Abstract
Objective:
To investigate whether cancer is associated with Alzheimer disease (AD) and vascular dementia (VaD).
Methods:
Cox proportional hazards models were used to test associations between prevalent dementia and risk of future cancer hospitalization, and associations between prevalent cancer and risk of subsequent dementia. Participants in the Cardiovascular Health Study–Cognition Substudy, a prospective cohort study, aged 65 years or older (n = 3,020) were followed a mean of 5.4 years for dementia and 8.3 years for cancer.
Results:
The presence of any AD (pure AD + mixed AD/VaD; hazard ratio [HR] = 0.41, 95% confidence interval [CI] = 0.20–0.84) and pure AD (HR = 0.31, 95% CI = 0.12–0.86) was associated with a reduced risk of future cancer hospitalization, adjusted for demographic factors, smoking, obesity, and physical activity. No significant associations were found between dementia at baseline and rate of cancer hospitalizations for participants with diagnoses of VaD. Prevalent cancer was associated with reduced risk of any AD (HR = 0.72; 95% CI = 0.52–0.997) and pure AD (HR = 0.57; 95% CI = 0.36–0.90) among white subjects after adjustment for demographics, number of APOE ε4 alleles, hypertension, diabetes, and coronary heart disease; the opposite association was found among minorities, but the sample size was too small to provide stable estimates. No significant association was found between cancer and subsequent development of VaD.
Conclusions:
In white older adults, prevalent Alzheimer disease (AD) was longitudinally associated with a reduced risk of cancer, and a history of cancer was associated with a reduced risk of AD. Together with other work showing associations between cancer and Parkinson disease, these findings suggest the possibility that cancer is linked to neurodegeneration.
GLOSSARY
- 3MSE
= modified Mini-Mental State Examination;
- AD
= Alzheimer disease;
- ADDTC
= Alzheimer Disease Diagnostic and Treatment Centers;
- CHD
= coronary heart disease;
- CHS
= Cardiovascular Health Study;
- CI
= confidence interval;
- HR
= hazard ratio;
- ICD-9
= International Classification of Diseases–Ninth Revision;
- MCI
= mild cognitive impairment;
- NINCDS-ADRDA
= National Institute of Neurological and Communicative Diseases and Stroke–Alzheimer's Disease and Related Disorders Association;
- PD
= Parkinson disease;
- VaD
= vascular dementia.
Basic research suggests that the development of Alzheimer disease (AD) and of many cancers may be related via one or more common molecular mechanisms.1–3 Common signaling pathways regulating cell death and survival also have been suggested to underlie the decreased risk of most cancers among persons with another age-associated neurodegenerative condition, Parkinson disease (PD).4–6
Despite hypothesized biologic links to AD, and demonstrated associations with another neurodegenerative disease,4,5 research investigating epidemiologic associations between cancer and AD is limited. We previously reported that older adults with prevalent clinical AD develop incident cancer at a slower rate compared to older adults without dementia, and that individuals without dementia with a cancer history may be slower to later develop clinical AD.7 Limitations included the use of a non-population-based sample; the use of informant reports of cancer diagnoses; the possibility that the results were an artifact of faster death rates among participants with AD; and the possibility that physicians may be less likely to thoroughly look for cancer among individuals with dementia.
This work used data from the Cognition Substudy of the Cardiovascular Health Study (CHS) to address these limitations by investigating associations between AD and cancer in a population-based sample, by defining incident cancer based on cancer hospitalization records, and by examining associations between cancer and 2 types of dementia with different etiologies, related to neurodegenerative processes in one (AD) and to cerebrovascular insults in the other (vascular dementia [VaD]).
METHODS
Design.
Two studies were conducted. We first investigated whether time to first cancer hospitalization was associated with having prevalent dementia of a particular type (any AD, pure AD, any VaD, pure VaD, or mixed AD/VaD) at baseline, and subsequently whether time to first diagnosis of dementia of a particular type was related to having a cancer history at baseline. The time of brain MRI, occurring 1992–1993, was used as baseline in both studies.
Participants.
