Abstract
Background:
Stereotactic radiosurgery (RS) is a promising treatment for intractable medial temporal lobe epilepsy (MTLE). However, the basis of its efficacy is not well understood.
Methods:
Thirty patients with MTLE were prospectively randomized to receive 20 or 24 Gy 50% isodose RS centered at the amygdala, 2 cm of the anterior hippocampus, and the parahippocampal gyrus. Posttreatment MRI was evaluated quantitatively for abnormal T2 hyperintensity and contrast enhancement, mass effect, and qualitatively for spectroscopic and diffusion changes. MRI findings were analyzed for potential association with radiation dose and seizure remission (Engel Ib or better outcome).
Results:
Despite highly standardized dose targeting, RS produced variable MRI alterations. In patients with multiple serial imaging, the appearance of vasogenic edema occurred approximately 9–12 months after RS and correlated with onset of seizure remission. Diffusion and spectroscopy-detected alterations were consistent with a mechanism of temporal lobe radiation injury mediated by local vascular insult and neuronal loss. The degree of these early alterations at the peak of radiographic response was dose-dependent and predicted long-term seizure remission in the third year of follow-up. Radiographic changes were not associated with neurocognitive impairments.
Conclusions:
Temporal lobe stereotactic radiosurgery resulted in significant seizure reduction in a delayed fashion which appeared to be well-correlated with structural and biochemical alterations observed on neuroimaging. Early detected changes may offer prognostic information for guiding management.
GLOSSARY
- ADC
= apparent diffusion coefficient;
- CI
= confidence interval;
- CVLT-LDFR
= Long Delay Free Recall score of the California Verbal Learning Test;
- FOV
= field of view;
- MTLE
= medial temporal lobe epilepsy;
- NAA
=N-acetylaspartate;
- QOLIE-10
= Quality of Life in Epilepsy 10 inventory;
- RS
= stereotactic radiosurgery;
- TE
= echo time;
- TMT
= Trail Making Test;
- TR
= repetition time;
- WMS
= Wechsler Memory Scale–Revised.
Stereotactic radiosurgery (RS) is currently under evaluation as an alternative to open surgery for mesial temporal lobe epilepsy (MTLE). Outcome in terms of seizure remission is variable.1–9 Recent European reports have demonstrated good long-term outcomes in larger series of patients.2,5 Similarly, we recently published high rates of seizure control after Gamma Knife® RS in a prospective multicenter pilot trial.10 In this study, 2 RS doses were compared; the overall seizure remission rate was 69% during the third follow-up year after treatment which is comparable to that reported for resective temporal lobectomy.11
While preliminary data on efficacy of RS exist, several important questions remain to be answered. One potential limitation of RS for MTLE is the 12- to 24-month latency from treatment to seizure remission.4 The antiepileptic effect of RS is much slower than remission of other conditions such as neoplasms or trigeminal neuralgia.7,12–14 As there is a morbidity and even a small mortality of uncontrolled seizures,15–18 an early marker of efficacy would be clinically useful, allowing interventions such as early open surgical “escape” resection to be judiciously applied. Furthermore, the mechanism by which RS renders an anticonvulsant effect is incompletely understood. While the cognitive effects of open surgery for MTLE have been extensively studied,19–21 they have not been adequately delineated for RS.22
In order to address these issues, we report in detail the serial MRI radiographic changes in our prospective, multicenter study of RS for MTLE.23 We chronicle the time course of the RS-induced changes and their relationships to RS dose, subsequent seizure remission, and changes on neuropsychometric testing.
METHODS
Human investigation approval, inclusion and exclusion criteria, RS protocol, and initial efficacy and safety of our multicenter, prospective trial of RS for MTLE are detailed elsewhere.23 Briefly, subjects had pharmacoresistant complex partial seizures and underwent standardized, presurgical evaluation that confirmed the presence of unilateral mesial temporal sclerosis (defined as either asymmetric increased hippocampal T2 signal or hippocampal atrophy) concordant with ipsilateral video-EEG lateralization confirming unilateral temporal lobe seizures.
Subjects were randomized to RS treatment with either a 20 Gy or 24 Gy dose containing a 50% isodose volume ranging from 5.5 to 7.5 mL comprising the amygdala, anterior 2 cm of hippocampus, and parahippocampal gyrus. Subjects were stratified according to center and gender. Radiation safety factors limited dose to a maximum of 10 Gy to the nearby brainstem and 8 Gy to the optic nerves and chiasm.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the NIH–National Institute of Neurological Disorders and Stroke, by the University of California, San Francisco Committee on Human Research, and by the respective institutional review boards at each of the other 6 treatment centers. Written informed consent was obtained from all patients.
MRI analysis.
