2 years. Although there appears to be a higher yield of MRI with infants with preexisting developmental delays or cerebral palsy, our sample size was too small to yield significance.
We found malformations of cortical development to be considerably more common than previous imaging reports examining children of all ages with a first unprovoked afebrile seizure (16% vs 4%–5%).19,32 Children with more severe epilepsy due to structural brain abnormalities may be more likely to present at an earlier age. Focal cortical dysplasia also changes with appearance and detectability with changing maturation of myelination.33,34 In contrast, some infants with focal cortical dysplasias have been found to be increasingly more difficult to identify with increasing myelination with age.33
In weighing choice of imaging modalities, several factors need to be considered. Accessibility of imaging modality is often a major determinant; CT is more widely available especially in urgent circumstances. CT involves the risk of radiation exposure while MRI involves the risks of sedation in most infants. Proper diagnosis, however, provides therapeutic and prognostic implications. Symptomatic epilepsies in the first year can correlate with more difficult-to-control seizures.35 In addition, the developmental outcome is reported to be poorer in symptomatic epilepsies beginning in the first years of life, as opposed to cryptogenic epilepsies.35–37 The higher rate of localization-related seizures in this age range, the higher rate of abnormalities in infants vs older children, the prognostic implications, and the superior yield of MRI compared to CT in identifying and defining abnormalities support the view that MRI should be obtained in all infants under 2 years old presenting with a new-onset afebrile seizure. An argument can be made for cases in which urgent neuroimaging is not indicated and the infant can be observed, to avoid CT, and thus unnecessary radiation, and instead arrange for a brain MRI.
There are several potential limitations to our study. There may be referral biases as our study was conducted at an urban tertiary referral center. Younger patients or infants with more severe seizures (frequent, prolonged, generalized) are more likely to be evaluated urgently in an ED rather than an outpatient office. The decision to obtain MRI is based upon clinician judgment. There was an increased tendency among our faculty to order MRI in the 1–6 month group vs the 12–24 month group. Some cortical dysplasias may be more difficult to identify with increasing myelination with age, thus limiting our diagnostic capabilities in older infants. Our data are also dependent upon parental and witness recollection. The accuracy of the initial clinician classification of epileptic seizure vs a nonepileptic spell was not formally evaluated; our study examined the acute presentation of the new-onset afebrile seizure in infants; long-term follow-up is necessary to confirm initial diagnostic impressions and is the subject of a future study. Our database was constructed for children of all ages, not specifically for infants, but provides a framework for collection of data to characterize seizures in infancy.
Based upon our findings, we recommend close observation of all infants presenting with new-onset afebrile seizures as seizures often recur. Imaging should be obtained in all infants less than 2 years old presenting with new-onset afebrile seizures as significant abnormalities are common and will dictate management; MRI is preferred to CT. CT may be more readily available in an urgent setting but may be falsely reassuring if normal. Imaging establishes etiology and prognosis in a substantial proportion of children and helps direct (urgent) medical management.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Madison M. Berl.
DISCLOSURE
Dr. Hsieh has received funding for travel for lectures not funded by industry and received royalties from publishing in Current Neurology and Neuroscience Reports (Current Medicine Group LLC, 2008). Dr. Chang has received honoraria for lectures or educational activities not funded by industry and receives research support from the NIH [5R01-HL060922–09 (Co-PI)]. Dr. Tsuchida and Dr. Vezina report no disclosures. Dr. Vanderver receives research support from the American Academy of Neurology Foundation [Clinical Training in Research Fellowship Award (PI)] and from the NIH/NINDS [K08 NS060695–01A1 (PI)]. J. Siedel holds stock in Eli Lilly and Company. Dr. Brown serves on the editorial board of Prehospital Emergency Care and has received honoraria for lectures or educational activities not funded by industry. Dr. Berl receives research support from the NIH [PCRS Scholar Award and NCRR 5K12RR017613–05 (PI)]. S. Stephens and A. Zeitchick report no disclosures. Dr. Gaillard has served on a scientific advisory board for General Electric and on an educational committee supported by Ovation (now Lundbeck) and Questcor; serves as an editor of Epilepsia; his department derives clinical income from the evaluation and management of children with epilepsy; receives research support from Lundbeck Inc., King Pharmaceuticals, PRA International, Eisai Inc., and Marinus Pharmaceuticals, Inc.; and is supported by federal funding from the NIH [NINDS 1R01NS44280–01 (PI) and NICHD 1P30HD40677–01 (IDDRC, core director), NCRR 1K12RR17613–01 (mentor), NIMH 1 R01 MH065395–01A2 (Co-I)] and CDC-APTR R-03 (Paid consultant).