Detailed descriptions of the CHS Cognition Study sample and assessment procedures are available.8–11 Briefly, the CHS is a population-based sample of participants aged 65 years and older from 4 communities (Forsyth County, NC; Washington County, MD; Sacramento County, CA; Pittsburgh, PA). Randomized Medicare eligibility lists were used to identify and recruit 5,201 participants during 1989–1990, and an additional 687 African American participants during 1992–1993. CHS participants were interviewed by telephone every 6 months, and completed up to 10 annual clinic visits through 1998–1999, where demographic, medical, functional, psychosocial, and neuropsychological data were collected.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from all participants, both at entry and at specified intervals during the study.12 Each CHS center obtained approval for the study from their respective institutional review boards.12 Researchers at Washington University completed the CHS Data Distribution Agreement and obtained approval for the study protocol from the Washington University Medical Center Human Subjects Committee.
The CHS Cognition Study.
This ancillary study, initiated in 1998, evaluated cognitive status among CHS participants who had completed MRI and a modified Mini-Mental State Examination (3MSE)13 during 1992–1993 (n = 3,602). Living participants at high risk for dementia based on previous CHS testing, all Pittsburgh site participants, and all participants of minority race were invited to participate in additional neuropsychological testing.
Determination of prevalent (i.e., at the time of the 1992–1993 MRI, which was also defined as the enrollment date of the CHS Cognition Study) and incident (i.e., between Cognition Study enrollment and June 30, 1999) cognitive status was made.9–11 For participants who were deceased, or who refused or were unable to come into the clinic for additional testing, cognitive status determination was based on information obtained from previously completed CHS clinic visits, along with new data from informant/proxy interviews, physician questionnaires, and medical records.
Without knowledge of the MRI results, cognitive status was first classified as normal, mild cognitive impairment (MCI),14 or dementia by a committee of study neurologists and psychiatrists. For participants with dementia, an estimated date of dementia symptom onset was assigned; participants for whom symptom onset occurred before the date of CHS Cognition Study enrollment were classified as having prevalent dementia, and those with symptom onset after enrollment as having incident dementia. The presumed etiology of the dementia (AD only, VaD only, mixed AD and VaD, or other) was then determined based on all available data, including the brain MRI results.9 The presumed etiology of dementia for the “AD only” group was believed to be AD, but not VaD, and dementia in the “VaD only” group was believed to have resulted from VaD, but not AD. The “mixed AD/VaD group” had features of both diseases. Standardized criteria were used to classify dementia subtype including the National Institute of Neurological and Communicative Diseases and Stroke–Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) for AD15 and the State of California Alzheimer's Disease Diagnostic and Treatment Centers (ADDTC) criteria for VaD.16 More detailed information on how dementia diagnoses were assigned has been published.9–11
CHS cancer hospitalization data17–19 were available from the time of CHS enrollment through June 2003. Prevalent (i.e., at the time of the 1992–1993 MRI) cancer was defined as answering “yes” to the CHS enrollment interview question “Have you ever been diagnosed with cancer?” or a hospitalization for cancer after CHS enrollment but before the date of the Cognition Study MRI. Cancer hospitalizations were identified using International Classification of Diseases–Ninth Revision (ICD-9) diagnostic codes on hospital discharge abstracts during the follow-up period, and included new primary, recurrent primary, metastatic (ICD-9 196–198), unspecified site (ICD-9 199), and in situ (ICD-9 230–234) cancers.
Statistical analyses.
Because the date of onset was not determined, data from the 577 participants diagnosed with MCI, and from 5 participants with missing data on baseline cancer status, were excluded. The number of participants in each analysis varied according to the inclusion criteria for that analysis.
Analyses testing whether prevalent dementia is associated with future hospitalizations for cancer.
Cox proportional hazards models were used to first examine associations between having any AD diagnosis (pure AD + mixed AD/VaD) vs no dementia at baseline and time from baseline to first cancer hospitalization among participants with no cancer history. Participants with prevalent dementia diagnoses of other types (i.e., pure VaD) were not included in these analyses. Participants without prevalent dementia, but who later received incident dementia diagnoses, were included in the analyses as part of the baseline “no dementia” group. The models were adjusted for demographic characteristics (sex, race, age, education, income, CHS clinic) and baseline risk factors associated with multiple cancer types20: tobacco use (current smoker, former smoker, or never smoked and number of cigarette pack-years smoked), obesity (more than 130% overweight), and physical inactivity (kilocalories expended in physical activity). Data from participants who were not hospitalized for cancer were censored at the date of last follow-up or death from other causes. Preliminary models tested whether the prevalent AD variable interacted with each of the other variables in the model in determining time to first cancer hospitalization. If the prevalent AD variable interacted significantly with another categorical variable, the analyses were then conducted separately for each level of the categorical variable.