All subjects underwent brain MRI at baseline, 12 and 24 months after treatment, and also at any other time at physician discretion. A highly standardized protocol for MRI was carried out as follows: 1) T1 sagittal localizer (repetition time [TR] = 600; field of view [FOV] = 22); 2) T2 axial images (TR = 2,500; echo time [TE] = 30/80; FOV = 22 × 16); 3) fast spin echo coronal image (TR = 4,000; TE = 102; FOV = 22 × 16); 4) MPGR coronal (TR = 787; TE = 25; flip angle = 20°; FOV = 22 × 16); 5) 3-mm coronal interleaved fluid-attenuated inversion recovery 2 (TR = 36; flip angle = 35°; FOV = 22 × 16); 6) 3-dimensional-SPGR coronal (TR = 36; flip angle = 35°; FOV = 22 × 16; slice thickness 1.5 mm and in-plane resolution of 0.9 mm); 7) 3-dimensional-SPGR coronal with gadolinium; 8) echoplanar–diffusion-weighted imaging (TR = 8,000; TE min; 1 shot 166.67; FOV = 36 × 27; 5 mm skip 0; matrix 256 × 128; b = 1,000). MRIs were reviewed centrally by evaluators blinded to dose and clinical condition (E.F.C., M.O., W.P.D.).
To assess the severity of vasogenic edema, we used complementary quantitative and qualitative techniques. The volumes of T2 hyperintensity and T1 contrast enhancement were measured using digital calipers on a PACS station. Volumes (cc) were approximated using the formula for an ellipsoid: 4/3π A × B × C, where A, B, and C are the mediolateral, dorsoventral, and anteroposterior dimensions (cm). Given the variability of radiographic responses between patients after RS, the volumes were normalized by values obtained at 12 months after RS and plotted as a function of time.
To capture the neuroanatomic extent and the clinical severity of changes, volume measurements were supplemented with objective, standardized severity scales ranking the anatomic extent of T2 hyperintensity and the degree of mass effect into 6-point ranges.24 The hyperintensity score rated T2 change severity (0 = none, 1 = confined to temporal lobe, 2 = temporal lobe + basal ganglia, 3 = temporal lobe + basal ganglia + [parietal lobe or occipital lobe], 4 = temporal lobe + basal ganglia + [parietal and occipital lobes], 5 = temporal lobe + basal ganglia+[parietal and occipital lobes] + frontal lobe). The mass effect scale rated edema in terms of swelling (0 = none, 1 = slight swelling, 2 = moderate local swelling without narrowing and 3 = with narrowing of the ambient cistern, 4 = important cerebral swelling with midline shift <10 mm, 5 = major cerebral swelling with midline shift ≥10 mm).
Apparent diffusion coefficient (ADC) was measured from the hippocampus and temporal stem. The ADC measures the magnitude of diffusion (of water molecules) within cerebral tissue. Diffusion-weighted datasets were acquired using a single-shot, echoplanar technique (b values of 0 and 1,000 s/mm2), from which maps of ADC were calculated with software provided by the system manufacturer (GE Healthcare, Milwaukee, WI).
Multi-time point spectroscopic data were acquired from both mesial temporal lobes using a single-voxel (8 mL), point-resolved spectroscopic technique (TE = 135 msec; TR = 1,600 msec). The postgadolinium T1-weighted 3-dimensional SPGR was used to prescribe the PRESS selected volume. Briefly, the data were filtered with a Lorentzian function and Fourier transformed, resulting in an array of spectra. The spectra were corrected for baseline variations, phase shifts, and frequency shifts within the region of each peak, employing a priori information about the relative location of each metabolic peak. Voxels were selected from the RS target region in the medial temporal lobe and compared to unaffected homologous control voxels from the contralateral hemisphere. Choline, creatine, N-acetylaspartate (NAA), lactate, and lipid resonance peaks in chosen voxels were visually identified.
Seizure remission.
Seizure remission was determined from standardized, physician-supervised seizure diaries. Seizure remission was defined as having no seizures (with or without auras, comparable to Engel Ib or better outcome25) during the year-long period between 24 and 36 months.
Neuropsychology.
Neuropsychological testing results acquired at baseline and at 12 and 24 months postoperatively were evaluated in relation to peak MRI changes. The Long Delay Free Recall score of the California Verbal Learning Test (CVLT-LDFR)26 and the delayed recall score of the Logical Memory subtest from the Wechsler Memory Scale–Revised (WMS-R DR)27 provided measures of noncontextual and contextual verbal memory.19 Assessments of global cognitive function were carried out with the use of the Trail Making Test (TMT)28 and quality of life was assessed with the Quality of Life in Epilepsy 10 inventory (QOLIE-10).29
Statistical analysis.
Nonparametric Mann-Whitney tests were used to statistically evaluate associations between variables such as volumes of T2 edema and T1 contrast enhancement, and seizure remission. Spearman correlations were used to evaluate associations between neuroimaging changes and neuropsychological findings. Statistical significance was determined at p values < 0.05.
RESULTS
Thirty subjects were randomized and treated. Three subjects did not complete the 36-month study: one subject was lost to follow-up (20 Gy), one required urgent temporal lobectomy at 15 months for dexamethasone-resistant papilledema following radiosurgery (24 Gy), and another was not seizure-free at 24 months and requested temporal lobectomy (20 Gy). The 2 subjects that underwent temporal lobectomy were considered as “no remission.” Seizure remission was defined as complete seizure control during the 1-year period between 24 and 36 months after treatment. In this study, 20 of 29 (69%) patients were considered to be in seizure remission (the patient who was lost to follow-up was excluded). Although a greater proportion of patients who received 24 Gy (76.9%) were seizure-free compared to 20 Gy (58.8%), the difference did not reach statistical significance.23
Neuroimaging.
