Abstract
Background:
Cognitive fluctuations are spontaneous alterations in cognition, attention, and arousal. Fluctuations are a core feature of dementia with Lewy bodies, but the impact of fluctuations in healthy brain aging and Alzheimer disease (AD) are unknown.
Methods:
Research participants (n = 511, age 78.1 ± 8 years, education 14.9 ± 3 years) enrolled in a longitudinal study of memory and aging at the Washington University Alzheimer Disease Research Center were assessed for the presence and severity of dementia with the Clinical Dementia Rating (CDR) and a neuropsychological test battery. Informant assessments of fluctuations with the Mayo Fluctuations Questionnaire and daytime level of alertness with the Mayo Sleep Questionnaire were completed.
Results:
After controlling for age and alertness level, participants with cognitive fluctuations (3 or 4 individual symptoms) were 4.6 times more likely to have dementia (95% confidence interval: 2.05, 10.40). Participants who presented with disorganized, illogical thinking were 7.8 times more likely to be rated CDR >0. The risk of being rated CDR 0.5 among those with fluctuations was 13.4 times higher than among those without fluctuations. The risk of being rated CDR 1 increased 34-fold among participants with fluctuations. Compared with participants without fluctuations, the presence of cognitive fluctuations corresponds to a decrease in performance across individual neuropsychological tests as well as composite scores.
Conclusions:
Cognitive fluctuations occur in Alzheimer disease and, when present, significantly affect both clinical rating of dementia severity and neuropsychological performance. Assessment of fluctuations should be considered in the evaluation of patients for cognitive disorders.
GLOSSARY
- AD
= Alzheimer disease;
- CDR
= Clinical Dementia Rating;
- CI
= confidence interval;
- DLB
= dementia with Lewy bodies;
- MCI
= mild cognitive impairment;
- OR
= odds ratio;
- SRT
= Selective Reminding Test;
- WAIS
= Wechsler Adult Intelligence Scale;
- WMS
= Wechsler Memory Scale.
Cognitive fluctuations, defined as spontaneous alterations in cognition, attention, and arousal, are considered a core feature of dementia with Lewy bodies (DLB).1,2 Features of disturbed arousal may include episodes of excessive daytime somnolence, staring spells, diminished awareness of surroundings, and incoherent or illogical thoughts.3,4 Such findings may suggest the presence of a delirium without identifiable precipitants. Fluctuating cognition and abilities have also been described as periods of behavioral confusion, inattention, and incoherent speech alternating with episodes of lucidity and capable task performance. Due to the waxing and waning nature of fluctuations, evaluation of the dementia patient may be confounded if a fluctuation occurs during the course of assessment of functional abilities and test performance.3–5
Because of the varied definitions of fluctuations and the absence of a standardized way of assessing their presence, it has been difficult to determine how much interference with cognitive performance can be directly attributable to fluctuations. Most of what is known about the effect of fluctuations has been described in DLB. It is unknown to what extent fluctuations occur in aging and Alzheimer disease (AD) and whether the presence of fluctuations impairs cognitive performance in AD compared with individuals who do not have features of fluctuations. Here we tested the hypothesis that fluctuations worsen dementia ratings and impair neuropsychological performance in AD.
METHODS
Participants.
Data were examined from 511 research participants aged 51 to 101 who were enrolled in a longitudinal study of memory and aging6 at the Washington University Alzheimer Disease Research Center between May 2007 and October 2008 (table 1). The longitudinal study focuses on the characterizing the transition between healthy brain aging and very mild dementia with a current number of 549 individuals being followed. We recruit only a small number of more impaired individuals annually to meet specific research needs. The Washington University Human Studies Committee approved all procedures.
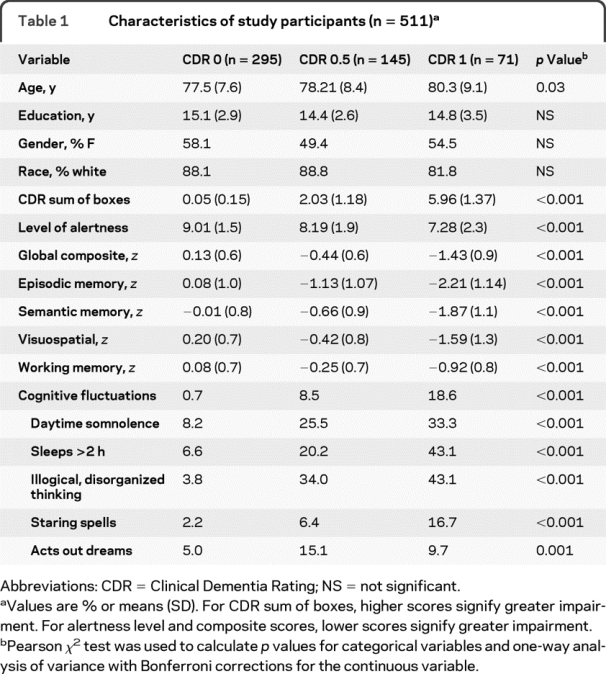
Table 1 Characteristics of study participants (n = 511)
Clinical evaluation.