The analyses were repeated 5 times, substituting the predictor variable “no dementia vs any AD” with variables reflecting different types, or combinations of types, of prevalent dementia diagnoses. Other prevalent dementia diagnoses tested were pure AD, any VaD (pure VaD + mixed AD/VaD), pure VaD, mixed AD/VaD, and any dementia (regardless of diagnosis type).
Because genetic factors may underlie links between cancer and AD, we conducted a similar set of analyses in which participants with no diagnosis of a particular dementia type at baseline who later developed that dementia were not considered part of the baseline no dementia group but were eliminated from the analysis.
Differences between the pure AD, pure VaD, and groups without dementia in baseline severity of dementia, as reflected in 3MSE scores, were tested using a mixed linear model. Using pure AD as the reference group, differences between these groups in time from baseline to death were tested using a Cox proportional hazards model. Both models were adjusted for the demographic and risk factor variables previously listed.
Analyses testing whether a history of cancer at baseline is associated with a future diagnosis of dementia.
Cox proportional hazards models were first used to examine associations between a baseline cancer history (yes vs no) and time from baseline to first diagnosis of any AD, after adjustment for demographic characteristics (sex, race, age, education, income, CHS clinic) and risk factors associated with AD and VaD21 (number of APOE ε4 alleles, hypertension, diabetes, and coronary heart disease [CHD, a summary variable that includes a history of angina, myocardial infarction, bypass surgery, or angioplasty]). Participants without a prevalent cancer history, but who later had an incident cancer hospitalization, were included in the baseline “no cancer history” group. Data from participants who did not develop AD were censored at the time of death or at June 30, 1999, when clinical observation was ended for the CHS Cognition Study. If preliminary testing indicated that the prevalent cancer variable interacted with another categorical variable, the analyses were then conducted separately for each level of the categorical variable. The analyses were repeated 5 times, substituting the “any AD” endpoint variable with variables reflecting different types, or combinations of types, of incident dementia diagnoses. In these analyses, data from participants who developed other dementia types were censored at the date of onset of those other dementia types. Like other CHS investigators,12 we did not adjust for stroke in our analyses, because evidence of stroke was used in making the dementia-type diagnoses and is therefore not an independent risk factor.
To provide evidence for or against the idea that genetic factors might underlie associations between AD and cancer, we conducted similar analyses in which participants with no diagnosis of cancer at baseline who later developed cancer were removed from the analysis.
We used a Cox proportional hazards model, adjusted for the variables listed above, to examine whether there was a difference in time to death for participants with and without a cancer history at baseline.
Several additional analyses were conducted to test the proportional hazards assumption, the impact of missing data, and the robustness of results (appendix e-1 on the Neurology® Web site at www.neurology.org).
RESULTS
In 3,020 eligible participants, there were 478 new dementia diagnoses and 376 hospitalizations for invasive cancer over the follow-up period (tables 1 and 2). Frequently occurring cancers are presented in table e-1. Participants were followed a mean of 5.4 years for dementia and 8.3 years for cancer.
Table 1 Demographics for participants meeting study criteria by dementia status (n = 3,020)
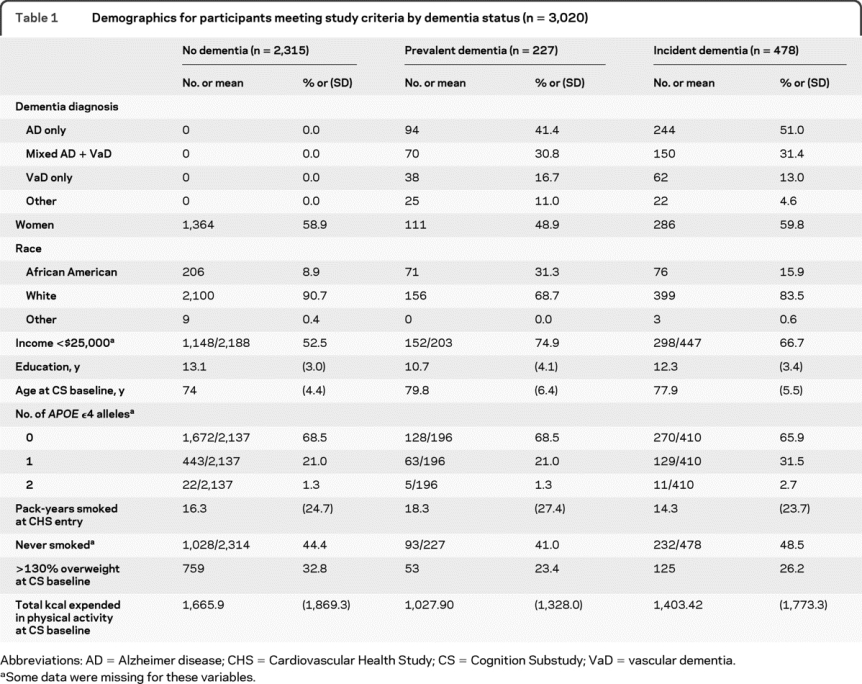
Table 2 Demographics for participants meeting study criteria by cancer status (n = 3,020)

Time to incident cancer hospitalization as it relates to having dementia at baseline.