Figure 1 demonstrates the time course of the RS-induced radiologic changes in relationship to clinical responses of seizures. MRI scans were obtained for all subjects at 12 and 24 months, and the intervening additional MRIs were done at discretion of the treating physician. The most striking effect was the degree of abnormal T2 hyperintensity associated with vasogenic edema. In those patients with multiple serial MRI, T2 hyperintensity appeared within the medial temporal lobe beginning around the ninth postoperative month and peaked in intensity at 12 months, corresponding to a decline in the proportion of patients experiencing complex partial seizures. Contrast enhancement followed a similar time course, except that it preceded T2 changes and diminished quickly after months 9–12. Enhancement was typically ring-enhancing and centered over the target region. Serial MRI changes are illustrated for a representative subject in figure 2.
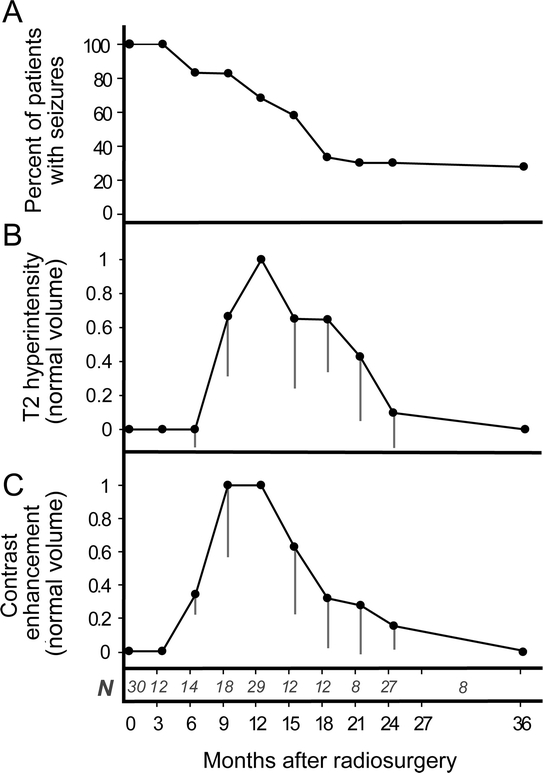
Figure 1 Time course of incidence of seizure remission (seizure-free with or without auras)
(A) The mean volumes of T2 hyperintensity (B) and contrast enhancement (C) were normalized by values obtained at 12 months after RS for each patient and the mean is plotted as function of time (gray lines are SEM). N indicates the number of patients who underwent neuroimaging between each examination interval since neuroimaging was standardized for 12- and 24-month visits and by physician request at other intervals.
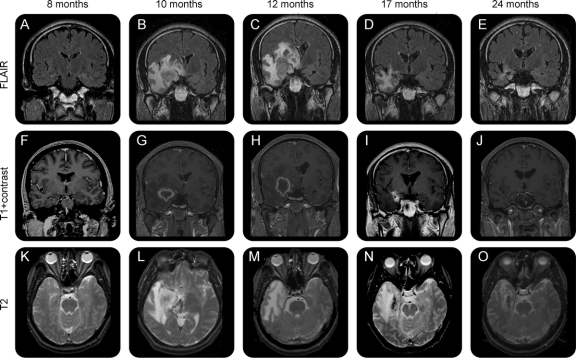
Figure 2 Development of radiologic changes in a representative patient treated with a 24-Gy dose
Fluid-attenuated inversion recovery coronal (A–E), T1 coronal with gadolinium (F–J), T2 axial (K–O). Abnormal T2 prolongation involving the right frontal and temporal lobes demonstrating central heterogeneity at the level of the right medial temporal lobe. Severe vasogenic edema resulting in a mild increase in midline shift. The central area of heterogeneity has peripheral enhancement. By 12 months, the swelling of the temporal lobe resulted in almost 1 centimeter of midline shift on the coronal view. The T2 abnormality is largely limited to the white matter. The corresponding volume of abnormal T2 hyperintensity is 703 mL, the volume of contrast enhancement is 31.4 mL, the T2 hyperintensity anatomic extent score is 5 (extends to parietal and frontal lobes), and the mass effect score is 3 (local swelling with narrowing of the ambient cistern). At 24 months, there was significant reduction in the extent of T2 hyperintensity (102 mL, score 1), with residual T2 prolongation only in the right anterior parahippocampal gyrus, fusiform gyrus, and extending superiorly along the right temporal stem into the inferior subinsular cortex. There was interval decrease in mass effect at 24 months (mass effect score = 0). The right temporal lobe now appears atrophic compared to the left temporal lobe.
