Abstract
Background:
Olfactory dysfunction and autonomic failure are gaining recognition as nonmotor manifestations of Parkinson disease (PD). This observational study assessed whether in PD anosmia and autonomic failure are related to each other or to neuroimaging evidence of striatal dopamine deficiency.
Methods:
Olfactory function was assessed by the University of Pennsylvania Smell Identification Test (UPSIT) in 23 patients with sporadic PD. Baroreflex-cardiovagal gain was quantified from the relationship between cardiac interbeat interval and systolic pressure during the Valsalva maneuver and baroreflex-sympathoneural function by responses of systolic pressure to the Valsalva maneuver and of hemodynamics and plasma norepinephrine (NE) and dihydroxyphenylglycol (DHPG) levels to orthostasis. 6-[18F]Fluorodopamine PET and plasma and skeletal muscle microdialysate NE and DHPG were used to indicate cardiac and extracardiac noradrenergic innervation and brain 6-[18F]fluorodopa PET to indicate striatal dopaminergic innervation. Parkinsonism was assessed by UPDRS scores.
Results:
Compared to patients with PD and normal to moderately decreased sense of smell, patients with anosmic PD had lower mean baroreflex-cardiovagal gain (p = 0.04), larger falls in systolic pressure during the Valsalva maneuver and orthostasis (p = 0.04, p = 0.02), smaller orthostatic increments in plasma NE and DHPG (p = 0.003, p = 0.03), lower cardiac septal:hepatic and renal cortical:hepatic ratios of 6-[18F]fluorodopamine-derived radioactivity (p = 0.01, p = 0.06), and lower microdialysate NE and DHPG (p = 0.01; p = 0.006). Neither clinical severity of parkinsonism nor the putamen:occipital cortex ratio of 6-[18F]fluorodopa-derived radioactivity was related to the UPSIT category.
Conclusions:
In Parkinson disease, anosmia is associated with baroreflex failure and cardiac and organ-selective extracardiac noradrenergic denervation, independently of parkinsonism or striatal dopaminergic denervation.
GLOSSARY
- DHPG
= dihydroxyphenylglycol;
- LSD
= least significant difference;
- NE
= norepinephrine;
- OH
= orthostatic hypotension;
- PD
= Parkinson disease;
- QSART
= quantitative sudomotor axon reflex test;
- UPDRS
= Unified Parkinson's Disease Rating Scale;
- UPSIT
= University of Pennsylvania Smell Identification Test.
There is growing realization that Parkinson disease (PD) is a more general disease than thought previously and involves prominent nonmotor manifestations. Two such manifestations are anosmia (absent sense of smell) and autonomic failure. Virtually all patients with PD have at least some loss of sense of smell,1 and about 90% have findings compatible with failure of one or more components of the autonomic nervous system.2 In particular, about 40% of patients with PD have orthostatic hypotension (OH) that is neurogenic, independent of levodopa treatment, and associated with cardiac sympathetic denervation.3 Moreover, olfactory dysfunction, OH, and neuroimaging evidence of cardiac sympathetic denervation can precede onset of the movement disorder,4–6 and so they might provide biomarkers of the pathogenetic process. This study addressed whether among patients with sporadic PD anosmia and autonomic failure are related to each other or to neuroimaging evidence of striatal dopamine deficiency, which is thought to cause the movement disorder that characterizes PD.
Recent findings suggest a degree of independence of olfactory dysfunction from nigrostriatal dopamine depletion in PD. Olfactory dysfunction is unrelated to the duration or severity of parkinsonism,7 and studies have disagreed about whether olfactory dysfunction is related to neuroimaging evidence for loss of striatal dopaminergic terminals.1,8 Symptomatic OH, a cardinal manifestation of failure of the sympathetic noradrenergic component of the autonomic nervous system, can come on late in the course of PD9 or can already be prominent in de novo PD.10–12 Both OH and cardiac sympathetic denervation13,14 seem unrelated to neuroimaging or neurochemical evidence of central dopamine deficiency.1
In contrast with weak or absent relationships of severity of parkinsonism with olfactory dysfunction or autonomic failure, recent reports have noted an association between loss of sense of smell and loss of noradrenergic innervation in the heart.1,15 Cardiac sympathetic neuroimaging is not available at most centers in the United States, and this modality assesses only one component of the autonomic nervous system, in one organ. Studies have not yet addressed whether anosmia is related to more generally available and accepted indices of autonomic failure, such as deficient baroreflex-cardiovagal function, abnormal beat-to-beat blood pressure responses to the Valsalva maneuver, orthostatic increments in plasma levels of norepinephrine (NE) and its neuronal metabolite dihydroxyphenylglycol (DHPG), decreased sweat production in the quantitative sudomotor axon reflex test (QSART), or OH. The main purpose of this study was to address this issue.