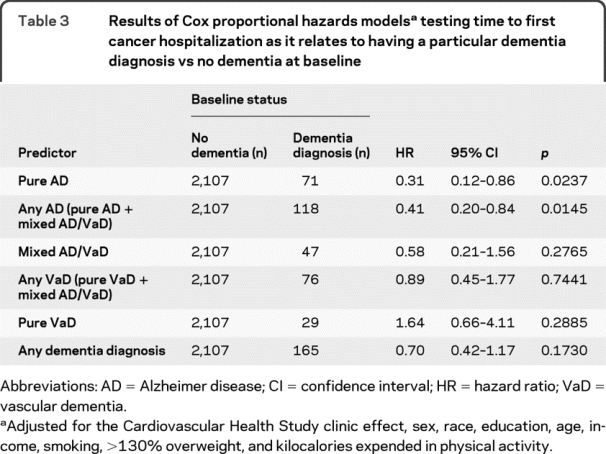
The prevalent dementia variables did not interact with the other independent variables to predict time to first cancer hospitalization. The results of the final models are shown (table 3). Compared to participants who had no dementia at baseline, individuals with any AD and pure AD had a slower rate of future cancer hospitalizations with time, whereas participants with pure VaD, any VaD, mixed AD/VaD, or dementia generally showed no significant associations between baseline dementia and rate of cancer hospitalizations with time. Of note, the point estimates of the hazard ratios (HRs) for the prevalent dementia variables increased as the ratio of VaD to AD in the prevalent dementia groups increased, regardless of the significance of the effect (table 3). Least square mean (95% confidence interval [CI]) 3MSE scores at baseline were 91.5 (90.1–92.9) for those without dementia, 73.6 (71.7–75.5) for pure AD, and 66.3 (63.8–68.8) for pure VaD groups (p < 0.0001 overall and for all pairwise group comparisons). The group without dementia had a slower (HR = 0.59, 95% CI = 0.44–0.79) and the pure VaD group had a faster (HR = 2.63, 95% CI = 1.64–4.22) rate of death with time compared to the pure AD group (p < 0.0001). The analyses excluding incident dementia cases from the baseline no dementia groups yielded similar results (table e-2).
Table 3 Results of Cox proportional hazards models testing time to first cancer hospitalization as it relates to having a particular dementia diagnosis vs no dementia at baseline
Time to first dementia diagnosis as it relates to a history of cancer at baseline.
Because preliminary modeling indicated that the prevalent cancer variable interacted with race in predicting time to diagnosis of any AD (p = 0.0002), pure AD (p = 0.0001), and any dementia (p = 0.0067), these models were repeated separately by race. White participants with a cancer history had a slower rate of receiving any AD or a pure AD diagnosis with time compared to white participants with no cancer history (table 4). The effect was in the opposite direction for minorities, but the number of minority participants with a history of cancer was small (n = 29). Cancer history was not associated with time to diagnosis of any VaD (HR = 1.01, 95% CI = 0.69–1.48, p = 0.9666), pure VaD (HR = 0.78, 95% CI = 0.36–1.66, p = 0.5125), or mixed dementia (HR = 1.06, 95% CI = 0.68–1.65, p = 0.8029). Results were generally similar for the analyses excluding incident cancer cases from the baseline no cancer groups (table e-3). Participants with a cancer history had a faster rate of death with time (HR = 1.18, 95% CI = 1.01–1.37, p = 0.0396).