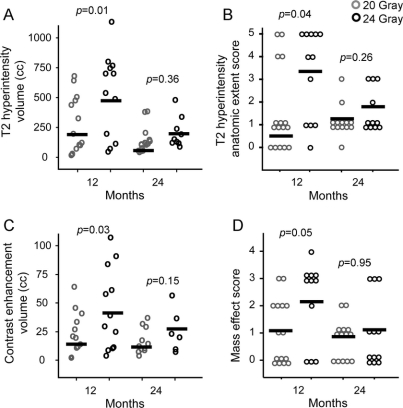
The distribution of T2 hyperintensity, contrast enhancement, and hyperintensity severity and mass effect scales are shown in figure 3, also comparing 20 Gy vs 24 Gy at 12 and 24 months. In general, 24 Gy doses induced significantly larger responses than 20 Gy when measured at 12 months. At 24 months, however, these differences were not significant. Notably, the T2 hyperintensity volume at 12 months did not predict subsequent volumes at 24 months (Spearman nonparametric correlation, p = 0.99), demonstrating that the radiosurgical response is a monophasic, subacute lesion.
Figure 3 Quantification of radiographic alterations stratified by dose
Distribution by stereotactic radiosurgery dose of (A) T2 volumes, (B) qualitative severity scores of T2 neuroanatomic extent, (C) contrast enhancement volumes, and (D) mass effect. Bars = mean. p Values from Mann-Whitney tests.
As graded by the T2 hyperintensity severity (HIS) scale, at 12 months substantial variability existed in the extent of edema (figure 3C), ranging from none to extensive white matter changes extending from the temporal lobe to occipital lobe. No signs of abnormal T2 hyperintensity were observed in the brainstem. Over the following 12–15 months, the degree of abnormal T2 hyperintensity subsided considerably. The course of mass effect was similar to hyperintensity changes, demonstrated by the correlation between mass effect and T2 severity scores at 12 months (Spearman correlation p value = 0.002). As with the edema, a large degree of variability existed for the mass effect. As it resolved, the swollen medial temporal lobe transitioned to a slightly atrophic morphology.
Diffusion and MRS.
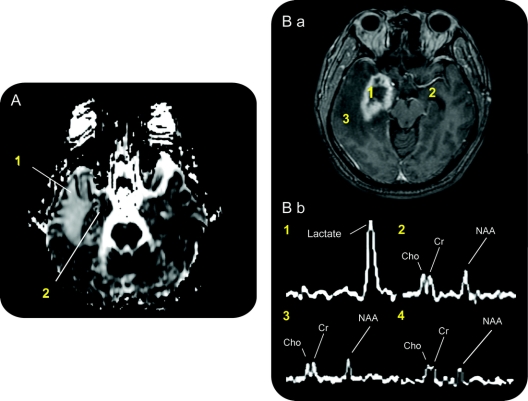
ADC values from the ipsilateral temporal lobes were approximately twice as high as the contralateral temporal lobes at 12 months (n = 14, median difference in ADC [ipsilateral-contralateral] = 934; p < 0.001, Wilcoxon signed rank test). Typically, the temporal lobe and surrounding areas demonstrated increased ADC values consistent with the transient vasogenic edema observed in T2 imaging. However, a heterogenous pattern with focal areas of reduced diffusion was also observed at the target site (figure 4A), indicating ongoing ischemia and local metabolic alterations at a relatively long timepoint after treatment.
Figure 4 Potential mechanisms of radiosurgery for medial temporal lobe epilepsy revealed by magnetic resonance diffusion and spectroscopy
(A) Diffusion magnetic resonance at 12 months after treatment shows increased diffusion throughout the temporal lobe (1). There is also an area of decreased diffusion, and heterogeneity in the medial temporal lobe (2) suggesting ongoing delayed ischemic changes. (B) Proton magnetic resonance spectroscopy. T1-weighted axial MRI after gadolinium administration for spectroscopy voxel measurements (B.a). Spectra obtained for individual voxels (1 mL) selected in the following areas (B.b): (1) radiosurgery target region in mesial temporal lobe at 12 months, (2) contralateral mesial temporal lobe at 12 months, (3) peri-target region edema at 12 months, and (4) target region in mesial temporal lobe before radiosurgery treatment (not shown, but same position as (1) before treatment). The right medial temporal lobe demonstrates a prominent lactate peak in the area of contrast enhancement, and the near absence of the N-acetylaspartate signal compared to other regions or before treatment.
Proton spectroscopy was available in 5 patients (pretreatment = 4 scans, postoperative months 8–11 = 4, month 12 = 5, month 24 = 4 scans, total = 19 scans). Pretreatment levels of choline, creatine, and NAA were either normal or slightly reduced in the ipsilateral mesial temporal lobe compared qualitatively to the contralateral side on pretreatment MRS. At 12 months, choline, creatine, and NAA peaks were essentially absent and a highly elevated lactate peak appeared at the center of the surgical target (figure 4B).
Predictors of seizure remission.
Beyond neuroimaging variables, we also examined treatment variables to evaluate which findings were best associated with subsequent seizure remission. The association between remission and treatment volume at the 50% isodose line (p = 0.08), or the volume of the surgical target with remission (p = 0.12) did not reach significance.