Supplementary Material
Address correspondence and reprint requests to Dr. William Davis Gaillard, Center for Neuroscience, Children's National Medical Center, Washington, DC 20010 wgaillar@cnmc.org
Supplemental data at www.neurology.org
The views expressed in this article are those of the author(s) and do not reflect the official policy or position of the United States Air Force, Department of Defense, or the U.S. Government.
Disclosure: Author disclosures are provided at the end of the article.
Received February 2, 2009. Accepted in final form October 27, 2009.
REFERENCES
- 1.Hauser WA. Epidemiology of epilepsy in children. In: Pellock JM, Dodson WE, Bourgeois BFD, eds. Pediatric Epilepsy: Diagnosis and Therapy, 2nd ed. New York: Demos Medical Publishing, Inc.; 2001: 81–96. [Google Scholar]
- 2.Hirtz D, Ashwal S, Berg A, et al. Practice parameter: evaluating a first nonfebrile seizure in children: report of the quality standards subcommittee of the American Academy of Neurology, the Child Neurology Society, and the American Epilepsy Society. Neurology 2000;55:616–623. [DOI] [PubMed] [Google Scholar]
- 3.Nordli DR, Bazil CW, Scheuer ML, Pedley TA. Recognition and classification of seizures in infants. Epilepsia 1997;38:553–560. [DOI] [PubMed] [Google Scholar]
- 4.Nordli DR, Kuroda MM, Hirsch LJ. The ontogeny of partial seizures in infants and young children. Epilepsia 2001;42:986–990. [DOI] [PubMed] [Google Scholar]
- 5.Nordli DR. Infantile seizures and epilepsy syndromes. Epilepsia 2002;43(S3):11–16. [DOI] [PubMed] [Google Scholar]
- 6.Nordli DR. Diagnostic difficulty in infants and children. J Child Neurol 2002;17:S28–S35. [DOI] [PubMed] [Google Scholar]
- 7.Korff CM, Nordli DR. The clinical-electrographic expression of infantile seizures. Epilepsy Res 2006;70S:S116–S131. [DOI] [PubMed] [Google Scholar]
- 8.Hamer HM, Wyllie E, Luders HO, Kotagal P, Acharya J. Symptomatology of epileptic seizures in the first three years of life. Epilepsia 1999;40:837–844. [DOI] [PubMed] [Google Scholar]
- 9.Acharya JN, Wyllie E, Luders HO, Kotagal P, Lancman M, Coelho M. Seizure symptomatology in infants with localization-related epilepsy. Neurology 1997;48:189–196. [DOI] [PubMed] [Google Scholar]
- 10.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia 1981;22:489–501. [DOI] [PubMed] [Google Scholar]
- 11.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993;34:453–468. [DOI] [PubMed] [Google Scholar]
- 12.Shinnar S, Kang H, Berg AT, Goldensohn ES, Hauser WA, Moshe SL. EEG abnormalities in children with a first unprovoked seizure. Epilepsia 1994;35:471–476. [DOI] [PubMed] [Google Scholar]
- 13.Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia 1999;40:445–452. [DOI] [PubMed] [Google Scholar]
- 14.Shahar E, Barak S, Andraus J, Kramer U. Primary generalized epilepsy during infancy and early childhood. J Child Neurol 2004;19:170–174. [PubMed] [Google Scholar]
- 15.Korff C, Nordli DR. Do generalized tonic-clonic seizures in infancy exist? Neurology 2005;65:1750–1753. [DOI] [PubMed] [Google Scholar]
- 16.Shinnar S, Berg AT, Moshe SL, Shinnar R. How long do new-onset seizures in children last? Ann Neurol 2001;49:659–664. [PubMed] [Google Scholar]
- 17.Sogawa Y, Maytal J. Emergency department admission of children with unprovoked seizure: recurrence within 24 hours. Pediatr Neurol 2006;35:98–101. [DOI] [PubMed] [Google Scholar]
- 18.King MA, Newton MR, Jackson GD, et al. Epileptology of the first-seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet 1998;352:1007–1011. [DOI] [PubMed] [Google Scholar]
- 19.Doescher JS, deGrauw TJ, Musick BS, et al. Magnetic resonance imaging and electroencephalographic findings in a cohort of normal children with newly diagnosed seizures. J Child Neurol 2006;21:491–495. [PMC free article] [PubMed] [Google Scholar]