Table 4 Results of Cox proportional hazards models testing time to diagnoses of any AD, pure AD, and any dementia diagnosis as a function of a history of cancer at baseline for white subjects and minority subjects separately

DISCUSSION
This study addresses a number of potential limitations regarding prior findings of a slower rate of AD development for individuals with a cancer history and a slower rate of incident cancer diagnosis for individuals with AD.7 First, associations between AD and cancer were found in a population-based sample, suggesting that relationships between the 2 diseases were not due to the use of a convenience sample from a particular geographic area. Second, a potential source of diagnostic bias related to the possibility that the collateral sources of participants with AD might be less likely to report cancer diagnoses compared to those of participants without dementia is eliminated because cancer diagnoses in this study were obtained from hospitalization records. Third, the possibility that persons with AD were slower to be diagnosed with cancer because physicians were less likely to look for cancer among individuals with dementia is controlled by comparing the associations between cancer and AD with the associations between cancer and another dementia, VaD, caused by a non-AD etiology.
Although often co-occurring with AD, VaD itself is thought not to be neurodegenerative in origin, but to result from brain damage due to vascular pathology. Because baseline dementia severity was even higher for individuals with VaD than those with AD, the finding that VaD was not related to rate of incident cancer diagnosis suggests that it is unlikely that cognitive impairment per se explains the association between AD and cancer. The related possibility, that physicians tend to overlook dementia among individuals with cancer, is unlikely because the purpose of the CHS Cognition Study was to identify dementia and its subtypes among CHS participants regardless of other comorbidities. However, in everyday clinical practice, the possibility that physicians may be less likely to thoroughly look for cancer among individuals with dementia or less likely to look for AD among those with cancer remains.
In cross-sectional research, differential death rates at earlier ages are possible explanations for a lower prevalence of cancer among individuals with AD, and a lower prevalence of AD among individuals with cancer. In this and our previous longitudinal study, all participants were alive at baseline and survival analysis techniques adjusted for age and other potential confounders were used to examine the rate of incident AD and cancer with time. The inclusion of participants with baseline VaD in this study allowed us to investigate whether individuals with AD showed a reduced rate of incident cancer because they died earlier during the follow-up period than participants without dementia. Previous research in the CHS sample indicated that individuals with VaD have a shorter interval than those with AD between disease onset and death.22 Likewise, we found that the time between baseline assessment and death was faster for those with VaD compared to those with AD, with both dementia groups dying at a faster rate than participants without dementia in this study. If the association of baseline AD with incident cancer was due to an earlier rate of death for the AD group compared to the group without dementia, then a similar effect should have been found for the VaD group.
The effect of a baseline cancer history on subsequent dementia diagnoses also differed for incident AD and incident VaD. No association between cancer history and VaD development was found. Among white subjects, a cancer history was associated with a slower rate of AD diagnoses. The opposite effect was found for minority participants, such that a history of cancer was related to a more rapid rate of AD diagnosis with time. Most of our minority participants were African American (table 1), a group that is at higher risk for cancers occurring at many sites and for cancer-related deaths.23 Reasons for these race-based disparities vary by cancer type, but may include differences in detection and treatment, underlying risk factors, cultural beliefs, lifestyle, and genetic factors.23,24 If a future study were to validate this finding, this would underscore the importance of research on racial and ethnic differences in cancer incidence and prognosis. However, given the small number of minority participants with a cancer history at baseline (n = 29), we cannot exclude the possibility that this is a spurious finding.
Cancers occurring at some sites (e.g., benign skin cancers), or that were untreated (e.g., some prostate cancers) or treated solely on an outpatient basis (e.g., cervical carcinoma in situ) are not represented. There may be differences between AD and VaD in the stage at which cancers were diagnosed. Replication using data that reflect both inpatient and outpatient cancer diagnoses and cancer stage is needed. Additionally, because participants in the incident dementia analyses with a history of cancer were those who survived cancer, incident AD may be related to factors associated with cancer survival, rather than cancer itself. Future research should also examine relationships between AD and cancers occurring at particular sites. Finally, issues of mortality and bias remain a major concern.
Despite these limitations, the results add to and support the small amount of existing evidence suggesting an association between the development of cancer and AD among older adults, and suggest that cancer does not share a similar relationship with VaD. Together with work linking the development of cancer and PD,4,5 these results suggest that the development of many cancers may be associated with the development of neurodegenerative disorders.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Catherine M. Roe.