In contrast, larger volumes of T2 hyperintensity and contrast enhancement at 12 months were strongly associated with subsequent seizure remission in the period evaluated between 24 and 36 months (figure 5). The specificity of T2 hyperintensity volume with cutoff at >200 mL was 100% for predicting eventual seizure remission, with sensitivity = 65%, positive predictive value = 100% (95% confidence interval [CI] = 75%–100%), and negative predictive value = 42% (95% CI = 0.15–0.72).
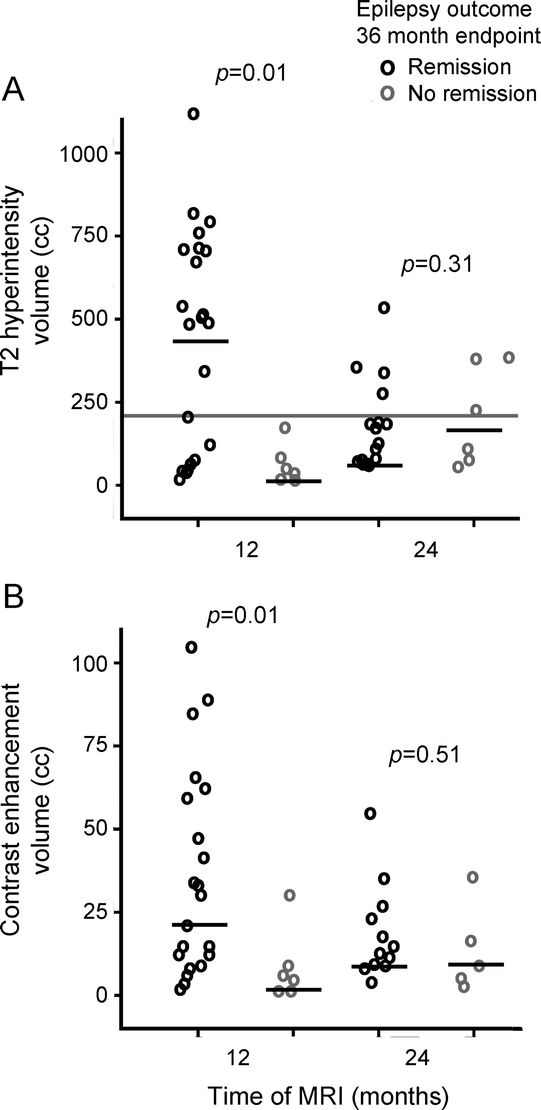
Figure 5 Radiographic predictors of seizure freedom after radiosurgery for medial temporal lobe epilepsy
Dose effects on T2 hyperintensity volumes (A) and contrast enhancement volumes (B) at 12 and 24 months after radiosurgery in relationship to seizure remission between postoperative months 24 and 36. Black bars denote means. In (A), gray bar demonstrates cutoff threshold volume denoting 100% specificity in predicting seizure remission. p Values from Mann-Whitney tests.
Neuropsychology.
We examined the relationship between magnetic resonance changes and language or global cognitive functions. We found no correlation between T2 hyperintensity volumes and changes in learning and memory or global cognition at either 12 or 24 months (CVLT-LDFR, WMS-R DR, TMT-A and TMT-B; Spearman rank correlation, p > 0.45). Interestingly, improvement in quality of life was correlated with greater T2 hyperintensity volumes at 12 months (QOLIE-10; coefficient = 0.47, 95% CI = 0.09–0.73, p = 0.01). We interpret this finding as a reflection of higher seizure remission in the responder group, since QOLIE-10 scores strongly follow seizure remission patterns.23
However, in the subset of patients with RS in the dominant hemisphere (n = 13), we found that worsening of the memory and language scores evaluated at 12 months appeared to have weak but insignificant correlation with concurrently measured peak T2 hyperintensity volumes (WMSR-DR, Spearman correlation coefficient = −0.55, p = 0.05; CVLT-LDFR = −0.53, p = 0.06; BNT = −0.47, p = 0.10).
T2 hyperintensity volumes at 12 months did not correlate with long-term memory and language outcomes at 24 months (WMSR-DR, r = 0.02, p = 0.92; CVLT-LDFR = 0.03, p = 0.94; BNT = −0.11, p = 0.71). This was confirmed by the lack of difference in T2 changes in the subgroup of dominant hemisphere patients who were classified as significantly impaired (as determined by relative change indices)10 compared to those who were not impaired (Mann-Whitney test, p = 0.59). Changes in global cognitive function at 24 months did not reveal an association with T2 hyperintensity volumes (TMT-A, p = 0.86; TMT-B, p = 0.15).
DISCUSSION
Our recently published pilot trial demonstrated that Gamma Knife® RS for MTLE was a well-tolerated procedure with seizure remission rates similar to those published for standard open temporal lobectomy. In the current report, we examined the development of the RS-induced radiologic changes and their association with clinical outcomes. We found that MRI indicators of vasogenic edema occurred approximately 9 months after RS and showed a significant relationship with seizure remission. Evidence of vasogenic changes, as well as diffusion and biochemical alterations detected by spectroscopy, are consistent with a mechanism of temporal lobe radiation injury mediated by local vascular insult and tissue necrosis. The extent of these alterations at 12 months was dose-dependent and predicted seizure remission in the third year of follow-up. The extent of the radiographic lesion did not correlate with long-term or sustained impairments in measures of language function or neurocognition.