We assessed whether patients with PD categorized as anosmic by the University of Pennsylvania Smell Identification Test (UPSIT) have dysregulation of autonomic outflows, indicated by baroreflex-cardiovagal or baroreflex-sympathoneural failure, or have neuroimaging or neurochemical evidence of loss of postganglionic sympathetic noradrenergic nerves. We also examined whether anosmia is related to subnormal QSART results (which would be expected if there were diffuse sympathetic cholinergic denervation), clinical parkinsonism measured by Unified Parkinson's Disease Rating Scale (UPDRS) scores, or neuroimaging evidence of loss of striatal dopaminergic terminals, measured by brain 6-[18F]fluorodopa PET.
METHODS
Subjects.
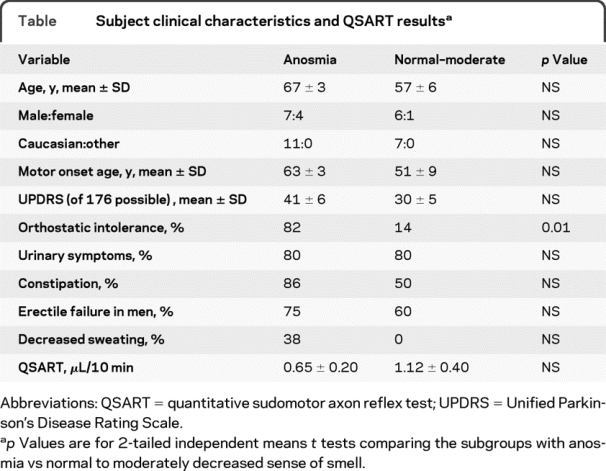
A total of 23 patients with PD completed the UPSIT and underwent autonomic function testing at the NIH Clinical Center, after giving informed written consent to participate in protocols approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board. Among the 23 patients with PD, 11 had anosmia, 5 severe microsmia, and 7 normal to moderately decreased sense of smell (normal–moderate microsmia). The table summarizes clinical characteristics and UPSIT categories of the subgroups with anosmia or normal–moderate microsmia.
Table Subject clinical characteristics and QSART results
All the patients in this study were referred by board-certified neurologists or internists and carried a diagnosis of PD that was confirmed by clinical examination and laboratory testing at the NIH. All the patients had bradykinesia, cogwheel rigidity, and either a resting “pill roll” tremor (n = 11) or excellent responses to treatment with levodopa/carbidopa (n = 14). In addition, all the patients had low putamen:occipital cortex ratios of 6-[18F]fluorodopa-derived radioactivity, confirming a striatal dopaminergic lesion. Nine patients with PD had neurogenic OH that was associated with neuroimaging and neurochemical or neuropharmacologic evidence of sympathetic noradrenergic denervation.
OH was defined by a decrease in systolic blood pressure of at least 20 mm Hg and in diastolic pressure at least 10 mm Hg between supine rest for at least 15 minutes and upright posture for 5 minutes (unless symptomatic or rapid hypotension necessitated return of the patient to the supine position before 5 minutes upright).
Patients were tested while on their usual medications, except that in most parkinsonian patients levodopa/carbidopa was withheld. UPDRS scoring was done when patients were off levodopa/carbidopa.
Procedures.
Each patient completed the 40-item UPSIT, purchased from the American Academy of Neurology.