ACKNOWLEDGMENT
The authors thank Dr. Alison Goate, Washington University, St. Louis, MO, for comments. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
STUDY FUNDING
Supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133, and grant U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke, grants 5 R01 AG15928-02, P50-AG05681, and P01-AG03991 from the National Institute on Aging of the NIH, Bethesda, MD, the Charles and Joanne Knight Alzheimer Research Initiative of the Washington University Alzheimer's Disease Research Center, St. Louis, MO, and the Postdoctoral Program of 1UL1RR024992-01 from the National Center for Research Resources.
DISCLOSURE
Dr. Roe receives research support from the NIH [N01-HC-85079 through N01-HC-85086 (Co-I), N01-HC-35129 (Co-I), N01 HC-15103 (Co-I), N01 HC-55222 (Co-I), N01-HC-75150 (Co-I), N01- HC-45133 (Co-I), NIHLBI U01 HL080295 (Co-I), NINDS 5 R01 AG15928-02 (Co-I), P50-AG05681 (Co-I), NIA P01-AG03991 (salary support), and 1UL1RR024992- 01 (Postdoctoral Program)], the National Center for Research Resources, and the Charles and Joanne Knight Alzheimer Research Initiative of the Washington University Alzheimer's Disease Research Center. Dr. Fitzpatrick has received travel expenses and/or honoraria for lectures or educational activities not funded by industry and receives research support from the NIH [NHLBI 1 R01 HL80698-01 (PI), 5U01HL080295 (Co-I), NCBI Gene-Environment Initiative Genome-Wide Association Study (GENEVA) (Co-I), NCI 1R01 CA116393 01 (PI of Subcontract), and NIMH R01 MH081757 (PI of Data Coordinating Center)] the CDC [1 U48 DP000312-01 (PI)] and the University of Washington Royalty Research Fund. Dr. Xiong serves as an Associate Editor of Biostatistics and receives research support from the NIH [NIA K25 AG025189 (PI), NIA P01 AG26276-01 (Biostatistics Component Leader), NIA 5 P01 AG03991 (Biostatistics Core Director), NIA 5P50 AG05681 (Biostatistics Core Director), NIA U01 AG032438 (Biostatistics Core Director), and R01 AG029672 (Subcontract PI)], and from the Alzheimer Association (New Investigator Research Award, PI). Dr. Sieh and Dr. Kuller report no disclosures. Dr. Miller serves on a DSMB for Ethicon; receives royalties from publishing Editor Handbook of Statistics (Elsevier, 2008); and receives research support from the NIH [P50 NS055977 (Core PI), U54 RR023496 (Program PI), R01-NS051631 (Co-I), U01 DK062401 (coordinating center PI), U01-AG023746 (coordinating center Co-I), P30 CA091842 (Core PI), and U10 EY09341 (coordinating center Co-Director)]. Dr. Williams serves on scientific advisory boards for Myriad Genetics, Inc. and Gentiva Home Health; has received honoraria for lectures or educational activities not funded by industry; serves as a consultant for Centene Corporation; serves on a speakers' bureau for the Alzheimer's Association St. Louis Chapter; and receives research support from the NIH [NCRR KL2RR024994 (subproject of UL1 RR024992) (Clinical Research Scholar), NIA P50AG05681 (Research Clinician, Director, African American Outreach Satellite), NIDDK 3 R01 DK063202–03S1 (Minority Scholar), and 5K12RR023249-03 (Clinical Research Scholar)]. Dr. Kopan serves as a consultant to Merck Serono; served on a scientific advisory board for Sigma; serves as an Associate Editor of Developmental Cell; receives research support from the NIH [GM55479 (PI), DK066408 (PI), AG025973 (PI), DK073453 (PI), P50 AG05681 (Project investigator), P50CA094056 (Project investigator), P30DK079333 (Project investigator), and R21NS0616680 (Co-I)]; has received license fee payments for delta E V1744L, V1744K, ICV1744, and Jagged-1 constructs license effective 9/21/2001 (GlaxoSmithKline, 2002), Cell Lines (Merck Serono, 2004), and delta E V1744L, V1744K, ICV1744, and Jagged-1 constructs license effective 5/31/2004 (Amgen, 2004); and receives royalty payments for MAN1 antibody license effective 12/18/01 (Merck Serono, 2001). Dr. Behrens has received research support from the Chilean government (Fondecyt #1080569). Dr. Morris serves on scientific advisory boards for Bristol-Myers Squibb, Elan Corporation, Genentech, Inc., Eli Lilly and Company, Merck Serono, Novartis, Pfizer Inc., Schering-Plough Corp., and Wyeth; serves on the editorial advisory board of Alzheimer's Disease and Associated Disorders; receives royalties from publishing Mild Cognitive Impairment and Early Alzheimer Disease: Detection and Diagnosis (Blackwell Publishing, 2009); and receives research support from Elan Corporation, Wyeth, Eli Lilly and Company, Novartis, Pfizer Inc., Avid Radiopharmaceuticals, Inc., the NIH/NIA [P50AG05681 (PI), P01AG03991 (PI), P01AG026276 (PI), U01AG032438 (PI) U01AG024904 (Neuropathology Core Leader), R01AG16335 (consultant), and P50NS006833 (investigator)], and the Dana Foundation.