Our results help to explain the outcomes reported in previous trials of RS for MTLE. With few exceptions, high-dose protocols (>20 Gy) yielded better short-term seizure remission than lower dose protocols.8 In the current report, high-dose RS treatments resulted in larger radiologic changes; accordingly, the presence of a “significant” T2 hyperintensity lesion (volume >200 mL) at the 12-month postoperative mark strongly predicted good seizure and quality of life outcomes.
While the neuroimaging changes documented in the current study are consistently associated with seizure control, the mechanisms by which RS reduces or eliminates seizures are unclear. Diffusion and proton spectroscopic data may help in this task, as well as shed light on the findings of “radionecrosis” in general. ADC maps at 12 months showed increased perilesional diffusion consistent with vasogenic edema, and within the target, decreased diffusion, consistent with the development of cytotoxic edema as seen with ischemia. Proton spectroscopic data support this interpretation: losses of NAA, choline, and creatine peaks and the development of lactate within the target indicated lack of normal oxidative metabolism, and thus, ischemic loss of neuronal parenchyma. Therefore, our data demonstrated a progression of ischemic vascular injury with subsequent necrosis, leading to increased vascular permeability, vasogenic edema within and around the radiosurgical target, and finally focal encephalomalacia. These findings are somewhat unexpected, since preclinical investigations with the use of rat models of limbic epilepsy demonstrate that seizure reduction is not associated with tissue necrosis or a concomitant loss of neurons,30–32 though most animals achieved improvement rather than remission of seizures. On the other hand, in humans, seizure remission after RS of other epileptic lesions—hypothalamic hamartomas or vascular malformations—does not require radiologic changes consistent with radionecrosis.33,34 Similarly, histopathology of hippocampal specimens obtained from open temporal lobectomy after failed RS for MTLE1,3,7 demonstrates perivascular sclerosis and hyalinization, rather than necrosis. However, since these specimens were obtained from unsuccessful RS, they may not be sufficient to explain the changes required for successful RS in MTLE. Our data indicate that RS does not result in remission of temporal lobe seizures unless there is significant injury to neural tissue in the presumed seizure focus. Whether there is an antiepileptic effect in the human temporal lobe that does not require neuronal damage has not been demonstrated.
Although our data provide guidance in the prediction of seizure remission by 12-month neuroimaging criteria, they do not explain the biologic variability among patients exposed to very similar treatment protocols. Of course, the factors that predict failure of standard temporal lobectomy may also apply to RS, including incomplete hippocampal “resection” or bilateral disease. On the other hand, sensitivities to radiation may differ among patients. Radiation injury mediated by vascular endothelial insult may be affected by genetic factors, such as DNA repair mechanisms, or even acquired conditions such as intracranial atherosclerosis.
The lack of neurocognitive impairments that correspond to peak neuroimaging changes has implications in the overall morbidity of RS. The development of transient, perilesional edema is expected after RS, but short-term cognitive morbidities have not been described.22 Studies of cognition or behavior after RS for tumors,35 vascular malformations,36 hypothalamic hamartomas,34 or mesial temporal lobe epilepsy4,23,37 all demonstrate stabilization, relative sparing, or improvements in various markers of cognitive function, but these studies focus on final outcomes with little emphasis on interim or peak effects. Studies of patients treated with RS for metastatic tumors show that the majority of survivors, most of whom are impaired in categories of executive function, motor dexterity, or learning and memory at presurgical baseline, demonstrate stability or improvements 200 days following treatment35; however, the majority of subjects died of their disease. Patients tested 1 year after RS for vascular malformations demonstrated no significant changes.36 We found a weak trend between the severity of temporal lobe edema and measures of language function when involving the dominant hemisphere, though the study was not powered to make definitive conclusions. A larger trial currently underway will address this issue in detail.
AUTHOR CONTRIBUTIONS
Statistical analyses: Edward Chang, Mark Quigg, Kathleen Lamborn. Data collection: Edward Chang, Mark Quigg, Michael Oh, Mariann Ward, Nicholas Barbaro. Data analysis: Edward Chang, Kathleen Lamborn, Mark Quigg, Donna Broshek. Preparation and editing of manuscript: Edward Chang, Mark Quigg, William P. Dillon, Mariann Ward, Kenneth Laxer, Donna Broshek, Nicholas M. Barbaro.
COINVESTIGATORS
The Epilepsy Radiosurgery Study Group includes David Larson and Lynn Verhey (Department of Radiation Oncology, University of California at San Francisco), Paul Garcia (Department of Neurology, University of California at San Francisco), Ladislau Steiner (Department of Neurological Surgery, University of Virginia), Christine Heck (Department of Neurology, University of Southern California), Douglas Kondziolka (University of Pittsburgh), Robert Beach (State University of New York, Upstate Medical Center), William Olivero (Illinois Neurological Institute), Thomas C. Witt (Department of Neurological Surgery, Indiana University), Vicenta Salanova (Department of Neurology, Indiana University), and Robert Goodman (Department of Neurological Surgery, Columbia University).