Experienced clinicians conducted semi-structured interviews with the participant and a knowledgeable collateral source (usually a spouse or adult child) at an initial visit and annually thereafter. The Clinical Dementia Rating (CDR) was used to determine the presence or absence of dementia and, if present, to stage its severity.7 The CDR evaluates cognitive function in each of 6 categories (memory, orientation, judgment and problem solving, performance in community affairs, home and hobbies, and personal care) without reference to psychometric performance or results of previous evaluations. CDR 0 indicates no dementia, and CDR 0.5, 1, 2, and 3 correspond to very mild, mild, moderate, and severe dementia, respectively. The CDR sum of boxes provides a quantitative expansion of the global CDR score, ranging from 0 (no impairment) to 18 (maximal impairment).7
The CDR has high interrater reliability,8 is sensitive to clinical progression, and is highly predictive (93%) of autopsy-confirmed AD.6,9 The clinical diagnostic criteria for probable AD were according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association.10 Individuals with a CDR greater than 0 but with clinical diagnoses of dementias other than AD were excluded from this study. Impaired individuals rated CDR 0.5 but who did not meet AD criteria (n = 58) were classified as uncertain dementia. These individuals have many characteristics of mild cognitive impairment (MCI)11 except their episodic memory testing is less impaired.12 Individuals with dementia with a CDR of 2 or greater were excluded as these individuals have difficulty completing psychometric assessment.
Psychometric assessment.
The psychometric battery was administered to all participants by trained psychometricians usually a week or 2 after the annual clinical assessment. The tests assess a broad spectrum of abilities across multiple cognitive domains and include all components of the Uniform Data Set used by the 29 federally funded AD Centers,13 as well as tests incorporated into our longitudinal studies. This test battery assesses most of the major cognitive domains that are compromised in AD and other neurodegenerative disorders and is effective for evaluating cognitive status in patients with known or suspected dementia. The battery includes Associate Learning and Mental Control from the Wechsler Memory Scale (WMS),14 Logical Memory I (Story A only), Digit Span forward and backward from the WMS-R,15 Information and Block Design from the Wechsler Adult Intelligence Scale (WAIS),16 Digit Symbol from the WAIS-R,17 Similarities from the WAIS-III, the Boston Naming Test (30 odd items),18 letter fluency for S and P,19 category fluency for animals and vegetables,13 Trail Making Test A and B,20 Free and Cued Selective Reminding Test (SRT),21 and Form D of the Benton Visual Retention Test.22 The Crossing-Off23 task was given as a test of simple motor speed.
Based on confirmatory factor analyses of these measures that were cross-validated across samples with and without dementia24 and subsequent work including more measures, we also report 5 composite scores for each person at time of assessment. The episodic memory composite included Logical Memory, Associate Learning, and free recall from the SRT. The semantic memory composite included Information, Boston Naming, and Animal Naming. The 4 measures in the working memory composite were Mental Control, Digit Span Forward and Backward, and Letter Fluency. The visuospatial composite included Block Design, Digit Symbol, and Trail Making A and B. A global composite included all 14 individual measures.
Assessment of cognitive fluctuations.
Cognitive fluctuations were assessed with the Mayo Fluctuations Questionnaire3 administered to the informant, who responded yes or no to 4 questions: 1) drowsiness and lethargy all the time or several times a day despite getting enough sleep the night before (daytime somnolence); 2) daytime sleep of 2 or more hours before 7 pm (sleeps >2 hours); 3) times when the patient's flow of ideas seems disorganized, unclear, or not logical (illogical, disorganized thinking); and 4) staring into space for long periods (staring spells).3 Although used to distinguish AD from DLB, here we explored the effect of both the total score and individual items on cognitive performance in older adults without dementia and those with AD. Affirmative responses to 3 or more items suggests cognitive fluctuations; therefore the total score was dichotomized; total scores of 0 through 2 represented absence of fluctuation and total scores of 3 or 4 represented presence of fluctuation.3
As a measure of participants' alertness and daytime somnolence, we used a question from the Mayo Sleep Questionnaire25 completed by the informants. This 1–10 Likert scale asks the informant to rate the participant's general level of alertness for the past 3 weeks ranging from “sleeps all day” to “fully awake and normal.” Because of the prevalence of REM sleep disorder in DLB, we used the question from the Mayo Sleep Questionnaire querying acting out dreams to test whether “subclinical” or undiagnosed DLB was contributing to effect of fluctuations on cognitive performance.