Supplementary Material
Address correspondence and reprint requests to Dr. Catherine M. Roe, Washington University School of Medicine, 660 S. Euclid Avenue, Campus Box 8111, St. Louis, MO 63110 cathyr@wustl.edu
Editorial, page 100
Supplemental data at www.neurology.org
e-Pub ahead of print on December 23, 2009, at www.neurology.org.
Study funding: Funding information is provided at the end of the article.
Dr. Catherine Roe had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure: Author disclosures are provided at the end of the article.
Received February 20, 2009. Accepted in final form August 3, 2009.
REFERENCES
- 1.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 2007;32:577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signaling and disease. Nat Rev Mol Cell Biol 2007;8:904–916. [DOI] [PubMed] [Google Scholar]
- 3.Li T, Wen H, Brayton C, et al. Moderate reduction of γ-secretase attenuates amyloid burden and limits mechanism-based liabilities. J Neurosci 2007;27:10849–10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driver JA, Logroscino G, Buring JE, Gaziano JM, Kurth T. A prospective cohort study of cancer incidence following the diagnosis of Parkinson's disease. Cancer Epidemiol Biomarkers Prev 2007;16:1260–1265. [DOI] [PubMed] [Google Scholar]
- 5.Inzelberg R, Jankovic J. Are Parkinson disease patients protected from some but not all cancers? Neurology 2007;69:1542–1550. [DOI] [PubMed] [Google Scholar]
- 6.Kim RH, Mak TW. Tumours and tremors: how PTEN regulation underlies both. Br J Cancer 2006;94:620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC. Alzheimer disease and cancer. Neurology 2005;64:895–898. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright PE, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 9.Lopez OL, Kuller LH, Fitzpatrick AL, et al. Evaluation of dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology 2003;22:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 2004;52:195–204. [DOI] [PubMed] [Google Scholar]
- 11.Lopez OL, Kuller LH, Becker JT, et al. Classification of vascular dementia in the Cardiovascular Health Study Cognition Study. Neurology 2005;64:1539–1547. [DOI] [PubMed] [Google Scholar]
- 12.Newman AB, Fitzpatrick AL, Lopez O, et al. Dementia and Alzheimer's disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc 2005;53:1101–1107. [DOI] [PubMed] [Google Scholar]
- 13.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 14.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman DA, Folstein MF, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 16.Chui HC, Victoroff JI, Margolin D, et al. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology 1992;42:473–480. [DOI] [PubMed] [Google Scholar]
- 17.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol 1995;5:278–285. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick AL, Daling JR, Furberg CD, Kronmal RA, Weissfeld JL. Use of calcium channel blockers and breast carcinoma risk in postmenopausal women. Cancer 1997;80:1438–1447. [DOI] [PubMed] [Google Scholar]
- 19.Sieh W, Edwards KL, Fitzpatrick AL, et al. Genetic susceptibility to prostate cancer: prostate-specific antigen and its interaction with the androgen receptor (United States). Cancer Causes Control 2006;17:187–197. [DOI] [PubMed] [Google Scholar]
- 20.American Cancer Society. Cancer Prevention & Early Detection Facts & Figures 2007. Atlanta: American Cancer Society; 2007. [Google Scholar]
- 21.Kuller LH, Lopez OL, Jagust WJ, et al. Determinants of vascular dementia in the Cardiovascular Health Study. Neurology 2005;64:1548–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Survival following dementia onset: Alzheimer's disease and vascular dementia. J Neurol Sci 2005;229–230:43–49. [DOI] [PubMed]
- 23.Jemal A, Siegel R, Ward E, Murray T, Xu J. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- 24.Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol 2007;177:444–449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


