Abstract
Background:
What makes a movement feel voluntary, and what might make it feel involuntary? Motor conversion disorders are characterized by movement symptoms without a neurologic cause. Conversion movements use normal voluntary motor pathways, but the symptoms are paradoxically experienced as involuntary, or lacking in self-agency. Self-agency is the experience that one is the cause of one's own actions. The matched comparison between the prediction of the action consequences (feed-forward signal) and actual sensory feedback is believed to give rise to self-agency and has been in part associated with the right inferior parietal cortex. Using fMRI, we assessed the correlates of self-agency during conversion tremor.
Methods:
We used a within-subject fMRI block design to compare brain activity during conversion tremor and during voluntary mimicked tremor in 8 patients.
Results:
The random effects group analysis showed that conversion tremor compared with voluntary tremor had right temporoparietal junction (TPJ) hypoactivity (p < 0.05 family-wise error whole brain corrected) and lower functional connectivity between the right TPJ, sensorimotor regions (sensorimotor cortices and cerebellar vermis), and limbic regions (ventral anterior cingulate and right ventral striatum).
Conclusions:
The right TPJ has been implicated as a general comparator of internal predictions with actual events. We propose that the right TPJ hypoactivity and lower TPJ and sensorimotor cortex interactions may reflect the lack of an appropriate sensory prediction signal. The lack of a match for the proprioceptive feedback would lead to the perception that the conversion movement is not self-generated.
GLOSSARY
- C
= conversion tremor;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- FWE
= family-wise error;
- R
= rest;
- TPJ
= temporoparietal junction;
- V
= voluntary mimic.
What makes a movement feel voluntary, and what might make it feel involuntary? Self-agency is the experience that we are the cause of our own actions. Contemporary motor theory postulates a feed-forward model that normal self-generated movement is accompanied by a sensory prediction of the motor outcome. The matched comparison of predicted outcome and visual or proprioceptive sensory feedback from the actual movement gives rise to a sense of self-agency.1,2 The monitoring of the discrepancy between the intended and actual outcome has been associated with the inferior parietal and prefrontal cortex and cerebellum.1–7
To understand the mechanisms underlying the sense of agency, we studied patients with conversion disorder,8–10 or involuntary neurologic symptoms not explained by a neurologic or medical disorder. Studies of conversion disorder date back to the work of Charcot and Freud, but unexplained neurologic symptoms remain common and poorly understood. Aberrant conversion motor symptoms such as tremor critically use voluntary motor pathways, but patients experience the movements as involuntary.9–11
We investigated the neurobiologic basis of lack of agency by comparing conversion tremor with voluntary mimicked tremor in a within-subject design using fMRI.
METHODS
Subjects.
Subjects were recruited over a 5-year period from patients assessed at the Human Motor Control Section, National Institute of Neurological Disorders and Stroke. Inclusion criteria included “clinically definite” psychogenic movement disorder, a form of conversion disorder,8,10 intention or postural tremor without resting tremor or head movements, ability to mimic movements without triggering symptoms, and absence of other major neurologic disorders (e.g., traumatic brain injury, stroke, central inflammatory diseases, tumors, dementia, neurodegenerative diseases). We did not exclude minor neurologic disorders as part of the study, but none were identified in our patients. All patients had diagnoses of conversion disorder.
Standard protocol approvals and patient consents.
The study was approved by the NIH Institutional Review Board, and all patients gave informed consent.
Task design.
While undergoing fMRI, subjects performed two 25-second pseudorandomized conditions interspersed with 25-second rest (R) periods: they positioned their affected forearm to trigger their conversion tremor (C) or they voluntarily reproduced their conversion tremor in the same arm at the same frequency and amplitude (V). Five C and 5 V conditions were repeated over 3 runs (total 27.5 minutes). Verbal instructions (“tremor,” “mimic,” “rest”) indicated the condition start. Imaging sessions were videotaped.
Imaging procedure.
