Abstract
Background:
Neuroimaging measures and chemical biomarkers may be important indices of clinical progression in normal aging and mild cognitive impairment (MCI) and need to be evaluated longitudinally.
Objective:
To characterize cross-sectionally and longitudinally clinical measures in normal controls, subjects with MCI, and subjects with mild Alzheimer disease (AD) to enable the assessment of the utility of neuroimaging and chemical biomarker measures.
Methods:
A total of 819 subjects (229 cognitively normal, 398 with MCI, and 192 with AD) were enrolled at baseline and followed for 12 months using standard cognitive and functional measures typical of clinical trials.
Results:
The subjects with MCI were more memory impaired than the cognitively normal subjects but not as impaired as the subjects with AD. Nonmemory cognitive measures were only minimally impaired in the subjects with MCI. The subjects with MCI progressed to dementia in 12 months at a rate of 16.5% per year. Approximately 50% of the subjects with MCI were on antidementia therapies. There was minimal movement on the Alzheimer's Disease Assessment Scale–Cognitive Subscale for the normal control subjects, slight movement for the subjects with MCI of 1.1, and a modest change for the subjects with AD of 4.3. Baseline CSF measures of Aβ-42 separated the 3 groups as expected and successfully predicted the 12-month change in cognitive measures.
Conclusion:
The Alzheimer's Disease Neuroimaging Initiative has successfully recruited cohorts of cognitively normal subjects, subjects with mild cognitive impairment (MCI), and subjects with Alzheimer disease with anticipated baseline characteristics. The 12-month progression rate of MCI was as predicted, and the CSF measures heralded progression of clinical measures over 12 months.
GLOSSARY
- AD
= Alzheimer disease;
- ADAS-Cog
= Alzheimer's Disease Assessment Scale–Cognitive subscale;
- ADNI
= Alzheimer's Disease Neuroimaging Initiative;
- CI
= confidence interval;
- MCI
= mild cognitive impairment;
- MMSE
= Mini-Mental State Examination.
Most investigators believe that Alzheimer disease (AD) is a slowly evolving process that likely begins years to decades before the clinical symptoms are manifest.1 There is a strong interest in identifying individuals at an earlier stage in the AD neuropathologic spectrum before the full clinical criteria for AD are met. Mild cognitive impairment (MCI) represents an attempt to characterize subjects at an early clinical phase and has been a target for clinical trials.2–5 Neuroimaging and chemical biomarkers may allow detection of the neurodegenerative process at an earlier point in the spectrum and increase our ability to detect treatment effects in clinical trials.6–8
The Alzheimer's Disease Neuroimaging Initiative (ADNI) is a consortium of universities and medical centers in the United States and Canada established to develop standardized imaging techniques and biomarker procedures in normal subjects, subjects with MCI, and subjects with mild AD.9 The major goals of ADNI are to develop improved methods that will lead to uniform standards for acquiring longitudinal, multisite MRI and PET data on patients with AD, patients with MCI, and elderly controls, to develop an accessible data repository that describes longitudinal changes in brain structure and metabolism while acquiring in parallel clinical, cognitive, and biochemical data, to develop methods that will maximize power to determine treatment effects in clinical trials, and to test a series of hypotheses based on clinical and biomarker data.
METHODS
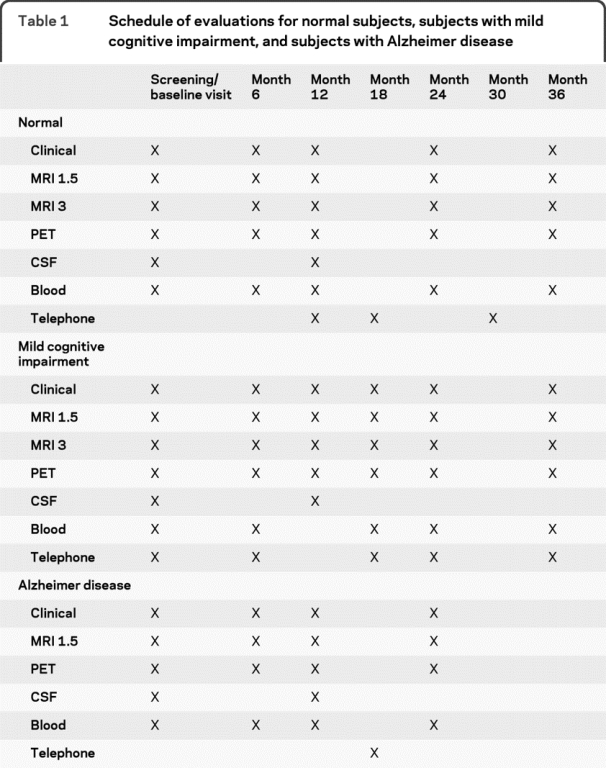
Table 1 describes the flow of subjects in each of the 3 clinical groups. Enrolled subjects were between 55 and 90 years of age (inclusive) and were required to have a study partner to provide an independent evaluation of functioning. Subjects could speak either English or Spanish. All subjects had to be willing to undergo all test procedures including neuroimaging and longitudinal follow-up. At least 20% of the subjects at each site had to be willing to undergo 2 lumbar punctures spaced 1 year apart. Psychoactive medications which were believed to possibly affect cognitive function were excluded. The general inclusion and exclusion criteria were as follows. All subjects had to have Hachinski Ischemic Score of less than or equal to 4; permitted medications stable for 4 weeks prior to screening; a Geriatric Depression Scale score of less than 6; a study partner with 10+ hours per week of contact either in person or on the telephone and who could accompany the participant to the clinical visits; visual and auditory acuity adequate for neuropsychological testing; good general health with no diseases precluding enrollment; 6 grades of education or work history equivalent; and ability to speak English or Spanish fluently.10,11 Women had to be sterile or 2 years past childbearing potential. Subjects had to be able to complete a 3-year imaging study (2 years for subjects with AD). Subjects agreed to DNA extraction for APOE testing and banking and agreed to blood and urine examination for biomarkers. Subjects could not have any medical contraindications to MRI and could not be enrolled in other trials or studies concurrently.