A typical sequence of testing was as follows. The patient reported to a clinical testing room at about 9 am after an overnight fast except for water. With the subject supine (head on pillow), an arm IV catheter was inserted and monitoring sensors applied to the neck, chest, arms, and fingers. For beat-to-beat blood pressure measurement, a Finometer (Finapres Medical Systems, Amsterdam, the Netherlands) or Nexfin (bmeye, Amsterdam, the Netherlands) device was used, with continuous recording using a PowerLab system (ADInstruments, Inc., Colorado Springs, CO). For measurement of cardiac stroke volume and thereby total peripheral resistance to blood flow, a BioZ impedance cardiograph was used (Cardiodynamics International Corp., San Diego, CA). The patient performed the Valsalva maneuver (12 seconds, 30 mm Hg) at least twice, until a technically adequate tracing was obtained. After the subject had been resting supine for at least 15 minutes, a baseline blood sample was drawn. The patient was then tilted to 90 degrees head-up using a motorized tilt table. After 5 minutes (less if there were a severe, sustained fall in blood pressure), another blood sample was drawn.
As measures of autonomic function, a variety of physiologic, neurochemical, and neuroimaging tests were used. Physiologic tests included 1) the orthostatic change in systolic blood pressure between at least 15 minutes of supine rest and head-up tilt; 2) the change in systolic pressure from the peak to the trough value during phase II of the Valsalva maneuver; 3) systolic pressure after at least 15 minutes of supine rest; 4) the fractional change in total peripheral resistance to blood flow, assessed by impedance cardiography, between at least 15 minutes of supine rest and head-up tilt; 5) baroreflex-cardiovagal gain, calculated from the slope of the line of best fit for the linear relationship between cardiac interbeat interval and systolic pressure between the peak and trough pressure values during phase II of the Valsalva maneuver; and 6) sweat production assessed by the QSART (WR Medical Electronics Co., Stillwater, MN) done on a forearm, as reported previously,16 with normal vs low values for microliters of sweat production per 10 minutes determined by comparison with previously published norms.
Neurochemical tests consisted of plasma NE and DHPG levels during supine rest, fractional changes in those levels during orthostasis, and quadriceps microdialysate levels of NE and DHPG, to examine noradrenergic innervation in skeletal muscle. Microdialysate samples were obtained via an indwelling commercially available probe (CMA 60, CMA Microdialysis, Inc., North Chelmsford, MA) placed percutaneously in quadriceps muscle after local anesthesia of the overlying skin. The perfusate, normal saline, was delivered continuously by an infusion pump at 3–5 μL/min in 30-minute aliquots. Analyte concentrations were averaged for the second aliquot through the end of testing (at least 4 aliquots). Levels of catechols were assayed in the Clinical Neurochemistry Laboratory of the Clinical Neurocardiology Section.17
Neuroimaging tests included 6-[18F]fluorodopamine PET, with interventricular septal myocardial, free wall, spleen, and renal cortex 6-[18F]fluorodopamine-derived radioactivity expressed as ratios of hepatic radioactivity at the midpoint of the 5-minute frame beginning about 5 minutes after initiation of the 3-minute infusion of the tracer. Thyroid and nasopharyngeal 6-[18F]fluorodopamine-derived radioactivity were measured for the 15-minute frame beginning about 30 minutes after initiation of the tracer infusion. On a separate day, all subjects also underwent brain 6-[18F]fluorodopa PET, with quantification of the ratio of 6-[18F]fluorodopa-derived radioactivity in the putamen and occipital cortex for the 15-minute frame ending about 120 minutes after initiation of 3-minute infusion of the tracer. Skeletal muscle microdialysis usually was done in conjunction with 6-[18F]fluorodopa PET.
Data analysis and statistics.
The UPSIT provides a numerical score; however, UPSIT scores vary with gender and age. The UPSIT Administration Manual contains nomograms assigning scores to categories based on previously established norms. In the present study, UPSIT categories were used for analyses of variance and absolute UPSIT scores for calculating correlation coefficients across individual patients.
Almost all patients with PD had at least some decrease in olfactory function. Of the 23 patients with PD, 11 were anosmic, 5 had severe microsmia, and only 1 (3%) had normal sense of smell. For statistical analyses, patients were therefore classified into 3 categories—anosmia, severe microsmia, and normal to moderately decreased sense of smell (microsmia).