Imaging was performed with a 1.5-T General Electric (Fairfield, CT) scanner using an 8-channel head coil. Twenty-one axial slices with a repetition time of 2.5 seconds were acquired (echo time 25 milliseconds, slice thickness/gap 5/1 mm, flip angle 90°, matrix size 64 × 64 mm). The first 6 dummy scans were discarded to allow for equilibrium effects.
fMRI data analysis was performed using SPM5 (Statistical Parametric Mapping; www.fil.ion.ucl.ac.uk/spm). Data preprocessing consisted of slice timing correction, within-subject realignment, spatial normalization, and smoothing using a 6-mm gaussian kernel. Twelve subjects were scanned. Data from 4 subjects were excluded because of excessive head motion artifact (>2 mm).
Blocks without sustained movement or with contralateral limb movement for more than 5 seconds were discarded. Videotaped tremor blocks were compared with mimic blocks within subjects. A rater blinded to subject and condition compared conditions on a visual analog scale for overall similarity in amplitude and frequency (1 = not similar; 10 = very similar).
A canonical hemodynamic response function was modeled to the block onset and used as a covariate in a general linear model. Contrasts were compared using a random effects group model. To assess main C and V activations, we assessed C-R and V-R contrasts using a single-sample t test. To assess overlapping activity in C and V, we used an inclusive mask (2-sample t test, mask p < 0.05). We compared differences between C and V by comparing C-R and V-R contrasts using a paired t test. A p value <0.05 family-wise error (FWE) whole brain corrected was considered significant. We assessed functional connectivity using a psychophysiologic interaction comparing C vs V (p < 0.001 uncorrected extent threshold >8 voxels was considered significant).
RESULTS
All subjects were diagnosed with conversion disorder (7/8 clinically assessed in person and 1/8 assessed by phone interview by a psychiatrist [V.V.]) (5 women, mean age 42 [SD 8.9] years; symptom duration mean 9.9 [SD 5.6] years [range 1–25 years]; 7 right handed; 6 right, 1 left, and 1 bilateral upper extremity tremor; psychological issues at symptom onset: 2/8 major depression, 3/8 generalized anxiety disorder, 4/8 psychosocial stressors; 1/8 taking antidepressants). None were clinically depressed at the time of the study (based on DSM-IV criteria based on assessment in person or by phone interview [V.V.]). Mean tremor similarity scores within individuals were 8.9 (SD 2.1).
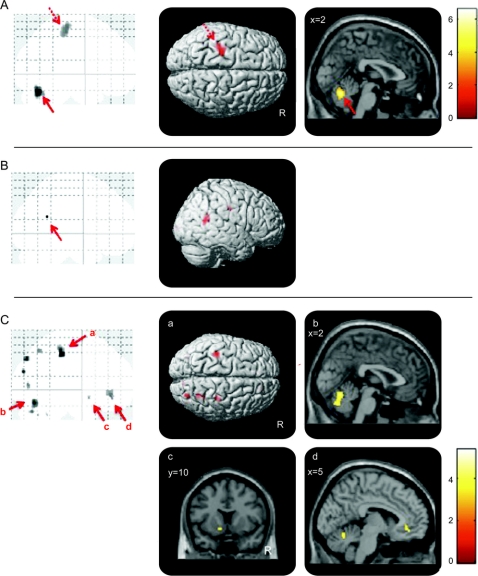
The global maximum in the C-R and V-R contrasts was the cerebellar vermis and secondarily the left sensorimotor cortex (figure, A). The paired t test comparison of C-R >V-R showed right temporoparietal junction (TPJ) hypoactivity (peak voxel: Montreal Neurological Institute x, y, z coordinates = 56, −56, 14 mm; Z score = 5.03; cluster size = 2) (p < 0.05 FWE whole brain corrected; figure, B). Given the low sample size, individual contrasts were also separately inspected (7/8 had right TPJ hypoactivity at a threshold of p < 0.01 uncorrected).