Table 1 Schedule of evaluations for normal subjects, subjects with mild cognitive impairment, and subjects with Alzheimer disease
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Institutional Review Boards of all of the participating institutions. Informed written consent was obtained from all participants at each site.
Subject selection.
The subjects for the study were classified as normal controls, subjects with MCI, or subjects with mild AD. The criteria for classification of the subjects were as follows. With respect to memory complaints, the normal subjects had none, while the subjects with MCI and subjects with AD both had to have complaints. On the Mini-Mental State Examination (MMSE), the range for the normal subjects and subjects with MCI was 24–30, and for AD 20–26; all are inclusive. The CDR score for normal subjects was 0 and for subjects with MCI was 0.5 with a mandatory requirement of the memory box score being 0.5 or greater, and the rating for subjects with AD was 0.5 or 1. For the memory criterion, delayed recall of 1 paragraph from the Logical Memory II subscale of the Wechsler Memory Scale–Revised (maximum score of 25)12 was used with cutoff scores as follows based on education: normal subjects ≥9 for 16 years of education, ≥5 for 8–15 years of education, and ≥3 for 0–7 years of education. For subjects with MCI and subjects with AD, these scores were ≤8 for 16 years of education, ≤4 for 8–15 years of education, and ≤2 for 0–7 years of education.
In addition, the normal control subjects were to be matched to the other subjects in age and could not have any significant impairment in cognitive functions or activities of daily living. The subjects with MCI had to be largely intact with regard to general cognition and functional performance, and could not qualify for the diagnosis of dementia.13 The subjects with AD had mild AD and had to meet the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria for probable AD.14
No subjects could be taking antidepressant medications with anticholinergic properties, and the regular use of narcotic agents had to be limited to fewer than 2 doses per week within 4 weeks of screening. Neuroleptic medications and other drugs with anticholinergic properties could not be used within 4 weeks of screening. Antiparkinsonian medications could not be used within 4 weeks of screening. Participants could not be enrolled in any other investigational drug studies within 4 weeks of screening, and diuretic drugs should not be started or discontinued within 4 weeks prior to screening. Cholinesterase inhibitors and memantine were permitted if the dose had been stable for 4 weeks prior to screening for subjects with MCI and AD. Estrogen and estrogen-like compounds and vitamin E were allowed if the dose had been stable for 4 weeks prior to screening. Participants were required to report any medication changes to the site investigators once they were enrolled in the study.
At the screening visit, all subjects were required to provide informed consent as compatible with the local sites (Institutional Review Board regulations). In addition, all subjects provided demographics, family history, and medical history. All subjects were given a physical examination and a neurologic examination, and vital signs were recorded. Screening laboratories were obtained as well as blood for DNA for APOE testing. As mentioned, all subjects had the MMSE and the ADNI administration of Logical Memory II.
At baseline, subjects were given the American National Adult Reading Test and the following cognitive measures were examined: digit span, category fluency, Trail Making A and B, Digit Symbol Substitution Test of the Wechsler Adult Intelligence Scale–Revised, Boston Naming Test, Auditory Verbal Learning Test, clock drawing, Neuropsychiatric Inventory Q, AD Assessment Scale–Cognitive Subscale, and Functional Assessment Questionnaire.15–20
All subjects received an MRI scan at 1.5 Tesla signal strength; 25% of the subjects also received an MRI scan at 3 Tesla, 50% received an FDG PET scan, and a minimum of 20% of the subjects at each site also received a lumbar puncture.
As noted in table 1, the normal subjects and subjects with MCI were evaluated approximately every 6 months for up to 3 years with telephone visits being performed at months 18 and 30. The subjects with AD were followed every 6 months for 24 months with a telephone contact at month 18. The primary outcome measures were the rate of progress from MCI to AD as well as a variety of imaging and chemical biomarkers. Rates of change were calculated for the imaging measures, each biomarker, and glucose metabolism for specified regions of interest on FDG PET scanning.
Data analysis.