Five patients with PD were determined to be on levodopa/carbidopa at the time of blood sampling for catechols, as evidenced by plasma DOPA levels greater than 5,000 pg/mL (about 25 nmol/L).
Physiologic, neurochemical, and neuroimaging measures were compared between the anosmic subgroup of patients with PD and the subgroups with severely decreased or normal to moderately decreased sense of smell, by 2-tailed, factorial analyses of variance, with post hoc comparisons among subgroups using the Fisher least significant difference (LSD) test (KaleidaGraph 4.01, Synergy Software, Reading, PA). Most mean data in the patient subgroup with severe microsmia were intermediate between those in the subgroup with anosmia and the subgroup with normal to moderately decreased sense of smell. The data analysis therefore focused on comparing the subgroups with anosmia vs normal–moderate microsmia. Analyses of data for baroreflex-cardiovagal gain used log-transformed data. Spearman correlation coefficients were calculated for relationships across individual subjects. Mean values ±1 SEM were displayed. A p value less than 0.05 defined statistical significance.
RESULTS
The subgroups with anosmia or normal to moderately decreased sense of smell did not differ in clinical characteristics, except for orthostatic intolerance, which was more frequent in the anosmic subgroup (table).
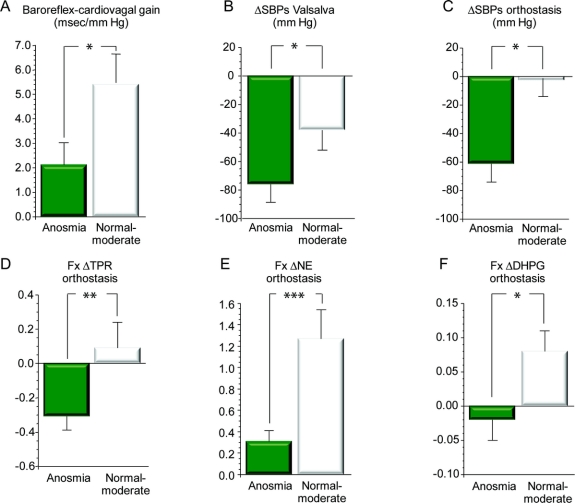
Baroreflex-cardiovagal gain was lower in patients with anosmic PD than in patients with PD and normal smell–moderate microsmia (p = 0.04; figure 1). Baroreflex-sympathoneural function assessed by a variety of methods was also lower in the anosmic subgroup (figure 1). Thus, the anosmic subgroup had larger decreases in systolic pressure during phase II of the Valsalva maneuver (p = 0.04) and orthostasis (p = 0.02) and had smaller fractional increments in total peripheral vascular resistance (p = 0.01) and in plasma NE and DHPG levels (p = 0.0008, p = 0.006) during orthostasis. Skeletal muscle microdialysate concentrations of both NE and DHPG were also lower in the anosmic subgroup (p = 0.03, p = 0.006).
Figure 1 Measures of baroreflex function vs olfactory category
(A) Mean (±SEM) data for baroreflex-cardiovagal gain, calculated from the slope of the relationship between cardiac interbeat interval and systolic blood pressure during the decline of pressure in phase II of the Valsalva maneuver. (B–F) Data for measures of baroreflex-sympathoneural function. *Significant difference between anosmia and normal–moderately decreased sense of smell, p < 0.05; **p < 0.01; ***p < 0.001. ΔSBPs = change in systolic blood pressure; Fx ΔTPR = fractional change in total peripheral resistance; Fx ΔNE = fractional change in plasma norepinephrine level; Fx ΔDHPG = fractional change in plasma dihydroxyphenylglycol level; moderate = moderate microsmia. Note that by all 6 measures, anosmic patients had evidence for decreased baroreflex function compared to patients with normal–moderately decreased sense of smell.
Consistent with the above findings based on UPSIT categories, individual values for UPSIT scores tended to correlate positively with the log of baroreflex-cardiovagal gain (r = 0.42, p = 0.08) and were positively correlated with the magnitude of fall in systolic blood pressure (r = −0.50, p = 0.02) and the fractional increments in plasma NE (r = 0.66, p = 0.0009) and in total peripheral resistance (r = 0.48, p = 0.02) during orthostasis.