Statistical analysis.
All analyses were conducted using SAS 9.1 (Cary, NC). Descriptive statistics were used to characterize and compare groups. The groups were compared using t tests and analysis of variance for quantitative variables and χ2 tests of independence for categorical variables. To control for multiple comparisons, we used Bonferroni correction. Because there were differences in age between groups, adjusted analyses controlled for age are presented. In each case, we explored the effect fluctuations have on CDR ratings and neuropsychological test performance. Our comparison groups for each analysis are those with fluctuations vs those without fluctuations with the control CDR 0 groups as the reference.
Proportional logistic regression was used to assess whether the level of alertness as reported by the informant or presence of fluctuation had an effect on the CDR rating given to the participant. Odds ratios (OR) were calculated to estimate the strength of the relationship between having fluctuations and dementia after controlling for age. For precision of estimates, 95% confidence intervals (95% CI) were computed. Multinomial logistic regression then was used to evaluate the associations between levels of alertness, cognitive fluctuations, and CDR, with participants without dementia as the reference category.
We then examined the relationship between the presence of cognitive fluctuations and neuropsychological test performance using Pearson correlations for CDR ratings and fluctuation composite scores and partial correlations (controlling for age) for individual variables. Finally, generalized linear modeling (controlling for age) was used to estimate the effects of fluctuations on psychometric performance. F statistics for the corrected model, R2, as well as beta estimates and t tests were calculated.
RESULTS
Sample characteristics.
Table 1 depicts the characteristics of the study participants as well as their composite cognitive performance and distributions for each of the cognitive fluctuation variables. The sample included 511 individuals (CDR 0 = 295, CDR 0.5 = 145, CDR 1 = 71). The mean age of participants was 78.1 ± 8 years with 14.9 ± 3 years of education. There were no differences in demographic characteristics among the study participants except the CDR 1 group was older than CDR 0 or 0.5. As also would be expected the CDR sum of boxes increased with increasing CDR. Performance on the psychometric composite scores decreased as CDR increased. The CDR 0 (no dementia) group had fewer cognitive fluctuations than did the 2 groups with dementia.
The presence of cognitive fluctuations as well as each of the component variables increased with the presence of cognitive impairment. Post hoc analyses revealed that the CDR 0.5 group and CDR 1 group were always different from the CDR 0 group. The CDR 0.5 group differed from the CDR 1 group in sleeps >2 hours, illogical thoughts, and staring spells, but not daytime drowsiness.
There was no relationship between the presence of cognitive fluctuations (Mayo Fluctuation Questionnaire) and the informant endorsing the question regarding acting out dreams (Mayo Sleep Questionnaire). Of the individual fluctuation questions, suggestion of a REM disorder was present in 18% of participants who experienced excessive daytime drowsiness (p = 0.001). No other fluctuation symptom was related to the endorsement of REM disorder features, supporting that participants were unlikely to have DLB.
Relationship between fluctuations and dementia rating.
Table 2 illustrates the adjusted OR for CDR 0, 0.5, and 1 among those with and without fluctuations. Controlling for age, cognitive impairment (CDR >0) was associated with a decreased level of daytime alertness (OR 1.42, 95% CI 1.28–1.56). Of note, informants reporting participants being more alert lowered the risk for being rated with dementia by 30%. After controlling for age, participants with cognitive fluctuations (3 or 4 individual symptoms) were 8.7 times more likely to be rated as having a cognitive impairment with a CDR >0. When individual fluctuation symptoms were evaluated separately after controlling for age, we found that participants who were drowsy and lethargic were 4 times more likely be rated CDR >0, while sleeping for 2 hours or more during the day accounted for a 5.1 times increased risk. Participants who presented with disorganized, illogical thinking were 7.8 times more likely to be rated CDR >0, and participants with staring spells were 5.1 times more likely to be rated as impaired. Within the impaired groups (CDR >0), individuals who fluctuated had high CDR sum of boxes scores (signifying more severe impairment) than individuals who did not fluctuate (3.22 ± 2.3 vs 4.34 ± 1.9, p = 0.02).