Baseline characteristics of the participants were summarized by diagnostic group (mean and SD for quantitative measures, proportion or percent for categorical variables). Group characteristics at baseline were compared by nonparametric tests (Kruskal-Wallis) for quantitative measures, using a Hochberg multiple comparison procedure to compare means, and using χ2 or exact tests to compare proportions. Change over time was summarized 3 different ways: conversion proportion, mean or percent difference in quantitative measure between baseline and 12-month follow-up, and rate of change per year estimated from mixed effects regression models for quantitative measures using all available follow-up data.21 Annual rates of conversion were estimated using a hybrid estimate of the distribution function as proposed by Wellner and Zahn22 for interval censored data. Differences in rate of change over time across groups were assessed in the mixed effects regression models by adding as covariates both a main effect term to estimate baseline differences and an interaction with time to test differences in rates of change. Similarly, the potential of biomarker or imaging marker level at baseline to account for within-group heterogeneity in rates of change was assessed by adding both a main effect term and an interaction term with time to the mixed effects regression models. All hypothesis tests were 2-sided at level 0.05, and models were validated both graphically and analytically. SAS/STAT® software23 and R were used for statistical analysis.24
RESULTS
Baseline characteristics.
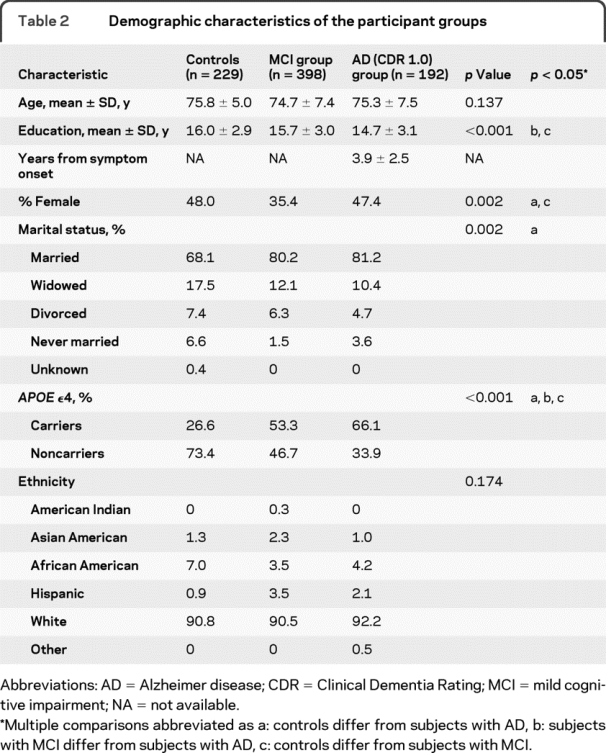
A total of 819 subjects were recruited and received a baseline evaluation as part of the ADNI study. There were 229 normal control subjects, 398 subjects with MCI, and 192 subjects with mild AD enrolled. As shown in table 2, the mean age of the 3 groups was equivalent at approximately 75 years. There were an approximately equal number of men and women in the normal control and AD groups, but there were more men than women in the MCI group (64.6% men vs 35.4% women). The estimated premorbid verbal IQ of these subjects was quite high, at almost 120 for the normal control subjects, 116 for the subjects with MCI, and 114 for the subjects with AD. Most of the subjects in the study were white, and this was approximately equivalent across the groups.
Table 2 Demographic characteristics of the participant groups
The mean scores for the screening measures (MMSE, Paragraph Recall, CDR, Geriatric Depression Scale, and Hachinski Scale) and the Functional Assessment Questionnaire are shown in table 3. In all cases except the Hachinski Scale and Geriatric Depression Scale, the mean scores for the 3 groups differed significantly at p < 0.001, with normal controls performing best, subjects with AD worst, and subjects with MCI in the middle. Overall, subjects with MCI had a mean total Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS-Cog) score of 11.5, while the normal control subjects had a score of 6.2 and the subjects with mild AD had a score of 18.7 (p < 0.001, table 3). The 3 groups also differed significantly in each of the subscales, with the MCI mean scores worse than those for normal participants but not as poor as those for participants with AD (table 3). These scores indicated, however, that the subjects with MCI gained most of their error points on memory items.
Table 3 Baseline assessments
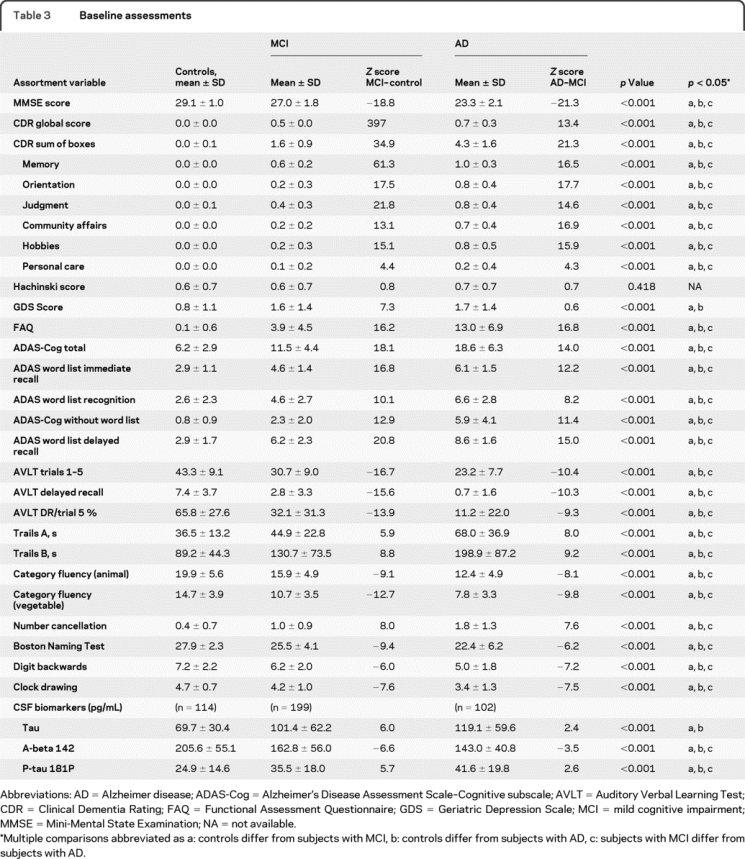
The neuropsychological battery indicated that, in general, subjects with MCI were more impaired than controls on the memory items and were only mildly impaired in nonmemory domains (table 3). The subjects with AD were impaired in virtually all of the cognitive areas tested. The next greatest area of impairment for the subjects with MCI resulted from the executive function domain.
