Abstract
In atherosclerosis and Alzheimer’s disease, deposition of the altered-self components oxidized low-density lipoprotein (LDL) and β-amyloid triggers a protracted sterile inflammatory response. Although chronic stimulation of the innate immune system is believed to underlie the pathology of these diseases, the molecular mechanisms of activation remain unclear. Here we show that oxidized LDL and β-amyloid trigger inflammatory signaling through a heterodimer of Toll-like receptors 4 and 6. Assembly of this novel heterodimer is regulated by signals from the scavenger receptor CD36, a common receptor for these disparate ligands. Our results identify CD36-TLR4-TLR6 activation as a common molecular mechanism by which atherogenic lipids and β-amyloid stimulate sterile inflammation and suggest a new model of TLR heterodimerization triggered by co-receptor signaling events.
The innate immune system protects the organism by discriminating molecular structures that are normally absent in the healthy host. Sensing of microbial or modified-endogenous ligands is mediated by germ line encoded pattern recognition receptors, such as the scavenger and Toll-like receptors (TLR), which recognize conserved non-self motifs1. Over the last decade, intense study of the mammalian TLRs has illuminated their critical role in the transcriptional regulation of genes essential for host defense against pathogens. The 12 members of this family are characterized by an extracellular leucine-rich repeat (LRR) domain and a cytoplasmic Toll-interleukin-1 receptor (TIR) domain that initiates signal transduction2. Upon activation, TLR signaling is propagated by recruitment of TIR-containing adaptor molecules such as MyD88, TIRAP (also called MAL), TRIF and TRAM, that link to conserved signaling pathways activating NF-κB- and interferon-regulated genes2. While these downstream signaling pathways have been well defined, the mechanisms facilitating ligand recognition by the TLRs remain poorly understood.
A notable aspect of the mammalian TLR family is the ability of this restricted group of receptors to recognize a wide spectrum of pathogen ligands from bacteria, viruses, fungi and protozoa, including lipopolysaccharide (LPS), flagellin, CpG DNA and viral RNA2. While the early identification of TLR2-TLR1 and TLR2-TLR6 heterodimers led to the speculation that combinatorial signaling among the TLRs might account for the breadth of this repertoire3, no other functional TLR heterodimers have been described to date. However, the recent identification of co-receptors that enhance TLR function suggest an emerging paradigm in which TLRs act together with other cell surface molecules to bridge microbe recognition and initiation of signaling responses. A prime example of this is the well-studied TLR4 co-receptor, CD14, which acts with soluble components such as LPS-binding protein and MD-2 to recognize LPS1.
The class B scavenger receptor CD36, as well as Dectin-1, integrin β3 and mannose binding lectin can coordinate responses to TLR2 agonists4-8. CD36 is an archetypal pattern recognition receptor that binds polyanionic ligands of both pathogen and self-origin. In addition to its recently ascribed function as a TLR2-TLR6 co-receptor for Staphylococcus aureus-derived lipoteichoic acid (LTA) and Mycoplasma macrophage-activating lipopeptide-2 (MALP-2)4,5, CD36 has established roles in the endocytic uptake of altered self-components, including oxidized phospholipids, apoptotic cells and amyloid proteins9. While important for the clearance of these host molecules from the circulation and tissues, CD36-dependent signaling has also been implicated in the pro-inflammatory effects of these modified endogenous ligands10-13. These observations led us to investigate whether CD36 cooperates with members of the TLR family to activate the innate immune response to sterile ligands that accumulate in atherosclerosis and Alzheimer’s disease plaques.
We demonstrate that recognition of oxidized low density lipoprotein (LDL) and β-amyloid peptide by CD36 triggers assembly of a novel heterotrimeric complex composed of CD36-TLR4-TLR6. Importantly, we determine that the earliest event in this inflammatory cascade is not ligation of the TLRs but rather CD36-mediated recognition, which signals via Src kinases to induce a previously undescribed TLR heterodimer of TLR4-TLR6. TLR4-TLR6 signaling is propagated by both the MyD88 and TRIF adaptors leading to the induction of proinflammatory mediators implicated in the deleterious effects of oxidized LDL (oxLDL) and β-amyloid in vivo, including the neurotoxicity that is pathognomonic of Alzheimer’s disease. These results identify a common molecular mechanism underlying innate immune activation in atherosclerosis and Alzheimer disease. Moreover, our data suggest a new model of TLR heterodimerization, regulated by signals from CD36 that acts as the common ligand binding receptor for oxLDL and β-amyloid. These observations support the notion that co-receptors can facilitate assembly of certain TLR heterodimers, and suggest that others may be identified if, as we demonstrate herein, the TLRs are co-expressed with the relevant co-receptors.