Table 2 Adjusted odds ratios and 95% confidence intervals for the associations between alertness and cognitive fluctuations and the Clinical Dementia Rating
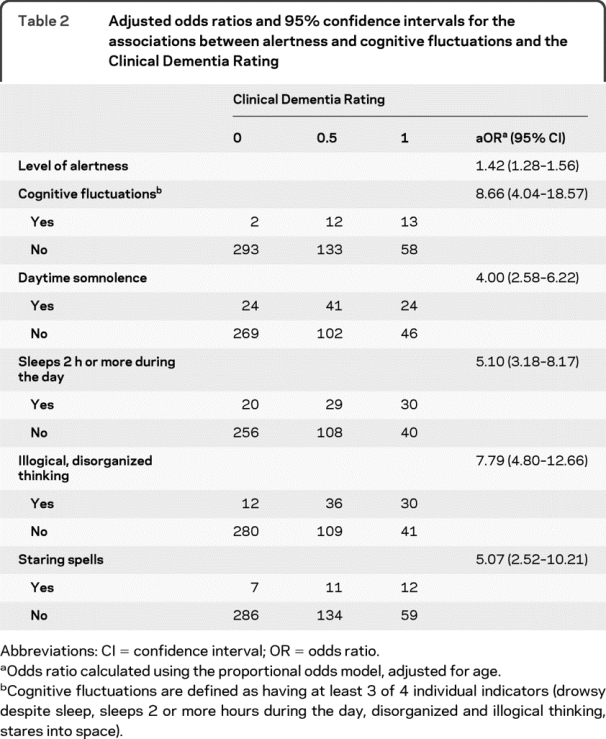
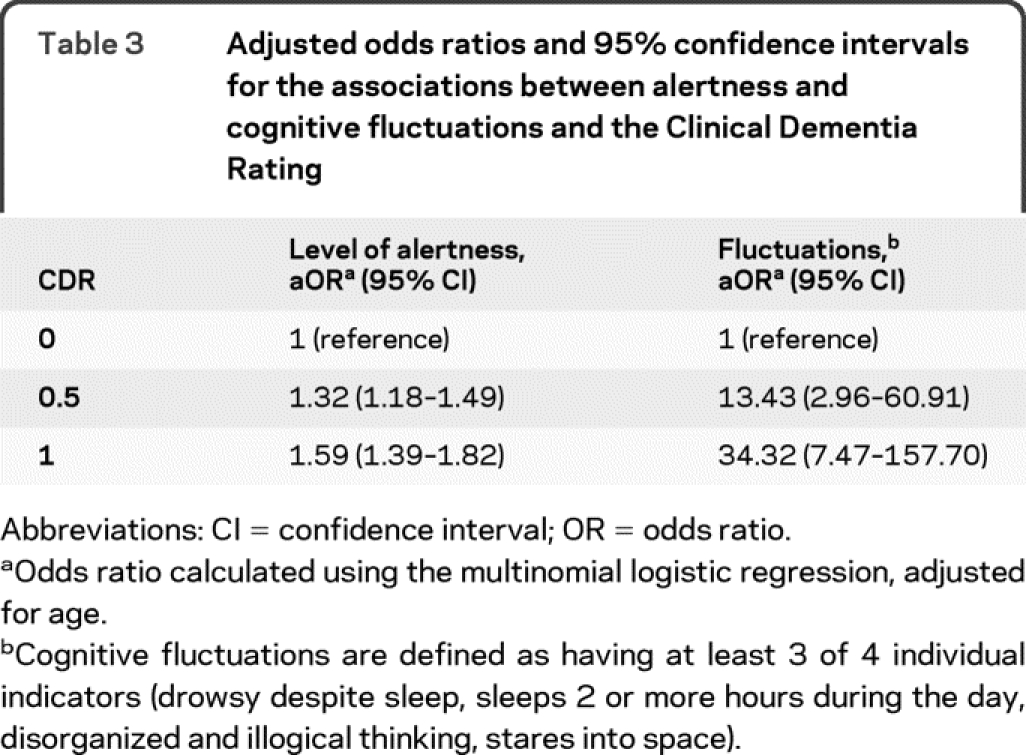
Table 3 illustrates the risk of being rated with cognitive impairment by CDR stage with reference to having no dementia (CDR 0). Diminished levels of daytime alertness were associated with an increased risk of being rated as having dementia (CDR >0). We found that the risk of a CDR 0.5 rating among those presenting with cognitive fluctuations was 13.4 times higher than among those without fluctuations. The risk increased to 34.3 among participants with fluctuations to be rated CDR 1. It is important to reiterate that none of the cognitively impaired individuals were diagnosed with DLB.