Concomitant medications.
Appropriate treatments for dementia were allowed in the subjects with MCI and subjects with AD. The following percentages of subjects were on therapy at baseline: cholinesterase inhibitors—normal controls 0%, subjects with MCI 43.7%, subjects with AD 84.9%; memantine—normal controls 0%, subjects with MCI 10.8%, subjects with AD 47.4%; combined cholinesterase inhibitors and memantine—normal controls 0%, subjects with MCI 8.8%, subjects with AD 40.6%.
Twelve-month follow-up measures.
The mean differences in performance of the subjects between baseline and 12 months are shown in table 4. In general, the normal group stayed nearly the same, and the AD group worsened significantly compared to the normal controls. The MCI group was more variable; in most cases they worsened more than the normal group but not as much as the AD group, but they looked more like the AD group for change in digit span, category fluency, and trail making, and more like the normal controls for the clock test and global CDR.
Table 4 12-Month change
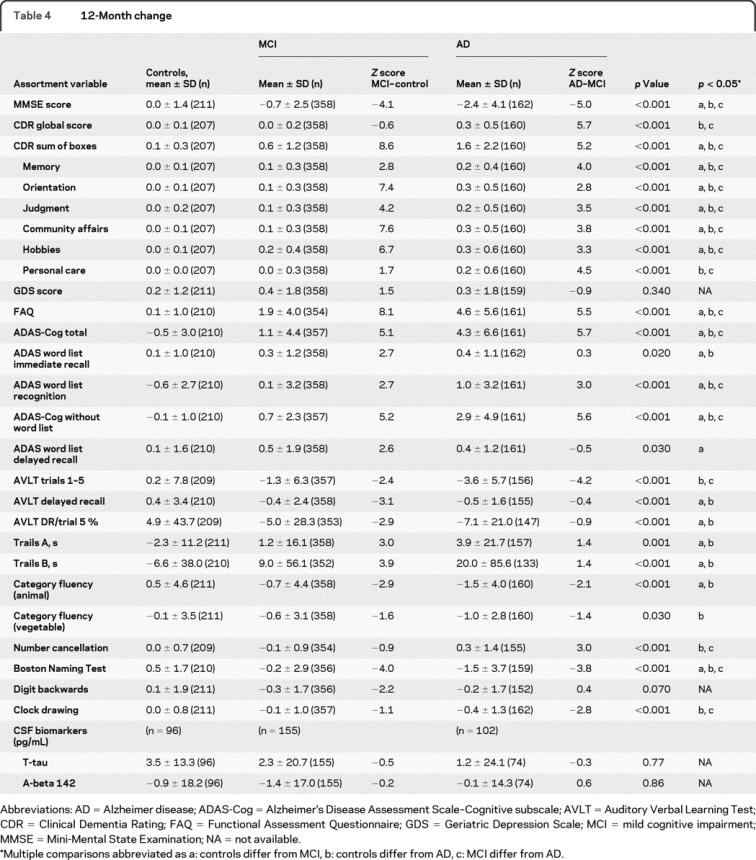
The CDR sum of the boxes essentially did not change in the normal control group (0.1) and increased by approximately 0.7 points in the subjects with MCI and 1.5 points in the subjects with AD (p < 0.001). On the memory measures such as the AVLT, there was virtually no change across 12 months in learning in the normal control group with a decreased learning of 1.3 items in the subjects with MCI and 3.7 fewer items learned in the AD group. Delayed recall improved by 5.3% in the normal control group and declined by 4.8% in the subjects with MCI and 7.2% in the subjects with AD.