Figure Conversion tremor and voluntary mimic tremor
(A) Inclusive mask of conversion and voluntary tremor. The glass brain and SPM image show cerebellar vermis hyperactivity (solid arrow) (Montreal Neurological Institute local maximum coordinates reported as x, y, z: 0, −66, −22 mm; Z score: 4.39) and left sensorimotor cortex (dashed arrow) (−26, −26, 58 mm; 3.51) during conversion tremor (C) vs rest (R) and voluntary mimic (V) vs R (2-sample t test). The glass brain and SPM image are shown at p < 0.001 uncorrected threshold >5 voxels. (B) Conversion vs voluntary tremor. The glass brain and SPM image show right temporoparietal junction hypoactivity in the contrast of C-R compared with V-R (paired t test). The glass brain is shown at p < 0.05 family-wise error whole brain corrected. The SPM image is shown at p < 0.001 uncorrected threshold >5 voxels. (C) Temporoparietal junction connectivity map for the contrast of conversion vs voluntary tremor. The glass brain and SPM images show decreased functional connectivity between the right temporoparietal junction (seed) and (a) left and right sensorimotor cortices, (b) bilateral cerebellar vermis, (c) left ventral striatum, and (d) bilateral ventral cingulate/medial prefrontal cortex during conversion vs voluntary tremor. The glass brains and SPM image are shown at p < 0.001 uncorrected threshold >5 voxels.
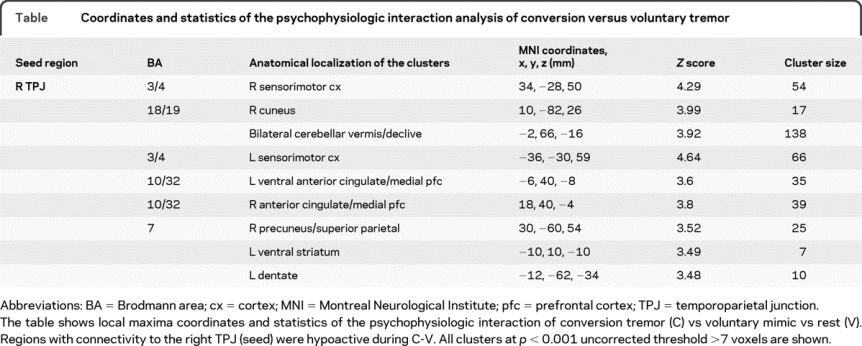
In the psychophysiological interaction (seed voxel based on right TPJ peak voxel, radius 0.8 mm) contrast of C-V, the TPJ showed less connectivity with bilateral sensorimotor cortices, cerebellar vermis and ventral cingulate/medial prefrontal cortex, and right precuneus/superior parietal and left ventral striatum (figure, C, and table).
Table Coordinates and statistics of the psychophysiologic interaction analysis of conversion versus voluntary tremor
DISCUSSION
We studied patients with conversion tremor using a within-subject comparison of involuntary conversion tremor and voluntary reproduction of their conversion tremor to assess for the correlates of loss of self-agency. We demonstrated that C-R vs V-R was associated with right TPJ hypoactivity, a region involved in multisensory integration. During C as compared with V, the TPJ had lower functional connectivity with sensorimotor regions and limbic regions.
From 156 patients in the database seen over a 5-year period, only 8 patients were included in the study because of technical demands of our study that would permit comparative analysis of voluntary vs involuntary movement. Recruiting for functional imaging studies on conversion disorder has been difficult, with reported sample sizes in the literature ranging from 1 to 8.12–18 The present study was limited by the lack of a healthy control group, which we did not include given the lack of involuntary tremors. We did not include a neurologic control group such as PD or essential tremor because the symptoms would not cease at rest and would be difficult to mimic without triggering their symptoms. Thus, having a patient with conversion disorder performing voluntary movement as a within-subject control was the optimal control condition to answer our question of interest. We controlled for movement differences with video recording and did not observe differences in the cortical motor areas in the contrast of C-V or V-C, confirming that possible differences in motor output were slight and involuntary and voluntary movement involves similar motor pathways. We also note that the inclusion of only patients with positionally triggered tremor symptoms may limit generalizability. Furthermore, the inclusion of patients with different lateralizing symptoms may present a limitation; however, we suggest that our findings may represent more general mechanisms that have been attributed to the right hemisphere.