Table 3 Adjusted odds ratios and 95% confidence intervals for the associations between alertness and cognitive fluctuations and the Clinical Dementia Rating
Relationship between fluctuations and cognitive performance.
We examined correlations between cognitive fluctuations and neuropsychological performance (table 4). Not surprisingly, there was a strong inverse correlation between CDR and most of the individual neuropsychological tests and composite factors showing poorer performance with advancing stages of dementia (−0.58 < r < −0.17). There were also small inverse correlations (−0.22 < r < −0.10) between the presence of cognitive fluctuations and performance on the composites and most of the individual tests. After controlling for age, we examined the partial correlations between each of the fluctuation variables and test performance. The strongest relationship with test performance was found with the illogical, disorganized thinking variable (−0.30 < r < −0.14). The weakest relationships, still inverse, were with the drowsy and lethargic (rs < 0.14) and stares into space (rs < 0.18) variables.
Table 4 Correlationsa among cognitive fluctuation, Clinical Dementia Rating, and neuropsychological test performance
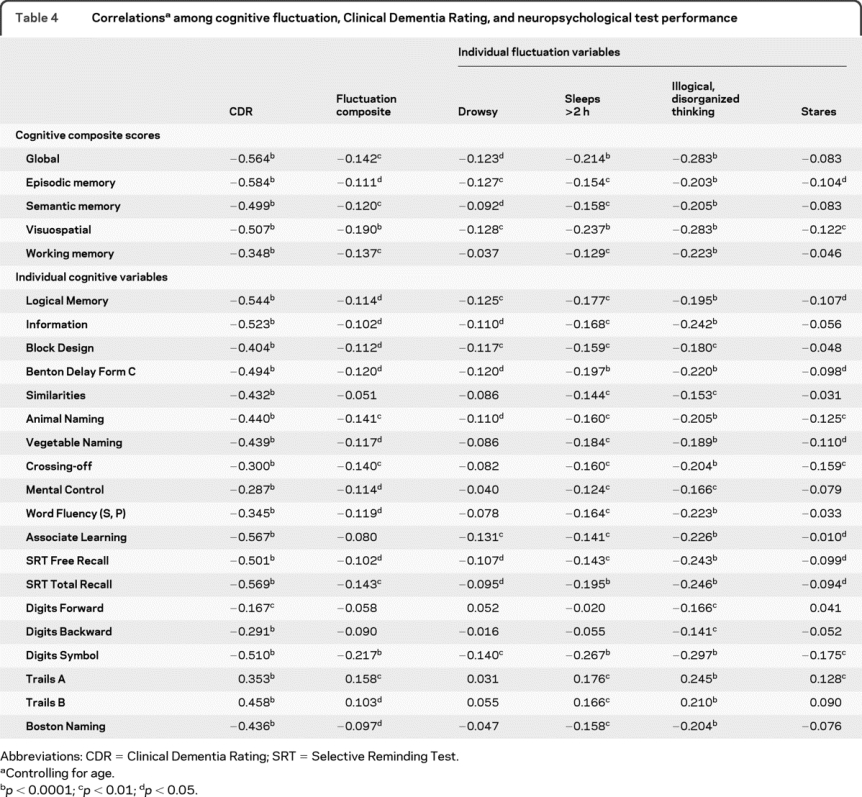
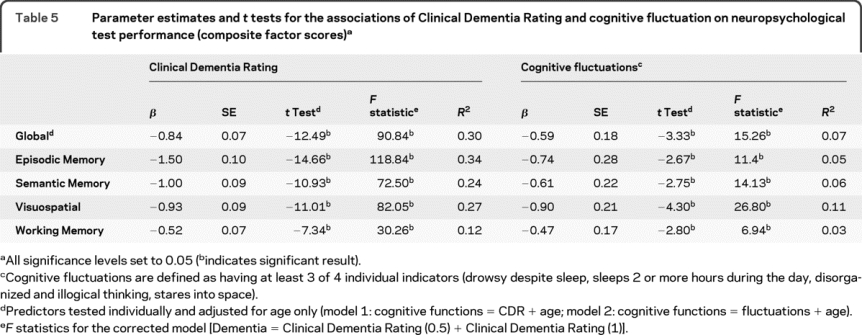
Table 5 shows the adjusted estimates of the individual effects of CDR and cognitive fluctuations in predicting the global, episodic, semantic, working, and visuospatial performance among the participants after controlling for age. Two individual models are shown. Model 1 tested the effect of CDR on performance controlling for age while model 2 tested the effect of cognitive fluctuation on performance controlling for age. Changes in the CDR correspond to increasing impairment in all domains. Compared with participants without fluctuations, the presence of cognitive fluctuations corresponds to a decrease in the global (0.59), episodic (0.74), semantic (0.61), visuospatial (0.90), and working memory (0.47) composites explaining 3%–11% of the variance in performance.