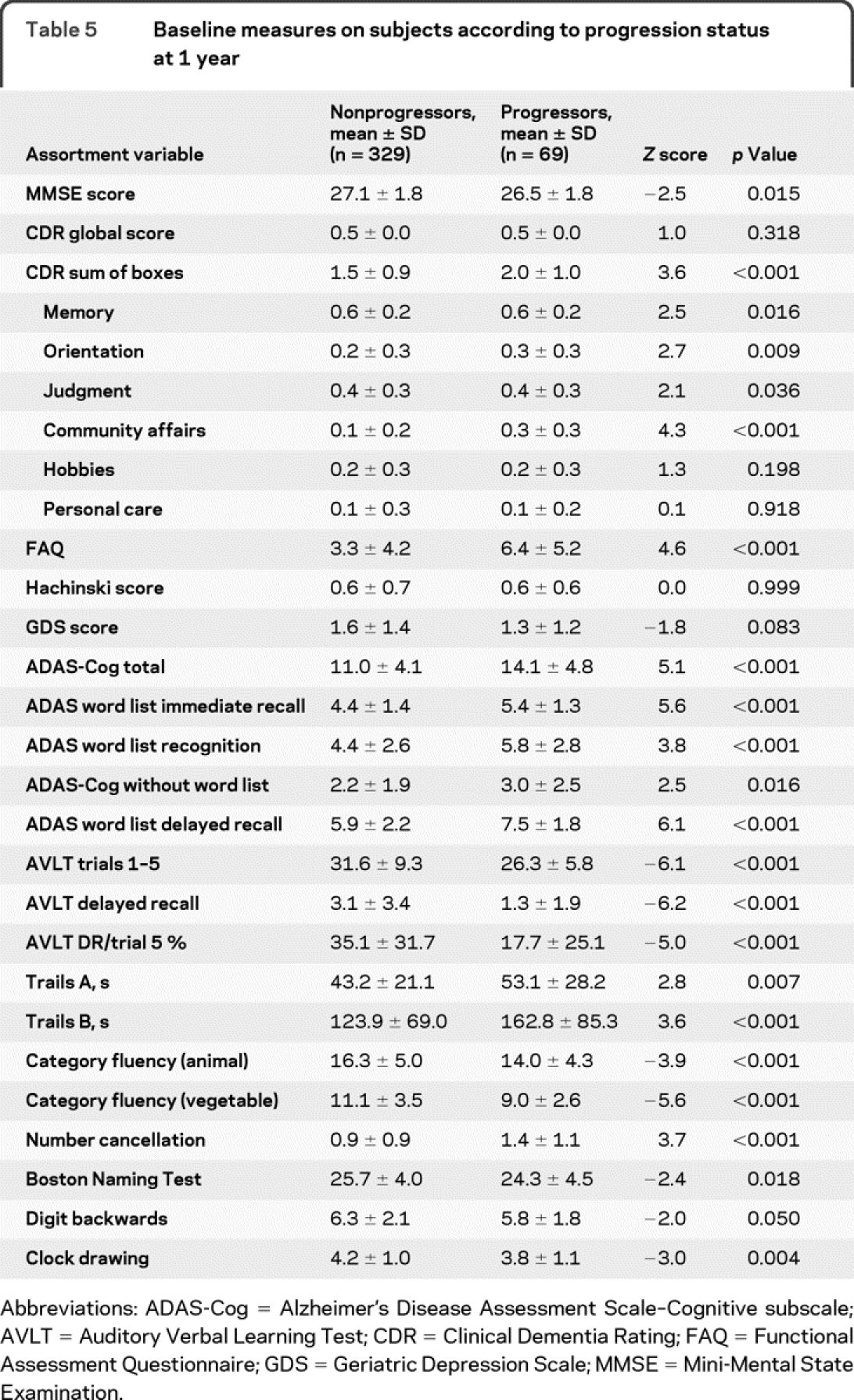
Table 4 shows the neuropsychological test changes over 12 months. Among the 229 subjects who were recruited in the normal control group, only 3 progressed to MCI over 12 months and none to AD; 17 did not have final diagnosis for 12-month follow-up. Allowing for interval-censored data, the estimated conversion rate was just 1.4% (95% confidence interval [CI] 0%–3.2%). Among the 398 subjects with MCI at baseline, 64 progressed to AD at 12 months for a progression rate of 16.5% (95% CI 11.9%–20.5%). An additional 8 subjects with MCI reverted to a normal classification over that interval, and 41 did not have a follow-up diagnosis. Of the 192 subjects with AD, virtually all of them remained in that category, while 2 reverted to MCI status at 12 months. Table 5 shows the baseline cognitive characteristics of the subjects with MCI who progress and do not progress to dementia over the course of the first 12 months.
Table 5 Baseline measures on subjects according to progression status at 1 year
Stratification by CSF Aβ-42 levels.
CSF Aβ-42 levels were assessed at baseline and were correlated with clinical diagnoses, performance on the ADAS-Cog, and changes in performance on the ADAS-Cog over 1 year (table 3). Baseline Aβ-42 levels decreased significantly across the diagnostic categories as follows (mean ± SE): normal controls 206 ± 5; MCI 164 ± 4; AD 143 ± 4 (p < 0.001). Higher levels of Aβ-42 were associated with better performance on the ADAS-Cog in normal subjects (r = −0.21; 95% CI −0.38 to −0.03) and subjects with MCI (r = −0.22; 95% CI −0.035 to −0.08). The correlation with the ADAS-Cog in AD was not significant. Similarly, the annual rate of change on the ADAS-Cog against Aβ-42 at screening suggested that higher baseline levels of Aβ-42 were associated with a smaller change over 12 months in the normal subjects (r = −0.23; 95% CI −0.40 to −0.05) and in MCI (r = −0.29; 95% CI −0.41 to −0.16). The mixed effects models suggested that higher levels of Aβ-42 were associated with improved performance on the ADAS-Cog in normal subjects (p = 0.01) and in subjects with MCI (p < 0.00). The interaction of Aβ-42 and time also had a significant effect on ADAS-Cog scores in subjects with MCI (p = 0.00), but not in normal subjects. There was no significant baseline Aβ-42 effect observed over 12 months in the subjects with AD. The CSF changes over 12 months are shown in table 4.