Decety and Lamm6 have proposed that the fundamental role of the right TPJ is a low-level computational process involving the prediction of external events by functioning as a general comparator of internal predictions with actual external events. This process is suggested to explain the various low- and high-level cognitive processes attributed to the right TPJ, including self-agency,2–4,6,19 theory of mind,20 and spatial reorienting of visual attention.21 Certainly, studies on theory of mind suggesting that the attribution of mental states to self and to others involves the right TPJ may be relevant in conversion disorder. Spatial attention may also be relevant, emphasizing that the semiautonomous generation of C is associated with a different awareness of spatial movement as compared with the voluntary generation of V. However, we suggest that our findings reflect a lack of self-agency, which is not only a symptom fundamental to the experience of conversion disorder but a feature core to the definition of conversion disorder. The mechanisms underlying self-agency, by definition, fit in well with the role of the right TPJ as a comparator of internal sensory prediction and the actual sensory state.
Stimulation of the inferior parietal cortex has been recently demonstrated to be associated with the illusion of controlling movement (i.e., the experience of controlling movement when no actual movement occurred), which the authors termed the sense of “conscious intention,” and has been suggested to be related to activation of the network involved in movement monitoring through forward modeling.3 In healthy volunteers, agency has been studied using self-generated action and visual feedback manipulation implicating the right inferior parietal cortex and TPJ.2,4–7 In this context, loss of agency is associated with right inferior parietal cortex hyperactivity, which is the opposite of what we observed. In visual feedback manipulation experiments of voluntary movement, the mismatch involves an intact higher-level motor intention. However, in our study, motor intention is almost certainly abnormal. The movement arises without conscious intention, and there may not be a feed-forward signal. The lack of feed-forward signal is a possible interpretation of the decreased connectivity of the TPJ and the sensorimotor cortices and cerebellar vermis. Thus, despite proprioceptive feedback from the movement, there is no mismatch detection, and activation is decreased. C vs V was also associated with lower connectivity between the TPJ and limbic regions (ventral anterior cingulate and ventral striatum), suggesting less limbic involvement in conversion movement evaluation.
The right TPJ has also been implicated in the pathologic states of vestibular illusions (of elevation, rotation), multisensory illusions (or visual shortening and movement of limbs), autoscopy (or the experience of seeing one's body in extrapersonal space), and out-of-body experiences (or the experience of seeing one's body and environment from a location outside of the physical body).22,23 For example, TPJ lesions22 and high-frequency stimulation23 targeting the superior temporal gyrus with increased functional activity of the right TPJ have been associated with out-of-body experiences. Similarly, mental imagery of an out-of-body experience in healthy volunteers is associated with TPJ activity, and transcranial magnetic stimulation impairs this specific mental imagery.24 The phenomenon of disembodiment has been suggested to be a failure to integrate proprioceptive, visual, and tactile information regarding one's body (disintegration in personal space) along with an additional disintegration between personal (vestibular) and extrapersonal (visual) space that occurs during impaired consciousness.22 There may indeed be similarities between these phenomena and conversion tremor on the general level of multisensory integration, hence implicating similar regions. However, we have confined our interpretation to the feed-forward model comparing sensory feedback and prediction to explain the clinical phenomena of the experience of subjective involuntary movement. Furthermore, conversion tremor does not involve integration within personal or extrapersonal space, and our findings suggest right TPJ hypoactivity, whereas out-of-body experiences are associated with the opposite. We cannot comment on whether the TPJ is intrinsically impaired and suggest rather that the process of generating the sensory prediction in conversion tremor may be abnormal. It is possible that a range of symptoms, from that of nonconscious “nervous” foot tapping/hand drumming (which presumably also uses voluntary pathways) to l- dopa–induced dyskinesias25 and other involuntary movement disorders (which are less likely to use voluntary pathways), may be perceived as involuntary in part because of reduced feed-forward signaling. Whether this mechanism holds for conversion paralysis or other conversion symptoms is not clear. Further studies will be able to clarify one of these hypotheses.