Table 5 Parameter estimates and t tests for the associations of Clinical Dementia Rating and cognitive fluctuation on neuropsychological test performance (composite factor scores)
DISCUSSION
The presence of cognitive fluctuations significantly worsened Clinical Dementia Ratings and was associated with diminished cognitive performance. After controlling for age, participants with cognitive fluctuations (3 or 4 individual symptoms) were at more than an eightfold risk of dementia compared with participants who did not fluctuate. When examined by individual symptoms, participants who presented with disorganized, illogical thinking had more than a sevenfold increased risk of being rated as cognitively impaired (CDR >0). We found that the risk of receiving a CDR 0.5 rating among those who presented with cognitive fluctuations was 13 times higher than among those individuals without fluctuations. The risk of being rated CDR 1 increased 34-fold among participants with fluctuations.
The presence of fluctuations was also associated with poorer performance on neuropsychological testing with the strongest relationship between disorganized or illogical thinking. Compared with participants who did not exhibit fluctuations, there were declines across composite measures of cognitive abilities. Because fluctuations may transiently affect attention, alertness, and concentration, we examined whether changes in daytime sleepiness (measured by the Mayo Sleep Questionnaire25) affected dementia ratings. Increased alertness as reported by an informant was associated with a lower risk of being rated with dementia by the examiner. However, there was no relationship between the REM behavior question (acting out dreams) and cognitive fluctuations, supporting the contention that the samples were free of unrecognized DLB cases.
Although thought to be a core feature of DLB and frequently present in PD dementia,26–28 fluctuations may occur in older adults without dementia and are present in 12% of AD cases in our sample. When present, fluctuations were associated with poorer rating on the CDR staging of the participant, and poorer performance on cognitive testing.
The Mayo Fluctuation Questionnaire3 captures 4 unique aspects of fluctuations and may reliably discriminate DLB from AD; however, we demonstrate here that asking these questions in assessment of cognitive abilities regardless of whether other DLB core features are present has utility. The presence of fluctuations worsens both the clinical rating of dementia and neuropsychological performance.
Few studies have assessed fluctuations in AD. In a comparison of 13 patients with DLB with 12 patients with AD, informants described DLB fluctuations as spontaneous and transient and as impacting functional abilities. Fluctuations in patients with AD highlighted episodes of memory failure and the concept of “good” vs “bad” days.29 A larger prospective study5 compared attentional deficits and fluctuations in 85 patients with DLB and 80 patients with AD. Cognitive processing speed, attention, and fluctuations were all more impaired in DLB compared with AD, although patients with AD at the moderate stage also demonstrated fluctuations. Although these studies correlated fluctuations with cognitive performance, the inclusion of a DLB group may have masked the impact of fluctuations on the cognitive performance of AD because the goal in each of these studies was to compare DLB with AD.
The biologic bases of fluctuations are not well understood.30 Ascending cholinergic pathways from the pedunculopontine nucleus and laterodorsal tegmental nuclei are involved in arousal.31 The loss of these projections has been described in DLB, but neuronal counts did not correlate with sleep disturbances or fluctuations. DLB is associated with greater attentional deficits compared with AD32; however, loss of attentional control is also an early finding in AD.33,34 EEG recordings of patients with AD and patients with DLB demonstrate differences in compressed spectral arrays favoring posterior alpha activity in patients with DLB with fluctuations.35 SPECT imaging of patients with DLB demonstrates significant correlation between fluctuations and increased thalamic and decreased occipital perfusion.36 Such studies have not been performed in patients with AD who experience cognitive fluctuations.
Our study has limitations. Although patients with clinically diagnosed DLB were excluded from this study, it is possible that patients with AD who fluctuate may go on to develop other core features of DLB. At the time of evaluation, however, no other core features (extrapyramidal signs, hallucinations, REM behavior disorder) were detected. It is difficult to accurately capture all aspects of cognitive fluctuation. Fluctuations may represent brief interruptions of consciousness, periods of increased confusion and cognitive impairment, episodes of diminished arousal, or what seem to be periods of prolonged sleep. Many issues regarding fluctuations are unresolved, including how important the assessment of fluctuations is to making a dementia diagnosis.37 We demonstrate here that fluctuations in AD worsen cognitive performance and lead to poorer CDR ratings. There are now several rating scales available to assess fluctuations, each of which assesses different aspects.3,38
The Mayo Fluctuation Questionnaire3 asks informants to indicate whether the symptom was present or absent; however, there is no consideration of the degree of change. The sample was not population-based; however, this sample is representative of our longstanding longitudinal study of over 3,000 individuals. As with any volunteer sample, there may be selection biases, thus limiting generalization of the results. Our convenience sample has demographic attributes and comorbid disorders that reflect those of the similarly aged population in the greater St. Louis metropolitan area, except they are slightly more educated.