DISCLOSURE
Dr. Chang reports no disclosures. Dr. Quigg has served on a speakers' bureau for GlaxoSmithKline and received research support from the NIH/NINDS [R01NS039280–01 (Treatment Site PI, Study Co-PI)]. Dr. Oh reports no disclosures. Dr. Dillon serves on a scientific advisory board as head of the core lab for Coaxia, Inc.; serves as Senior Editor of the American Journal of Neuroradiology; receives honoraria for speaking or educational activities not funded by industry; and estimates that 100% of his clinical effort is spent on neuroimaging. M.M. Ward reports no disclosures. Dr. Laxer serves on scientific advisory boards, speakers' bureaus, and received speaker honoraria from GlaxoSmithKline, UCB, and Pfizer Inc.; serves as a consulting editor for Epilepsy Research; serves as consultant to Lundbeck Inc. (formerly Ovation) and Chairman of Independent Data Monitoring Committee; and receives research support from Genentech, Inc. and the NIH [RO1-NS031966 (PI)]. Dr. Broshek reports no disclosures. Dr. Barbaro has received research support from Elekta AB (Stockholm, Sweden) and from the NIH/NINDS [NS39280–03 (PI)].
Address correspondence and reprint requests to Dr. Nicholas M. Barbaro, Department of Neurological Surgery, University of California San Francisco, 505 Parnassus Avenue, M779, San Francisco, CA 94143 barbaron@neurosurg.ucsf.edu
Study funding: Sponsored by NIH NINDS [NS39280–03] and Elekta AB (Stockholm, Sweden).
Disclosure: Author disclosures are provided at the end of the article.
Received February 5, 2009. Accepted in final form November 2, 2009.
REFERENCES
- 1.Kawai K, Suzuki I, Kurita H, Shin M, Arai N, Kirino T. Failure of low-dose radiosurgery to control temporal lobe epilepsy. J Neurosurg 2001;95:883–887. [DOI] [PubMed] [Google Scholar]
- 2.Rheims S, Fischer C, Ryvlin P, et al. Long-term outcome of gamma-knife surgery in temporal lobe epilepsy. Epilepsy Res 2008;80:23–29. [DOI] [PubMed] [Google Scholar]
- 3.Srikijvilaikul T, Najm I, Foldvary-Schaefer N, Lineweaver T, Suh JH, Bingaman WE. Failure of gamma knife radiosurgery for mesial temporal lobe epilepsy: report of five cases. Neurosurgery 2004;54:1395–1402; discussion 1402–1394. [DOI] [PubMed]
- 4.Regis J, Rey M, Bartolomei F, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia 2004;45:504–515. [DOI] [PubMed] [Google Scholar]
- 5.Bartolomei F, Hayashi M, Tamura M, et al. Long-term efficacy of gamma knife radiosurgery in mesial temporal lobe epilepsy. Neurology 2008;70:1658–1663. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen DK, Spencer SS. Recent advances in the treatment of epilepsy. Arch Neurol 2003;60:929–935. [DOI] [PubMed] [Google Scholar]
- 7.Hoggard N, Wilkinson ID, Griffiths PD, Vaughan P, Kemeny AA, Rowe JG. The clinical course after stereotactic radiosurgical amygdalohippocampectomy with neuroradiological correlates. Neurosurgery 2008;62:336–344; discussion 344–336. [DOI] [PubMed]
- 8.Quigg M, Barbaro NM. Stereotactic radiosurgery for treatment of epilepsy. Arch Neurol 2008;65:177–183. [DOI] [PubMed] [Google Scholar]
- 9.Cmelak AJ, Abou-Khalil B, Konrad PE, Duggan D, Maciunas RJ. Low-dose stereotactic radiosurgery is inadequate for medically intractable mesial temporal lobe epilepsy: a case report. Seizure 2001;10:442–446. [DOI] [PubMed] [Google Scholar]
- 10.Barbaro NM, Quigg M, Broshek DK, et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol 2009;65:167–175. [DOI] [PubMed] [Google Scholar]
- 11.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 12.Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med 1998;339:1426–1433. [DOI] [PubMed] [Google Scholar]
- 13.Kondziolka D, Lunsford LD, Flickinger JC. The application of stereotactic radiosurgery to disorders of the brain. Neurosurgery 2008;62 suppl 2:SHC707–SHC719; discussion SHC719–SHC720. [DOI] [PubMed]
- 14.Regis J, Metellus P, Hayashi M, Roussel P, Donnet A, Bille-Turc F. Prospective controlled trial of gamma knife surgery for essential trigeminal neuralgia. J Neurosurg 2006;104:913–924. [DOI] [PubMed] [Google Scholar]
- 15.Sperling MR, Feldman H, Kinman J, Liporace JD, O'Connor MJ. Seizure control and mortality in epilepsy. Ann Neurol 1999;46:45–50. [DOI] [PubMed] [Google Scholar]
- 16.Behrens E, Zentner J, van Roost D, Hufnagel A, Elger CE, Schramm J. Subdural and depth electrodes in the presurgical evaluation of epilepsy. Acta Neurochir (Wien) 1994;128:84–87. [DOI] [PubMed] [Google Scholar]