Conversion movements use voluntary motor pathways and yet are paradoxically experienced as involuntary. Our study highlights a potential abnormality of integration of the internal sensory prediction with the actual sensory state in conversion tremor. We note that this mechanism does not address the question of how or why the conversion tremor is initiated, but may give insight into why it is experienced as involuntary. This theory is further compatible with other theories put forward in conversion motor disorders, including that of abnormal motor conceptualization,13 limbic interference26 with motor function, and hyperactive monitoring of internal states.27 This mechanism may reflect a more general process of comparison of internal predictions with actual events attributed to the right TPJ.6 The absence of a feed-forward signal in conversion tremor would lead to a lack of a match in the TPJ, thus leading to the crux of conversion movements, the feeling that one is not the cause of one's actions.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Valerie Voon, MD.
DISCLOSURE
Dr. Voon serves on a scientific advisory board for Boehringer Ingelheim; serves on the editorial board of Movement Disorders; and was supported by an intramural National Institute of Neurological Disorders and Stroke fellowship. Dr. Gallea is supported by an intramural National Institute of Neurological Disorders and Stroke fellowship. Dr. Hattori receives research support from the Japan Science and Technology Agency. Dr. Bruno reports no disclosures. Dr. Hallett serves as Chair of the Medical Advisory Board for and receives funding for travel from the Neurotoxin Institute; serves as Chair of the Medical Advisory Board of the Benign Essential Blepharospasm Foundation; has received honoraria and/or funding for travel for lectures or educational activities not funded by industry; serves on editorial advisory boards for Clinical Neurophysiology, Western Hemisphere, Brain, Acta Neurologica Scandinavica, Journal of Clinical Neurophysiology, Italian Journal of Neurological Sciences, Medical Problems of Performing Artists, Annals of Neurology, Neurology and Clinical Neurophysiology, The Cerebellum, NeuroRx, Current Trends in Neurology, Faculty of 1000 Biology, European Neurology, Faculty of 1000 Medicine, Brain Stimulation, Journal of Movement Disorders (Korea), and World Neurology; may accrue revenue on US Patent 6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent 7,407,478 (Issued: August 5, 2008): Coil for magnetic stimulation and methods for using the same; receives royalties from publishing from Blackwell Publisher, Cambridge University Press, Springer Verlag, Taylor & Francis Group, Oxford University Press, John Wiley & Sons, and Elsevier; receives research support from Ariston Pharmaceuticals, NIH/NINDS (Intramural Program) and the US Department of Defense (Army); has received license fee payments from the NIH (from Brainsway) for licensing the patent for the H-coil; and with his spouse held stock in Agilent Technologies, Amgen, Amylin Pharmaceuticals, Merck & Co., Monsanto Co New Del, sanofi-aventis, Coventry Health Care Inc., Sigma Aldrich Corp., Warner Chilcott Ltd., Pfizer Inc, Genentech, Inc., United Health Group, St. Jude Medical, and Eli Lilly and Company.
Address correspondence and reprint requests to Dr. Valerie Voon, NIH, 10 Center Dr., Building 10, Room 7D42, Bethesda, MD 20892-1428 voonval@gmail.com
Editorial, page 190
Study funding: The study was supported and conducted at the National Institute of Neurological Disorders and Stroke, NIH.
Disclosure: Author disclosures are provided at the end of the article.
Received June 11, 2009. Accepted in final form September 16, 2009.
