Until recent years, autoimmune limbic encephalitis (LE) was mostly viewed as a paraneoplastic disorder associated with onconeuronal antibodies to intracellular antigens (mainly Hu, Ma2). Except for some patients with Ma2 antibodies, the outcome was considered poor.1,2 Currently, a growing number of immune responses against cell surface neuronal receptors are being described in patients previously considered antibody-negative.3–5 One of these new antigens is the GluR1/2 alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (or AMPA receptor [AMPAR]).6 Recognizing the syndrome associated with AMPAR antibodies is important because symptoms are often fully reversible. Here we report the clinical features of one of the patients whose serum and CSF were used to isolate this antigen.
Case reports.
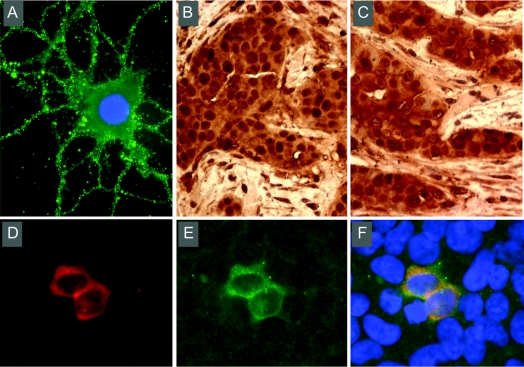
A 67-year-old woman came to our attention because of behavior and memory problems. She had been well until March 2008, when a right breast ductal infiltrating adenocarcinoma (T1, N1, M0) was diagnosed after routine screening mammography. In the ensuing hours after breast surgery she developed confusion, hypersomnia, visual hallucinations, and combativeness. On the following days she improved slightly, and was discharged. Due to persistent symptoms she was admitted to the neurology ward 2 weeks later. On examination she was calm, alert, and cooperative, with a mild depressed affect. She had decreased verbal fluency, but language function was otherwise normal. She knew her name and recognized family members without difficulty. She knew that she was in the hospital and was oriented to the year, but not to the day and month. She was able to count backwards and recite the months in reverse. Memory testing showed that she was unable to recall words, pictures, faces, or short stories after 5 minutes. She was also unable to recall details of her daily life in the hospital. She could not remember any event of the previous 3 years. The remainder of the neurologic and physical examination was unremarkable. Routine serum analyses were normal, including thyroid hormones, antithyroid antibodies, antinuclear antibodies, and vitamin B12 and folic acid levels. HIV and syphilis serology were negative. Brain MRI demonstrated very mild bilateral fluid-attenuated inversion recovery medial temporal lobe hyperintensities. EEG showed transient bilateral temporal sharp waves without clinical seizures. CSF analysis revealed 32 white blood cells/mm3 (90% lymphocytes), normal protein and glucose levels, absent oligoclonal bands, and negative cytology for neoplastic cells. CSF studies for syphilis, herpes simplex 1 and 2, human herpesvirus 6, and mycobacterium tuberculosis were negative. Paraneoplastic antibody studies in serum and CSF were negative. Studies for novel antibodies revealed serum and CSF reactivity with cell surface antigens predominantly expressed in the neuropil of hippocampus; further characterization using reported techniques6 demonstrated the antigens to be the GluR2 subunit of the AMPAR. Paraffin sections of the patient's tumor showed robust expression of GluR1/R2 (figure).
Figure GluR2 antibody specificity and expression of GluR2 in the patient's tumor
(A) Reactivity of patient's CSF (diluted 1:10) with nonpermeabilized cultures of rat hippocampal neurons developed with immunofluorescence (×800). Note the intense immunolabeling of the neuronal cell surface. (B, C) Patient's tumor immunolabeled with patient's biotinylated immunoglobulin G (IgG) (B) and a mouse monoclonal antibody against GluR2 (MAB397, Chemicon) (C) developed with immunoperoxidase (×200). Note the similar pattern of reactivities between the patient's IgG and the GluR2 antibody. No reactivity was detected with IgG from a normal individual (not shown). (D, E) Immunofluorescence of HEK293 cells (×400) transfected with GluR2 reacting with the GluR2 monoclonal antibody (D) and the patient's antibody (E). Merged reactivities are shown in (F). All techniques used for these studies have been previously reported by the authors.4,6
The patient was discharged after receiving a 5-day course of high-dose IV immunoglobulins (2 g/kg) followed by chemotherapy with Adriamycin and cyclophosphamide. Three months later, her memory had improved, but she had persistent severe apathy. Task planning was poor. Her mood was depressed and she had severe insomnia. Formal neuropsychological evaluation showed decreased spontaneous speech production and low scores on verbal fluency tests (word fluency FAS test score: 18, “set test” of Isaacs global score: 9). The rest of the examination was normal, except for a retrograde amnestic gap of 2 years. At the 1-year follow-up the serum GluR1/2 AMPAR antibody titers were undetectable. Her mood and neuropsychological evaluation were normal but she had partial amnesia of the illness and the previous 2 years.
Discussion.
In a series of 45 patients with paraneoplastic or idiopathic LE, the presence of antibodies only directed to cell surface antigens (including NMDA receptors, VGKC, and yet to be identified antigens) correlated with better outcome.5 One of these antigens was recently identified as the GluR1/2 AMPAR, which are the predominant subtype of AMPAR in the hippocampus.6 Patients' antibodies caused a decrease of pre- and postsynaptic GluR1/2 receptor clusters in cultures of rat hippocampal neurons. Given that the levels of receptors were more affected at synapses than along dendrites, the findings suggested a mechanism whereby patients' antibodies disrupted receptor trafficking/turnover, relocating them from synaptic to extrasynaptic sites/intracellular pool. These effects are similar to neuronal plasticity models that decrease synaptic strength, also called long-term depression.7 The effects of the antibodies were shown to be reversible. Of interest, our patient's ability to form new memories returned as the AMPA antibody titer decreased.
Study funding: Supported by FIS-PI06/0804 and NIH/NCI RO1CA107192 (J.D.).
Disclosure: Dr. Bataller, Dr. Galiano, Dr. García-Escrig, Dr. Martínez, Dr. Sevilla, Dr. Blasco, and Dr. Vílchez report no disclosures. Dr. Dalmau has received honoraria for lectures not funded by industry; receives research support from EUROIMMUN and the NIH/NCI [RO1CA107192 (PI) and RO1CA89054-06A2 (PI)]; has received license fee payments from EUROIMMUN for an NMDA receptor autoantibody test (patent pending PCT/US07/18092, filed: August 15, 2007); and has received royalty payments and may accrue revenue for US Patent 6,387,639, issued: May 14, 2002: Patent for Ma2 autoantibody test.
Received July 27, 2009. Accepted in final form November 16, 2009.
Address correspondence and reprint requests to Dr. Luis Bataller, Service of Neurology, Hospital Universitari la Fe, Avenida de Campanar 21, 46009 Valencia, Spain; bataller_lui@gva.es
&NA;
- 1.Gultekin SH, Rosenfeld MR, Voltz R, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain 2000;123:1481–1494. [DOI] [PubMed] [Google Scholar]
- 2.Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2 associated encephalitis. Brain 2004;127:1831–1844. [DOI] [PubMed] [Google Scholar]
- 3.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 2005;128:1764–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graus F, Saiz A, Lai M, et al. Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology 2008;7:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies associate with limbic encephalitis and alter receptor localization. Ann Neurol 2009;65:424– 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann NY Acad Sci 2003;1003:1–11. [DOI] [PubMed] [Google Scholar]