Fluctuations are common in DLB1–5; however, it was unknown what effect fluctuations play in AD. To address this, we compared patients with AD with older adults without dementia. Cognitive fluctuations do occur in AD and their presence significantly worsens CDR ratings by expert dementia clinicians and worsens cognitive performance across all domains. The inclusion of fluctuation scales such as the Mayo Fluctuations Questionnaire3 in the assessment of older adults for cognitive disorders may capture these clinically important events.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Noor Al-Hammadi and Dr. James E. Galvin.
ACKNOWLEDGMENT
The authors thank the clinicians from the Clinical Core of the Washington University Alzheimer's Disease Research Center (John C. Morris, MD, Director and PI) for performing the longitudinal clinical and cognitive assessments and the research participants who contributed data. The authors also thank Martha Storandt, PhD, of the ADRC Psychometric Core for expert analytic feedback.
DISCLOSURE
Dr. Escandon reports no disclosures. Dr. Al-Hammadi reports no disclosures. Dr. Galvin serves on a scientific advisory board for the American Federation for Aging Research and on the Board of Directors and the Scientific Advisory Council for the Lewy Body Dementia Association; serves on the editorial boards of Alzheimer Disease and Associated Disorders and Acta Neuropathologica; serves on speakers' bureaus for Pfizer Inc., Eisai Inc., Novartis, and Forest Laboratories, Inc.; has served as a consultant for Novartis, Forest Laboratories, Inc., Pfizer Inc., Eisai Inc., and Medivation, Inc.; has received license fee payments for AD8 dementia screening test (copyrighted): license agreements between Washington University and Pfizer Inc., Eisai Inc., and Novartis; and receives research support from Novartis, Eli Lilly and Company, Elan Corporation, Wyeth, Bristol-Myers Squibb, the NIH/NIA [P01 AG03991 (Investigator), P50 AG05681 (Investigator), and P01 AG026276 (Investigator)], and the Alzheimer Association.
Address correspondence and reprint requests to Dr. James E. Galvin, Alzheimer Disease Research Center, Washington University School of Medicine, 4488 Forest Park, Suite 130, St. Louis, MO 63108 galvinj@neuro.wustl.edu
Study funding: Supported by NIH/NIA grants P01 AG03991, P50 AG05681, and P01 AG026276.
Disclosure: Author disclosures are provided at the end of the article.
Received September 11, 2009. Accepted in final form October 28, 2009.
REFERENCES
- 1.McKeith IG, Dickson DW, Lowe J, et al, Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 2.Tarawneh R, Galvin JE. Distinguishing Lewy body dementias from Alzheimer's disease. Expert Rev Neurother 2007;7:1499–1516. [DOI] [PubMed] [Google Scholar]
- 3.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology 2004;62:181–187. [DOI] [PubMed] [Google Scholar]
- 4.Walker MP, Ayre GA, Cummings JL, et al. Quantifying fluctuation in dementia with Lewy bodies, Alzheimer's disease, and vascular dementia. Neurology 2000;54:1616–1625. [DOI] [PubMed] [Google Scholar]
- 5.Ballard C, O'Brien J, Gray A, et al. Attention and fluctuating attention in patients with dementia with Lewy bodies and Alzheimer disease. Arch Neurol 2001;58:977–982. [DOI] [PubMed] [Google Scholar]
- 6.Berg L, McKeel DW, Jr., Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 1998;55:326–335. [DOI] [PubMed] [Google Scholar]
- 7.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 8.Burke WJ, Miller JP, Rubin EH, et al. Reliability of the Washington University Clinical Dementia Rating. Arch Neurol 1988;45:31–32. [DOI] [PubMed] [Google Scholar]
- 9.Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol 2005;62:758–765. [DOI] [PubMed] [Google Scholar]
- 10.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectr 2008;13:45–53. [DOI] [PubMed] [Google Scholar]
- 12.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology 2006;67:467–473. [DOI] [PubMed] [Google Scholar]
- 13.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wechsler D, Stone CP. Manual: Wechsler Memory Scale. New York: Psychological Corporation; 1973. [Google Scholar]
- 15.Wechsler D. Manual: Wechsler Memory Scale–Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 16.Wechsler D. Manual: Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1955. [Google Scholar]