REFERENCES
- 1.Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: the role of sensorimotor context estimation. J Neurosci 1998;18:7511–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blakemore SJ, Sirigu A. Action prediction in the cerebellum and in the parietal lobe. Exp Brain Res 2003;153:239–245. [DOI] [PubMed] [Google Scholar]
- 3.Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement intention after parietal cortex stimulation in humans. Science 2009;324:811–813. [DOI] [PubMed] [Google Scholar]
- 4.Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. Neuroimage 2003;18:324–333. [DOI] [PubMed] [Google Scholar]
- 5.Farrer C, Frey SH, Van Horn JD, et al. The angular gyrus computes action awareness representations. Cereb Cortex 2008;18:254–261. [DOI] [PubMed] [Google Scholar]
- 6.Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist 2007;13:580–593. [DOI] [PubMed] [Google Scholar]
- 7.Fink GR, Marshall JC, Halligan PW, et al. The neural consequences of conflict between intention and the senses. Brain 1999;122(pt 3):497–512. [DOI] [PubMed] [Google Scholar]
- 8.Williams DT, Ford B, Fahn S. Phenomenology and psychopathology related to psychogenic movement disorders. Adv Neurol 1995;65:231–257. [PubMed] [Google Scholar]
- 9.Bhatia KP, Schneider SA. Psychogenic tremor and related disorders. J Neurol 2007;254:569–574. [DOI] [PubMed] [Google Scholar]
- 10.Hallett M, Cloninger CR, Fahn S, Jankovic J, Lange AE, Yudofsky SC, eds. Psychogenic Movement Disorders. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 11.Krem MM. Motor conversion disorders reviewed from a neuropsychiatric perspective. J Clin Psychiatry 2004;65:783–790. [DOI] [PubMed] [Google Scholar]
- 12.Marshall JC, Halligan PW, Fink GR, Wade DT, Frackowiak RS. The functional anatomy of a hysterical paralysis. Cognition 1997;64:B1–B8. [DOI] [PubMed] [Google Scholar]
- 13.Spence SA, Crimlisk HL, Cope H, Ron MA, Grasby PM. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet 2000;355:1243–1244. [DOI] [PubMed] [Google Scholar]
- 14.Vuilleumier P, Chicherio C, Assal F, Schwartz S, Slosman D, Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain 2001;124:1077–1090. [DOI] [PubMed] [Google Scholar]
- 15.Cojan Y, Waber L, Carruzzo A, Vuilleumier P. Motor inhibition in hysterical conversion paralysis. Neuroimage 2009;47:1026–1037. [DOI] [PubMed] [Google Scholar]
- 16.Stone J, Zeman A, Simonotto E, et al. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom Med 2007;69:961–969. [DOI] [PubMed] [Google Scholar]
- 17.Kanaan RA, Craig TK, Wessely SC, David AS. Imaging repressed memories in motor conversion disorder. Psychosom Med 2007;69:202–205. [DOI] [PubMed] [Google Scholar]
- 18.Ghaffar O, Staines WR, Feinstein A. Unexplained neurologic symptoms: an fMRI study of sensory conversion disorder. Neurology 2006;67:2036–2038. [DOI] [PubMed] [Google Scholar]
- 19.Farrer C, Frey SH, Van Horn JD, et al. The angular gyrus computes action awareness representations. Cereb Cortex 2008;18:254–261. [DOI] [PubMed] [Google Scholar]
- 20.Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One 2009;4:e4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex 2008;18:262–271. [DOI] [PubMed] [Google Scholar]
- 22.Blanke O, Landis T, Spinelli L, Seeck M. Out-of-body experience and autoscopy of neurological origin. Brain 2004;127:243–258. [DOI] [PubMed] [Google Scholar]
- 23.De Ridder D, Van Laere K, Dupont P, Menovsky T, Van de Heyning P. Visualizing out-of-body experience in the brain. N Engl J Med 2007;357:1829–1833. [DOI] [PubMed] [Google Scholar]
- 24.Blanke O, Mohr C, Michel CM, et al. Linking out-of-body experience and self processing to own-body imagery at the temporoparietal junction. J Neurosci 2005;25:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shenker JI, Wylie SA, Fuchs K, Manning CA, Heilman KM. On-line anosognosia: unawareness for chorea in real time but not on videotape delay. Neurology 2004;63:159–160. [DOI] [PubMed] [Google Scholar]
- 26.Marshall JC, Halligan PW, Fink GR, Wade DT, Frackowiak RS. The functional anatomy of a hysterical paralysis. Cognition 1997;64:B1–B8. [DOI] [PubMed] [Google Scholar]
- 27.de Lange FP, Roelofs K, Toni I. Increased self-monitoring during imagined movements in conversion paralysis. Neuropsychologia 2007;45:2051–2058. [DOI] [PubMed] [Google Scholar]