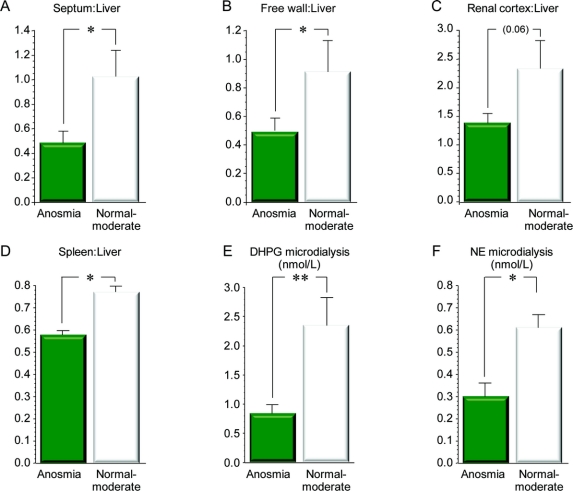
Patients with anosmic PD also had evidence of loss of cardiac and extracardiac noradrenergic nerves, with decreased interventricular septum:liver (p = 0.01), renal cortex:liver (p = 0.06), and spleen:liver (p = 0.05) ratios of 6-[18F]fluorodopamine-derived radioactivity (figure 2) compared to patients with PD and normal–moderate microsmia. Subgroup differences in left ventricular free wall:liver ratios of 6-[18F]fluorodopamine-derived radioactivity were not significant, because in 5 of the anosmic patients radioactivity in the free wall could not be resolved from that in the left ventricular chamber. Assuming equal radioactivity in the free wall and chamber in these patients, the anosmic subgroup had decreased free wall:liver ratios of 6-[18F]fluorodopamine-derived radioactivity (p = 0.008).
Figure 2 Measures of sympathetic noradrenergic innervation vs olfactory category
(A–D) Results for organ ratios of 6-[18F]fluorodopamine-derived radioactivity. (E, F) Results from skeletal muscle microdialysate concentrations of norepinephrine (NE) and dihydroxyphenylglycol (DHPG). *Significant difference between anosmia and normal–moderately decreased sense of smell, p < 0.05; **p < 0.01. Microdialysis = skeletal muscle microdialysate. Note that by all 6 measures, anosmic patients had evidence for decreased sympathetic noradrenergic innervation compared to patients with normal–moderately decreased sense of smell.
Individual values for UPSIT scores correlated positively with septum:liver (r = 0.47, p = 0.03), free wall:liver (r = 0.64, p = 0.003), and renal cortex:liver (r = 0.58, p = 0.02) ratios of 6-[18F]fluorodopamine-derived radioactivity and with microdialysate DHPG (r = 0.71, p = 0.02) and NE (r = 0.65, p = 0.04).
6-[18F]Fluorodopamine-derived radioactivity in the thyroid and nasopharynx and plasma NE and DHPG during supine rest did not differ between the anosmic and normal-moderate microsmia subgroups, with or without exclusion of neurochemical data from patients on levodopa/carbidopa at the time of testing.
Patients with anosmic PD had larger falls in systolic blood pressure during orthostasis than did patients with PD and normal–moderate microsmia (p = 0.02; figure 1). Across patients with PD, the magnitude of orthostatic decline in systolic pressure was strongly positively correlated with supine systolic pressure (r = 0.82, p < 0.0001), and patients with anosmic PD had higher supine systolic pressures than did patients with PD and normal–moderate microsmia (171 ± 13 vs 130 ± 14 mm Hg, p = 0.03), although they did not have significantly higher supine diastolic or mean arterial pressures.
Among patients with PD, the UPSIT category was unrelated to UPDRS scores off levodopa (table) or to putamen 6-[18F]fluorodopa-derived radioactivity, with or without adjustment for occipital cortex radioactivity. Putamen 6-[18F]fluorodopa-derived radioactivity at about 120 minutes after tracer injection averaged 1,434 ± 134 nCi-kg/mL-mCi in the anosmic subgroup compared to 1,178 ± 209 nCi-kg/mL-mCi in the subgroup with normal–moderate microsmia; and putamen:occipital ratios averaged 2.00 ± 0.10 and 2.07 ± 0.18 in the 2 subgroups.