DISCUSSION
The recruitment for ADNI was designed to simulate a clinical trial population. As such, the baseline characteristics of the subjects indicated that the 3 groups were generally well educated, intelligent, and mostly white, and perform in a fashion similar to subjects recruited for typical clinical trials.25–28
The summary of the baseline characteristics of the subjects indicated that the subjects with MCI were memory impaired compared to the normal controls and their memory performance was slightly better than that of the subjects with mild AD by design. With respect to nonmemory domains, the subjects with MCI were more similar to the normal control subjects than they were to the subjects with AD. As such, the MCI group recruited in this study represents individuals in a transitional state between cognitive changes of normal aging and clinical features and the clinical criteria for probable AD. The subjects recruited for ADNI likely do not represent “typical” subjects in the community. They tend to be more highly educated and the proportion of APOE4 carriers was quite high in the MCI and AD groups but is consistent with subjects recruited for clinical trials.25
The 12-month change data indicated little movement on many of the global scales. As would be expected, the control subjects essentially remained stable or improved slightly. The subjects with MCI also remained relatively stable as a group, and the subjects with mild AD showed a decline on most of these measures. The variability among the subjects increased across the 3 clinical groups from normal to MCI to AD. There was somewhat less variability on the global measures, while some of the individual neuropsychological tests such as the AVLT showed considerable variability among the subjects despite relatively modest mean group changes.
The diagnostic conversions over the 12-month period must be viewed with some caution. A 12-month change period is not a sufficient amount of time to draw conclusions regarding the likelihood of clinical change. However, there was considerable movement from the MCI group to the AD group over the 12 months at the progression rate of 16% per year as projected. This was in part by design since the criteria used to recruit subjects with MCI required a rather stringent degree of memory impairment to be included in the diagnostic classification group. As such, these subjects were likely further down the clinical spectrum with respect to the underlying AD process such that they would be more likely to progress than subjects with less impairment in the MCI spectrum.
The CSF Aβ-42 data correlated with the diagnostic groups in the expected fashion with levels being lower in the subjects with MCI than in the normal subjects. Correspondingly, the subjects with AD had lower Aβ-42 levels. In addition, the lower the CSF Aβ-42 level at baseline, the greater the cognitive change over 12 months in both the normal subjects and subjects with MCI, suggesting that baseline Aβ levels might be used to stratify subjects' likelihood of progressing more rapidly. This might be a useful technique to reduce the numbers of subjects used in clinical trials to detect a clinical effect of therapeutic interventions.
ADNI was successful in recruiting a cohort of subjects that was very similar to those seen in MCI and mild AD clinical trials.25–27 This subject population serves as an excellent resource for the study of the role of imaging and chemical biomarkers in tracking the AD disease process. The entire ADNI dataset, including demographic, clinical, neuropsychological, neuroimaging, and biochemical biomarker data, is available online for analysis by investigators (www.loni.ucla.edu/adni).
DISCLOSURE
Dr. Petersen serves on scientific advisory boards for Elan Corporation, Wyeth, and GE Healthcare; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH/NIA (U01 AG06786 [PI], P50 AG16574 [PI], U01 AG 024904 [Subcontract PI], and R01 AG11378 [Co-I]). Dr. Aisen serves on a scientific advisory board for NeuroPhage; serves as a consultant to Elan Corporation, Wyeth, Eisai Inc., Neurochem Inc., Schering-Plough Corp., Bristol-Myers Squibb, Eli Lilly and Company, NeuroPhage, Merck & Co., Roche, Amgen, Genentech, Inc., Abbott, Pfizer Inc, Novartis, and Medivation, Inc.; receives research support from Pfizer Inc, Baxter International Inc., Neuro-Hitech, Abbott, Martek, and the NIH (NIA U01-AG10483 [PI], NIA U01-AG024904 [Coordinating Center Director], NIA R01-AG030048 [PI], and R01-AG16381 [Co-I]); and has received stock options from Medivation, Inc. and NeuroPhage. Dr. Beckett received funding for travel to attend a conference not funded by industry; and receives research support from the NIH (2 P30 AG10129 [Director of Biostatistics Core], 2 P30 CA93373 [Director of Biostatistics Shared Resource], 1 U01 AG24904 [PI, UC Davis site; Biostatistics Team Leader], 1KL2RR024144-01 [Director of Biostatistics Core], 5R01EB002138-07 [Co-I], and 1RC2AG036535-01 [PI, UC Davis site]). Dr. Donohue receives research support from the NIH (AG010483 [Statistician] and AG024904 [Statistician]), the General Clinical Research Center, UCSD, National Center for Research Resources, and from the United States Public Health Service. Dr. Gamst served on an expert review board for Neurochem Inc; and receives research support as an investigator from the NIH [MH22005, CA104573, AG024904, MH062512, DK075128, AG010483, MH079752, AG031224, MH083552, MH083506, HL095089], and