RESULTS
Cooperative signaling of TLR4 and TLR6
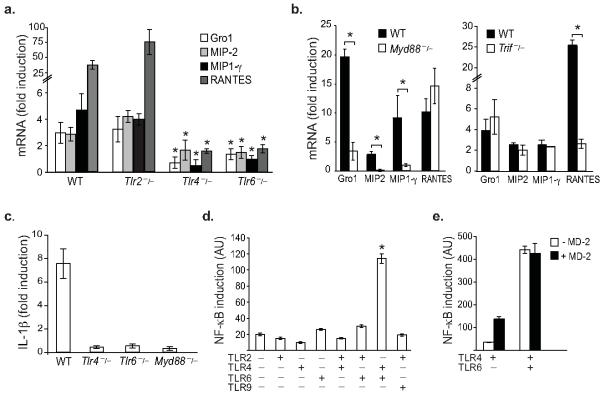
Lipoproteins accumulate abnormally in the artery wall during hypercholesterolemia setting off a cascade of pro-inflammatory events, particularly the expression of chemokines that initiate an influx of monocytes14. While this inflammation is sterile in nature, production of these inflammatory mediators requires the common Toll-interleukin-1 receptor signaling adaptor, MyD8815,16. To search for the upstream TLRs required for pro-atherosclerotic chemokine production, macrophages deficient in various TLRs were stimulated with a key inflammatory component of atherosclerotic plaques, oxLDL. Array screening of a complete panel of chemokines identified 4 genes induced by oxLDL but not native LDL (Supplementary Fig. 1) in wild type macrophages (Supplementary Table 1). In response to oxLDL, these cells show 3-30 fold increases in Gro1 (Cxcl1), MIP-2 (Cxcl2), MIP-1γ (Ccl9) and RANTES (Ccl5) mRNA (Fig. 1a). By contrast, macrophages deficient in TLR4 or TLR6, but not TLR2, failed to increase expression of these chemokines (Fig. 1a). Transcriptional upregulation of Gro1, MIP-2, MIP-1γ was similarly abolished in macrophages lacking MyD88, whereas oxLDL induction of RANTES mRNA required the alternative TLR adaptor TRIF (Fig. 1b). In addition to these chemokines, TLR4, TLR6 and MyD88 were also essential for upregulated expression of the cytokine interleukin-1β (IL-1β) in response to oxLDL (Fig. 1c). A notable consequence of MyD88 signaling is the activation of the transcriptional regulator NF-κB. A cell-based NF-κB-luciferase reporter assay was used to determine the requirements for oxLDL signalling via TLR4 and TLR6. Whereas oxLDL was unable to induce expression of a reporter construct driven by NF-κB in HEK293 cells expressing TLR2, TLR4 or TLR6 individually, a 6-fold increase in NF-κB activation was observed in cells co-expressing TLR4 and TLR6 (Fig. 1d). The signalling cooperation of TLR4 and TLR6 was specific, as oxLDL did not increase NF-κB-dependent reporter expression in cells co-expressing TLR2-TLR6, TLR2-TLR4 or TLR4-TLR9 (Fig. 1d). Notably, TLR4-TLR6-mediated NF-κB activation occurred in the absence of MD-2, an essential co-factor of the TLR4 complex that recognizes LPS (Fig. 1d), and was not augmented by the addition of MD-2 (Fig. 1e). Moreover, TLR4-TLR6 signaling was not altered in the presence of polymyxin B or the MD-2 inhibitor B-1287, which block the biological effects of LPS (Supplementary Fig. 1), further indicating that our observations were not due to LPS contamination. These data demonstrate that TLR4 and TLR6, acting together through MyD88 and TRIF-dependent signaling pathways, are required for macrophage chemokine production in response to atherogenic lipids, invoking the existence of a hitherto undescribed TLR heterodimer.
Fig 1.
TLR4 and TLR6 cooperatively mediate the macrophage inflammatory response to oxLDL.
OxLDL-induced chemokine gene expression in (a) wild-type, Tlr2−/−, Tlr4−/−, Tlr6−/− and (b) Myd88−/− and Trif−/− macrophages. Gene expression was analyzed by QRT-PCR and the fold increase in mRNA levels in oxLDL stimulated cells (50 μg/ml, 12h) compared to unstimulated cells is reported (*p<0.05, compared to wild type). (c) Expression of IL-1β mRNA in wild type, Tlr4−/−, Tlr6−/− and Myd88−/− macrophages stimulated with oxLDL (50 μg/ml, 12h). (d) OxLDL-induced expression of an NF-κB luciferase reporter gene in HEK293 cells expressing the indicated TLRs. (e) Effect of sMD-2 on oxLDL-induced NF-κB luciferase reporter gene expression. All data are mean ± s.d. of triplicate samples and are representative of an experimental n of 3-4.