Three of 9 anosmic patients had subnormal QSART values, compared to 2 of 5 patients with normal–moderate microsmia—a nonsignificant difference. Anosmic patients had lower mean forearm sweat production in the QSART than did patients with normal–moderate microsmia (table); however, the subgroup difference was not significant.
Among patients with PD, the log of the baroreflex-cardiovagal slope was correlated with the fall in systolic blood pressure during the Valsalva maneuver (r = −0.51, p = 0.03), the change in systolic pressure during orthostasis (r = 0.62, p = 0.004), and the fractional increments in plasma NE (r = 0.54, p = 0.02) and DHPG (r = 0.45, p = 0.05) and total peripheral resistance (r = 0.48, p = 0.04) during orthostasis.
DISCUSSION
The present findings support an association of anosmia with autonomic failure in sporadic PD. Patients with anosmic PD had consistent evidence of worse autonomic function by several physiologic, neurochemical, and neuroimaging tests than did patients with PD and normal to moderately decreased sense of smell.
Anosmic patients had baroreflex failure that involved both the cardiovagal and sympathetic efferent limbs. Thus, the patients had low baroreflex-cardiovagal gain assessed by the relationship between cardiac interbeat interval and systolic pressure during phase II of the Valsalva maneuver. By 5 different measures, anosmic patients also had baroreflex-sympathoneural failure, indicated by the change in systolic pressure from the peak to the trough value during phase II of the Valsalva maneuver, by orthostatic changes in systolic pressure and total peripheral resistance, and by orthostatic increments in plasma NE and DHPG levels. Across individual patients with PD, values for baroreflex-cardiovagal gain were correlated with values for all these indices of baroreflex-sympathoneural function.
Anosmia was also associated with neuroimaging evidence of postganglionic cardiac and organ-selective extracardiac noradrenergic denervation as indicated by relatively low ratios of interventricular septum:liver, left ventricular free wall:liver, renal cortex:liver, and spleen:liver ratios of 6-[18F]fluorodopamine-derived radioactivity and by low concentrations of NE and DHPG in skeletal muscle microdialysate samples. Anosmia was not associated with generalized noradrenergic denervation, because anosmic patients did not differ from patients with normal to moderately decreased sense of smell in mean plasma levels of either NE or DHPG during supine rest.
The sympathetic lesion attending anosmia in PD seems to be neurotransmitter type-specific, since most anosmic patients had values for the QSART that were within the normal range, indicating generally intact sympathetic cholinergic function. Since we conducted the QSART only on the skin of the forearm, the present results do not exclude possible abnormalities at other sites.
All patients with PD had low putamen:occipital cortex ratios of 6-[18F]fluorodopa-derived radioactivity, consistent with striatal dopaminergic denervation; however, the subgroup with anosmia did not differ from the subgroup with normal to moderately decreased sense of smell in terms of putamen 6-[18F]fluorodopa-derived radioactivity, with or without adjustment for radioactivity in the occipital cortex. Thus, the link between anosmia and autonomic failure in PD seems to be independent of the striatal dopamine deficiency that characterizes the movement disorder.
We and others have noted relationships of olfactory dysfunction with neuroimaging indices of cardiac sympathetic denervation. The present report builds importantly on these findings by including several other physiologic and neurochemical measures and extracardiac sympathetic neuroimaging. Previous studies also did not address associations of the other indices with putamen 6-[18F]fluorodopa-derived radioactivity.
Is there a unitary explanation for associations of anosmia with baroreflex-cardiovagal failure, baroreflex-sympathoneural failure, OH, and cardiac and extracardiac noradrenergic denervation in PD, relatively independently of striatal dopaminergic denervation? We propose that one common factor may be loss of noradrenergic neurons in the brain and periphery. Consistent with this view, patients with pure autonomic failure, which like PD is a Lewy body disease but does not entail parkinsonism or striatal dopaminergic denervation,18,19 have a strikingly similar pattern of abnormalities to that reported here in patients with anosmic PD. Testing the concept of different noradrenergic and dopaminergic lesions will require more information about NE and dopamine contents of the human olfactory bulb and striatum in control subjects and in patients with PD. The present findings seem to justify future studies about possible differences between NE and dopamine synthesis, storage, reuptake, or metabolism that might help explain relative independence of anosmia and autonomic failure from striatal dopamine deficiency and motor signs of PD.