the National Science Foundation. Dr. Harvey serves as an Associate Editor of Statistics for Alzheimer Disease and Associated Disorders; and receives research support from the NIH (NIA 2P30AG10129 [Biostatistician], NIA R01AG029672 [Biostatistician], NIA 1U01AG24904 [Member of Biostatistics Core], NRCC RL1NS062412 [Biostatistician], NIA R01AG031252 [Biostatistician], 1RC2AG036535–01 [Member of Biostatistics Team]) and from the Hillblom Foundation, 2007A005NET (Biostatistician). Dr. Jack served on a scientific advisory board for Elan Corporation; receives research support from Pfizer Inc, the NIH (NIA AG11378 [PI], P50-AG16574 [Co-I], and U01 AG024904 [Co-I]) and from the Mayo U of MN Biotechnology Partnership; and holds stock in GE Healthcare. Dr. Jagust has served on a scientific advisory boards for Genentech, Inc.; has served as a consultant for Synarc, Elan Corporation, Genentech, Inc., Ceregene, Schering Plough, and Merck & Co; and receives research support from the NIH (AG027859 [PI], AG027984 [PI], and AG 024904 [Co-I]) and from the Alzheimer's Association. Dr. Shaw has received funding for travel and speaker honoraria from Pfizer Inc; serves on the editorial board of Therapeutic Drug Monitoring; may potentially receive revenue for patent pending (application 10/192,193): O-methylated rapamycin derivatives for alleviation and inhibition of lymphoproliferative disorders, licensed by the University of Pennsylvania to Novartis; receives royalties from publication of Applied Pharmacokinetics and Pharmacodynamics: Principles of Therapeutic Drug Monitoring, Wolters Kluwer/Lippincott Williams & Wilkins, 2005; receives research support from the NIH (AG024904 [Co-PI Biomarker Core Laboratory]); and receives board of directors' compensation and holds stock options in Saladax Biomedical. Dr. Toga received a speaker honorarium from St. Jude Children's Hospital; serves in an editorial capacity for NeuroImage, InSight, The Cerebellum, Neuroimaging, Neuroinformatics, Anatomy & Embryology, Current Medical Imaging Reviews, Biology Image Library, Biomedical Computation Review, Brain Structure and Function, and Journal of Neural Regeneration Research; has served on scientific and/or external advisory boards for Wellcome Trust, Allen Institute for Brain Science, University of Texas at Austin, Oklahoma IDeA Network for Biomedical Research Excellence, Takeda Global Research & Development Center, and the University of Pittsburgh; and has received/receives research support from the NIH (1R01 HD053893-01 [Co-I], NIBIB 2 R01 LM005639 [Co-I], NCRR 5 P41 RR013642 [PI], R01 HD050735-01 [Co-I], 1 R01 NS049194 [Co-I], 1 R01 EB 006266-01 [Subcontract PI], NIMH/UCI, R24 RR021992 [Subcontract PI], 2P50AG005133 [Consultant: Core-Neuroimaging], NCRR 5 U24 RR021760 [PI], NIMH 2 P50 AG016570 [Co-I], NIMH 1R01MH072641-01A1 [Co-I], NIMH 5 R01 MH071940 [PI], NIMH/NIA 1 U01 AG024904 [Subcontract PI], NIMH/MGH, R24 RR021382 [Subcontract PI], NIBIB/BWH 1 U54 EB05149 [Subcontract PI], 1 R01 DA017830 [Co-I], NCRR 5 U54 RR021813 [PI], R01 MH069259 [Subcontract PI], 1 R01 NS050792 [Subcontract PI], MH069433 [Co-I], NIMH 9 P01 EB 001955-11 [Co-PI]), 2005 Equipment Grant, and an Academic Excellence Grant–SUN Microsystems, and from High-Q Foundation and the National Multiple Sclerosis Society. Dr. Trojanowski has received funding for travel and honoraria from Takeda Pharmaceutical Company Ltd. and to attend numerous conferences not funded by industry; serves as an Associate Editor of Alzheimer's & Dementia; holds 14 patents that may accrue revenue: US Patent 5,281,521, issued 25 Jan 1994: Modified Avidin-Biotin Technique; US Patent 5,580,898, issued 3 Dec 1996: Method of Stabilizing Microtubules to Treat Alzheimer's Disease; US Patent 5,601,985, issued 11 Feb 1997: Method of Detecting Abnormally Phosphorylated Tau; US Patent 5,733,734, issued 31 Mar 1998: Method of Screening for Alzheimer's Disease or Disease Associated with the Accumulation of Paired Helical Filaments; US Patent 5,792,900, issued 11 Aug 1998: Compositions and Methods for Producing and Using Homogeneous Neuronal Cell Transplants; US Patent 5,849,988, issued 15 Dec 1998: Rat Comprising Straight Filaments in Its Brain; US Patent 6,214,334, issued 10 Apr 2001: Compositions And Methods for Producing and Using Homogeneous Neuronal Cell Transplants to Treat Neurodegenerative Disorders and Brain and Spinal Cord Injuries; US Patent 6,358,681, issued 19 Mar 2002: Diagnostic Methods for Alzheimer's Disease by Detection of Multiple MRNAs; US Patent 6,727,075, issued 27 Mar 2004: Methods and Compositions for Determining Lipid Peroxidation Levels in Oxidant Stress Syndromes and Diseases; US Patent 7,011,827, issued 14 Mar 2006: Compositions and Methods for Producing and Using Homogenous Neuronal Cell Transplants; Penn 0652, K1828, filed 5 Aug 1998: Method of Identifying, Diagnosing and Treating Alpha-synuclein Positive Neurodegenerative Disorders; Penn L1986, Filed 13 Nov 1998: Mutation-specific Functional Impairments in Distinct Tau Isoforms of Hereditary Frontotemporal Dementia and Parkinsonism Linked to Chromosome-17: Genotype Predicts Phenotype; Penn R3868 (UPN-4439), filed 28 Feb 2005: Microtubule Stabilizing Therapies for Neurodegenerative Disorders; and Penn S-4018, DB&R 46406-217282, filed 22 Nov 2005: Treatment of Alzheimer's and Related Diseases with an Antibody; and