- 20.Berg AT, Testa FM, Levy SR, Shinnar S. Neuroimaging in children with newly diagnosed epilepsy: a community-based study. Pediatrics 2000;106:527–532. [DOI] [PubMed] [Google Scholar]
- 21.Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA 1999;282:36–39. [DOI] [PubMed] [Google Scholar]
- 22.Garvey MA, Gaillard WD, Rusin JA, et al. Emergency brain computed tomography in children with seizures: who is most likely to benefit? J Pediatr 1998;133:664–669. [DOI] [PubMed] [Google Scholar]
- 23.Sharma S, Riviello JJ, Harper MB, Baskin MN. The role of emergent neuroimaging in children with new-onset afebrile seizures. Pediatrics 2003;111:1–5. [DOI] [PubMed] [Google Scholar]
- 24.Maytal J, Krauss JM, Novak G, Nagelberg J, Patel M. The role of brain computed tomography in evaluating children with new onset of seizures in the emergency department. Epilepsia 2000;41:950–954. [DOI] [PubMed] [Google Scholar]
- 25.McAbee GN, Barasch ES, Kurfist LA. Results of computed tomography in “neurologically normal” children after initial onset of seizures. Pediatr Neurol 1989;5:102–106. [DOI] [PubMed] [Google Scholar]
- 26.Stroink H, Brouwer OF, Arts WF, Geerts AT, Boudewyn Peters AC, van Donselaar CA. The first unprovoked, untreated seizure in childhood: a hospital based study of the accuracy of the diagnosis, rate of recurrence, and long term outcome after recurrence: Dutch study of epilepsy in childhood. J Neurol Neurosurg Psychiatry 1998;64:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang PJ, Berger PE, Cohen ME, Duffner PK. Computed tomography and childhood seizure disorders. Neurology 1979;29:1084–1088. [DOI] [PubMed] [Google Scholar]
- 28.Gibbs J, Appleton RE, Carty H, Beirne M, Acomb BA. Focal electroencephalographic abnormalities and computerised tomography findings in children with seizures. J Neurol Neurosurg Psychiatry 1993;56:369–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warden CR, Brownstein DR, Del Beccaro MA. Predictors of abnormal findings of computed tomography of the head in pediatric patients presenting with seizures. Ann Emerg Med 1997;29:518–523. [DOI] [PubMed] [Google Scholar]
- 30.Bronen RA, Fulbright RK, Spencer DD, et al. Refractory epilepsy: comparison of MR imaging, CT, and histopathologic findings in 117 patients. Radiology 1996;201:97–105. [DOI] [PubMed] [Google Scholar]
- 31.Resta M, Palma M, Dicuonzo F, et al. Imaging studies in partial epilepsy in children and adolescents. Epilepsia 1994;35:1187–1193. [DOI] [PubMed] [Google Scholar]
- 32.Shinnar S, O'Dell C, Mitnick R, Berg AT, Moshe SL. Neuroimaging abnormalities in children with an apparent first unprovoked seizure. Epilepsy Res 2001;43:261–269. [DOI] [PubMed] [Google Scholar]
- 33.Eltze CM, Chong WK, Bhate S, Harding B, Neville BG, Cross JH. Taylor-type focal cortical dysplasia in infants: some MRI lesions almost disappear with maturation of myelination. Epilepsia 2005;46:1988–1992. [DOI] [PubMed] [Google Scholar]
- 34.Takanashi J, Barkovich AJ. The changing MR imaging appearance of polymicrogyria: a consequence of myelination. AJNR Am J Neuroradiol 2003;24:788–793. [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderlinden L, Lagae LG. Clinical predictors for outcome in infants with epilepsy. Pediatr Neurol 2004;31:52–55. [DOI] [PubMed] [Google Scholar]
- 36.Battaglia D, Rando T, Deodato F, et al. Epileptic disorders with onset in the first year of life: neurological and cognitive outcome. Eur J Paediatr Neurol 1999;3:95–103. [DOI] [PubMed] [Google Scholar]
- 37.Cavazzuti GB, Ferrari P, Lalla M. Follow-up study of 482 cases with convulsive disorders in the first year of life. Dev Med Child Neurol 1984;26:425–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