- 17.Wechsler D. Manual: Wechsler Adult Intelligence Scale–Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 18.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders, 2nd ed. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 19.Thurstone LL, Thurstone LG. Examiner Manual for the SRA Primary Mental Abilities Test. Chicago: Science Research Associates; 1949. [Google Scholar]
- 20.Armitage SG. An analysis of certain psychological tests used in the evaluation of brain injury. Psychol Monogr 1946;60:1–48. [Google Scholar]
- 21.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology 1988;3:900–903. [DOI] [PubMed] [Google Scholar]
- 22.Benton AL. The Revised Visual Retention Test: Clinical and Experimental Applications. New York: Psychological Corporation; 1963. [Google Scholar]
- 23.Botwinick J, Storandt M. Speed functions, vocabulary ability, and age. Percept Mot Skills 1973;36:1123–1128. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DK, Storandt M, Morris JC, Langford ZD, Galvin JE. Cognitive profiles in dementia: Alzheimer disease vs healthy brain aging. Neurology 2008;71:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caselli RJ, Reiman EM, Hentz JG, Osborne D, Alexander GE, Boeve BF. A distinctive interaction between memory and chronic daytime somnolence in asymptomatic APOE e4 homozygotes. Sleep 2002;25:447–453. [PubMed] [Google Scholar]
- 26.Ballard CG, Aarsland D, McKeith I, et al. Fluctuations in attention: PD dementia vs DLB with parkinsonism. Neurology 2002;59:1714–1720. [DOI] [PubMed] [Google Scholar]
- 27.Serrano C, García-Borreguero D. Fluctuations in cognition and alertness in Parkinson's disease and dementia. Neurology 2004;63(8 suppl 3):S31–S34. [DOI] [PubMed] [Google Scholar]
- 28.Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology 2006;67:1605–1611. [DOI] [PubMed] [Google Scholar]
- 29.Bradshaw J, Saling M, Hopwood M, Anderson V, Brodtmann A. Fluctuating cognition in dementia with Lewy bodies and Alzheimer's disease is qualitatively distinct. J Neurol Neurosurg Psychiatry 2004;75:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis PT, Perry EK. Cholinergic and other neurotransmitter mechanisms in Parkinson's disease, Parkinson's disease dementia, and dementia with Lewy bodies. Mov Disord 2007;22 suppl 17:S351–S357. [DOI] [PubMed] [Google Scholar]
- 31.Schmeichel AM, Buchhalter LC, Low PA, et al. Mesopontine cholinergic neuron involvement in Lewy body dementia and multiple system atrophy. Neurology 2008;70:368–373. [DOI] [PubMed] [Google Scholar]
- 32.Bradshaw JM, Saling M, Anderson V, Hopwood M, Brodtmann A. Higher cortical deficits influence attentional processing in dementia with Lewy bodies, relative to patients with dementia of the Alzheimer's type and controls. J Neurol Neurosurg Psychiatry 2006;77:1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castel AD, Balota DA, Hutchison KA, Logan JM, Yap MJ. Spatial attention and response control in healthy younger and older adults and individuals with Alzheimer's disease: evidence for disproportionate selection impairments in the Simon task. Neuropsychology 2007;21:170–182. [DOI] [PubMed] [Google Scholar]
- 34.Castel AD, Balota DA, McCabe DP. Memory efficiency and the strategic control of attention at encoding: impairments of value-directed remembering in Alzheimer's disease. Neuropsychology 2009;23:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonanni L, Thomas A, Tiraboschi P, Perfetti B, Varanese S, Onofrj M. EEG comparisons in early Alzheimer's disease, dementia with Lewy bodies and Parkinson's disease with dementia patients with a 2-year follow-up. Brain 2008;131:690–705. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien JT, Firbank MJ, Mosimann UP, Burn DJ, McKeith IG. Change in perfusion, hallucinations and fluctuations in consciousness in dementia with Lewy bodies. Psychiatry Res 2005;139:79–88. [DOI] [PubMed] [Google Scholar]
- 37.Cummings JL. Fluctuations in cognitive function in dementia with Lewy bodies. Lancet Neurol 2004;3:266. [DOI] [PubMed] [Google Scholar]
- 38.Walker MP, Ayre GA, Cummings JL, et al. The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale. Br J Psychiatry 2000;177:252–256. [DOI] [PubMed] [Google Scholar]