ACKNOWLEDGMENT
Tereza Jenkins coordinated patient travel. Sandra Pechnik, RN, assisted with clinical procedures and scheduling. Drs. Basil Eldadah, Richard Imrich, and Yehonatan Sharabi served as post-doctoral Fellows when the work was done.
DISCLOSURE
Dr. Goldstein received the Pioneer Award from the Bakken Heart-Brain Institute; has received funding for sponsored travel for official US Government duties; and receives royalties from publication of the books Stress, Catecholamines, and Cardiovascular Disease (Oxford University Press, 1995), The Autonomic Nervous System in Health and Disease (Taylor & Francis, 2000), and Adrenaline and the Inner World: An Introduction to Scientific Integrative Medicine (The Johns Hopkins University Press, 2006). L. Sewell and C. Holmes report no disclosures.
Address correspondence and reprint requests to Dr. David S. Goldstein, Clinical Neurocardiology Section, National Institute of Neurological Disorders and Stroke, NIH, 10 Center Drive MSC-1620, Building 10 Room 5N220, Bethesda, MD 20892-1620 goldsteind@ninds.nih.gov
Supported by the Intramural Research Program of NIH/NINDS.
Disclosure: Author disclosures are provided at the end of the article.
Received July 23, 2009. Accepted in final form October 29, 2009.
REFERENCES
- 1.Goldstein DS, Holmes C, Bentho O, et al. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord 2008;14:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufmann H, Biaggioni I. Autonomic failure in neurodegenerative disorders. Semin Neurol 2003;23:351–363. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DS, Eldadah BA, Holmes C, et al. Neurocirculatory abnormalities in Parkinson disease with orthostatic hypotension: independence from levodopa treatment. Hypertension 2005;46:1–7. [DOI] [PubMed] [Google Scholar]
- 4.Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol 2004;56:173–181. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann H, Nahm K, Purohit D, Wolfe D. Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology 2004;63:1093–1095. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DS, Sharabi Y, Karp BI, et al. Cardiac sympathetic denervation preceding motor signs in Parkinson disease. Clin Auton Res 2007;17:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 1988;38:1237–1244. [DOI] [PubMed] [Google Scholar]
- 8.Siderowf A, Newberg A, Chou KL, et al. [99mTc] TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology 2005;64:1716–1720. [DOI] [PubMed] [Google Scholar]
- 9.Senard JM, Rai S, Lapeyre-Mestre M, et al. Prevalence of orthostatic hypotension in Parkinson's disease. J Neurol Neurosurg Psychiatry 1997;63:584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonuccelli U, Lucetti C, Del Dotto P, et al. Orthostatic hypotension in de novo Parkinson disease. Arch Neurol 2003;60:1400–1404. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DS. Orthostatic hypotension as an early finding in Parkinson disease. Clin Auton Res 2006;16:46–64. [DOI] [PubMed] [Google Scholar]
- 12.Micieli G, Martignoni E, Cavallini A, Sandrini G, Nappi G. Postprandial and orthostatic hypotension in Parkinson's disease. Neurology 1987;37:386–393. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO 3rd. Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med 2000;133:338–347. [DOI] [PubMed] [Google Scholar]
- 14.Amino T, Orimo S, Takahashi A, Uchihara T, Mizusawa H. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol 2005;15:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee PH, Yeo SH, Kim HJ, Youm HY. Correlation between cardiac 123I-MIBG and odor identification in patients with Parkinson's disease and multiple system atrophy. Mov Disord 2006;21:1975–1977. [DOI] [PubMed] [Google Scholar]
- 16.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol 1983;14:573–580. [DOI] [PubMed] [Google Scholar]
- 17.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Applic 1994;653:131–138. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DS, Holmes C, Sato T, et al. Central dopamine deficiency in pure autonomic failure. Clin Auton Res 2008;18:58–65. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DS, Sewell L. Olfactory dysfunction in pure autonomic failure: Implications for the pathogenesis of Lewy body diseases. Parkinsonism Relat Disord 2009;15:516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]