receives research support from the NIH (NIA P01 AG 09215-20 [PI], NIA P30 AG 10124-18 [PI], NIA PO1 AG 17586-10 [Project 4 Leader], NIA 1PO1 AG-19724-07 [Core C Leader], NIA 1 U01 AG 024904-05 [Co-PI Biomarker Core Laboratory], NINDS P50 NS053488-02 [PI], NIA UO1 AG029213-01 [Co-I]; RC2NS069368 [PI], RC1AG035427 [PI], and NIA P30AG036468 [PI]), and from the Marian S. Ware Alzheimer Program. Dr. Weiner serves on scientific advisory boards for Bayer Schering Pharma, Eli Lilly and Company, CoMentis, Inc., Neurochem Inc, Eisai Inc., Avid Radiopharmaceuticals Inc., Aegis Therapies, Genentech, Inc., Allergan, Inc., Lippincott Williams & Wilkins, Bristol-Myers Squibb, Forest Laboratories, Inc., Pfizer Inc, McKinsey & Company, Mitsubishi Tanabe Pharma Corporation, and Novartis; has received funding for travel from Nestlé and Kenes International and to attend conferences not funded by industry; serves on the editorial board of Alzheimer's & Dementia; has received honoraria from the Rotman Research Institute and BOLT International; serves as a consultant for Elan Corporation; receives research support from Merck & Co., Radiopharmaceuticals Inc., the NIH (U01AG024904 [PI], P41 RR023953 [PI], R01 AG10897 [PI], P01AG19724 [Co-I], P50AG23501 [Co-I], R24 RR021992 [Co-I], R01 NS031966 [Co-I], and P01AG012435 [Co-I]), the US Department of Defense (DAMD17-01-1-0764 [PI]), the Veterans Administration (MIRECC VISN 21 [Core PI]), and from the State of California; and holds stock in Synarc and Elan Corporation.
Supplementary Material
Address correspondence and reprint requests to Dr. Ronald C. Petersen, Department of Neurology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905 peter8@mayo.edu
Supplemental data at www.neurology.org
e-Pub ahead of print on December 30, 2009, at www.neurology.org.
Study funding: This study was funded by NIH grant AG024904.
Data used in the preparation of this article were obtained in part from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). As such, the investigators within ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or the writing of this report. ADNI investigators are listed in appendix e-1 on the Neurology® Web site at www.neurology.org.
Disclosure: Author disclosures are provided at the end of the article.
Received March 10, 2009. Accepted in final form October 28, 2009.
REFERENCES
- 1.Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci 1997;20:154–159. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathology of amnestic mild cognitive impairment. Arch Neurol 2006;63:665–672. [DOI] [PubMed] [Google Scholar]
- 3.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathological outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol 2006;63:674–681. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 5.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet 2006;367:1262–1270. [DOI] [PubMed] [Google Scholar]
- 6.Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov 2007;6:295–303. [DOI] [PubMed] [Google Scholar]
- 7.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006;5:228–234. [DOI] [PubMed] [Google Scholar]
- 8.Jack CR Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999;52:1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller SG, Weiner MW, Thal LJ, et al. The Alzheimer's Disease Neuroimaging Initiative. In: Pettrella JR, Doraiswamy PM, eds. Neuroimaging Clinics of North America: Alzheimer's Disease: 100 Years of Progress. Philadelphia: Elsevier Saunders; 2005:869–877. [Google Scholar]
- 10.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975;32:632–637. [DOI] [PubMed] [Google Scholar]
- 11.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler DA. Wechsler Memory Scale–Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- 13.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 15.Reitan R. Validity of the Trail-Making Test as an indication of organic brain damage. Percept Mot Skills 1958;8:271–276. [Google Scholar]
- 16.Wechsler DA. Wechsler Adult Intelligence Scale–Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- 17.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test, 2nd ed. Philadelphia: Lea & Febiger; 1982. [Google Scholar]
- 18.Rey A. l'Examen Clinique en Psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 19.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314. [DOI] [PubMed] [Google Scholar]
- 20.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- 22.Wellner JA, Zahn Y. A hybrid algorithm for computation of the nonparametric maximum likelihood estimator from censored data. J Am Stat Assoc 1997;92:945–959. [Google Scholar]
- 23.SAS/STAT® version 9. Cary, NC: SAS Institute, Inc.; 2004. [Google Scholar]
- 24.R Foundation for Statistical Computing/R Development Core Team. A Language and Environment for Statistical Computing. Vienna, Austria; 2006.
- 25.Petersen RC, Thomas RG, Grundman M, et al. Donepezil and vitamin E in the treatment of mild cognitive impairment. N Engl J Med 2005;352:2379–2388. [DOI] [PubMed] [Google Scholar]
- 26.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer's disease from mild cognitive impairment: the InDDEx study. Lancet Neurol 2007;6:501–512. [DOI] [PubMed] [Google Scholar]
- 27.Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology 2005;30:1204–1215. [DOI] [PubMed] [Google Scholar]
- 28.Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008;70:2024–2035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