- 17.Rydenhag B, Silander HC. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990–1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery 2001;49:51–56; discussion 56–57. [DOI] [PubMed]
- 18.Siegel AM, Cascino GD, Meyer FB, Marsh WR, Scheithauer BW, Sharbrough FW. Surgical outcome and predictive factors in adult patients with intractable epilepsy and focal cortical dysplasia. Acta Neurol Scand 2006;113:65–71. [DOI] [PubMed] [Google Scholar]
- 19.Stroup E, Langfitt J, Berg M, McDermott M, Pilcher W, Como P. Predicting verbal memory decline following anterior temporal lobectomy (ATL). Neurology 2003;60:1266–1273. [DOI] [PubMed] [Google Scholar]
- 20.Seidenberg M, Hermann B, Wyler AR, Davies K, Dohan FC Jr, Leveroni C. Neuropsychological outcome following anterior temporal lobectomy in patients with and without the syndrome of mesial temporal lobe epilepsy. Neuropsychology 1998;12:303–316. [DOI] [PubMed] [Google Scholar]
- 21.Glosser G, Zwil AS, Glosser DS, O'Connor MJ, Sperling MR. Psychiatric aspects of temporal lobe epilepsy before and after anterior temporal lobectomy. J Neurol Neurosurg Psychiatry 2000;68:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong CL, Gyato K, Awadalla AW, Lustig R, Tochner ZA. A critical review of the clinical effects of therapeutic irradiation damage to the brain: the roots of controversy. Neuropsychol Rev 2004;14:65–86. [DOI] [PubMed] [Google Scholar]
- 23.Barbaro NM, Quigg M, Broshek DK, et al. A multicenter, prospective pilot study of Gamma Knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol 2009;65:167–175. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi M, Bartolomei F, Rey M, Farnarier P, Chauvel P, Regis J. MR changes after gamma knife radiosurgery for mesial temporal lobe epilepsy: evidence for the efficacy of subnecrotic doses. In: Kondziolka D, ed. Radiosurgery. Basel: Kargel; 2002:192–202. [Google Scholar]
- 25.Engel JJ, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Surgical Treatment of the Epilepsies, 2nd ed. New York: Raven Press; 1993: 609–621. [Google Scholar]
- 26.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test: Research Edition. San Antonio: Psychological Corp.; 1987. [Google Scholar]
- 27.Wechsler D. Wechsler Memory Scale–Revised Manual. New York: Psychological Corp.; 1987. [Google Scholar]
- 28.Gordon NG. The Trail Making Test in neuropsychological diagnosis. J Clin Psychol 1972;28:167–169. [DOI] [PubMed] [Google Scholar]
- 29.Cramer JA, Perrine K, Devinsky O, Meador K. A brief questionnaire to screen for quality of life in epilepsy: the QOLIE-10. Epilepsia 1996;37:577–582. [DOI] [PubMed] [Google Scholar]
- 30.Maesawa S, Kondziolka D, Dixon CE, Balzer J, Fellows W, Lunsford LD. Subnecrotic stereotactic radiosurgery controlling epilepsy produced by kainic acid injection in rats. J Neurosurg 2000;93:1033–1040. [DOI] [PubMed] [Google Scholar]
- 31.Mori Y, Kondziolka D, Balzer J, et al. Effects of stereotactic radiosurgery on an animal model of hippocampal epilepsy. Neurosurgery 2000;46:157–165; discussion 165–158. [PubMed]
- 32.Chen ZF, Kamiryo T, Henson SL, et al. Anticonvulsant effects of gamma surgery in a model of chronic spontaneous limbic epilepsy in rats. J Neurosurg 2001;94:270–280. [DOI] [PubMed] [Google Scholar]
- 33.Schauble B, Cascino GD, Pollock BE, et al. Seizure outcomes after stereotactic radiosurgery for cerebral arteriovenous malformations. Neurology 2004;63:683–687. [DOI] [PubMed] [Google Scholar]
- 34.Regis J, Scavarda D, Tamura M, et al. Epilepsy related to hypothalamic hamartomas: surgical management with special reference to gamma knife surgery. Childs Nerv Syst 2006;22:881–895. [DOI] [PubMed] [Google Scholar]
- 35.Chang EL, Wefel JS, Maor MH, et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery 2007;60:277–283; discussion 283–274. [DOI] [PubMed]
- 36.Blonder LX, Hodes JE, Ranseen JD, Schmitt FA. Short-term neuropsychological outcome following Gamma Knife radiosurgery for arteriovenous malformations: a preliminary report. Appl Neuropsychol 1999;6:181–186. [DOI] [PubMed] [Google Scholar]
- 37.McDonald CR, Norman MA, Tecoma E, Alksne J, Iragui V. Neuropsychological change following gamma knife surgery in patients with left temporal lobe epilepsy: a review of three cases. Epilepsy Behav 2004;5:949–957. [DOI] [PubMed] [Google Scholar]