CD36 is a TLR4-TLR6 co-receptor
To understand how oxLDL acts as a TLR-agonist the roles of CD14 and CD36, two lipid-binding receptors known to be TLR-co-receptors, were tested. Notably, whereas the prototypic TLR4 co-receptor CD14 did not augment oxLDL-induced NF-κB activation, CD36 increased NF-κB-driven reporter expression 3-fold in TLR4-TLR6 expressing cells (Fig. 2a). Furthermore, as observed in Tlr4−/− and Tlr6−/− macrophages, CD36-deficient macrophages failed to upregulate Gro1, MIP-2 and RANTES transcription in response to oxLDL (Fig. 2b). To determine whether CD36 is also required for induction of these inflammatory mediators in vivo, we performed gene expression profiling of atherosclerosis-prone Apoe−/− and Cd36−/−Apoe−/− mice fed a western diet for 12 weeks. Gro1, MIP-2 and RANTES mRNA expression was greatly reduced in the descending aorta of mice lacking CD36 (Fig. 2c), which showed a corresponding decrease in atherosclerotic lesion formation (Supplementary Fig. 2). Thus CD36, like MyD8815, is a key component of the signaling pathway that upregulates the expression of these pro-atherosclerotic chemokines in vivo. To further explore the role of CD36, TLR4 and TLR6 in promoting macrophage inflammatory responses to oxLDL, the requirement of this signaling complex in the production of reactive oxygen species (ROS) was measured. Wild-type macrophages exposed to oxLDL (Fig. 2d), but not native LDL (Supplementary Fig. 2), exhibit a robust respiratory burst and consequently, this heightens oxidative stress in the artery wall. Whereas Cd36−/−, Tlr4−/− and Tlr6−/− macrophages exhibit a competent respiratory burst in response to zymosan, as previously described8,11, these cells fail to produce ROS when stimulated with oxLDL (Fig. 2d). Collectively, these data demonstrate that Cd36−/− macrophages phenocopy Tlr4−/− and Tlr6−/− cells, suggesting that these receptors act in a common signaling pathway to initiate macrophage inflammatory responses to oxLDL.
Fig 2.
The co-receptor CD36 is required for TLR4-TLR6-dependent responses. (a) Effect of CD36 and CD14 on oxLDL-induced expression of an NF-κB luciferase reporter gene in TLR4-TLR6 expressing HEK293 cells. (b-c) Chemokine gene expression analyzed by QRT-PCR in (b) wild type and Cd36−/− macrophages stimulated with oxLDL (50 μg/ml, 6h), and (c) aortas of Apoe−/− and Cd36−/−Apoe−/− mice (n=3) fed a high fat diet for 12 weeks to induce atherosclerotic lesion formation. (d) Reactive oxygen species production in wild type, Tlr4−/−, Tlr6−/− and Cd36−/− macrophages stimulated with oxLDL (50 μg/ml) or zymosan (1 μg/ml) for 45 min. Data are the mean ± s.d. of triplicate samples in a single experiment and are representative of an experimental n of 3. *p≤0.05.
β-amyloid peptide triggers CD36-TLR4-TLR6 signaling
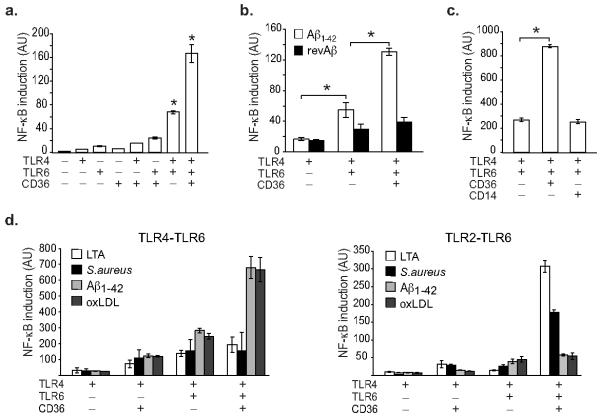
Like atherosclerosis, Alzheimer’s disease is characterized by a protracted inflammatory response driven by cells of the monocyte lineage in the brain called microglia17. Moreover, the known inflammatory component of Alzheimer’s disease, β-amyloid, has also been shown to bind CD3611. We therefore tested whether fibrillar β-amyloid peptide (1-42) (Aβ1-42) could activate TLR4-TLR6 signaling via CD36. Notably, Aβ1-42 induced a 3-5-fold activation of an NF-κB-dependent luciferase gene in HEK293 cells co-expressing TLR4 and TLR6 (Fig. 3a), whereas the control peptide reverse Aβ 42-1 was unable to activate NF-κB (Fig. 3b). The additional expression of CD36 in these cells caused a 2-3-fold amplification of TLR4-TLR6-signaling, however activation of NF-κB was not augmented by CD14 (Fig. 3a, c). Aβ1-42 was unable to induce expression of an NF-κB-dependent luciferase gene in HEK293 cells co-expressing other TLR pairs including TLR2-TLR6, TLR2-TLR4 or TLR4-TLR9 (data not shown). These data indicate that CD36 recognition of Aβ1-42 and oxLDL triggers TLR signaling via a shared pathway. Of note, this cooperation differs from that used by the bacterial ligands of CD36, S. aureus and LTA, which preferentially activate TLR2-TLR6-dependent signalling (Fig. 3d), suggesting that endogenous ligands of CD36 specifically trigger the TLR4-TLR6 heterodimer. Moreover, because simultaneous expression of CD36, TLR4 and TLR6 was required for maximal induction of these inflammatory responses, our data suggest that a macromolecular signaling complex containing these molecules mediates the innate immune response to endogenous host ligands.
Fig 3.
The Alzheimer’s disease peptide β-amyloid activates CD36-TLR4-TLR6 signaling.
(a-b) β-amyloid induced expression of an NF-κB luciferase reporter gene in HEK293 cells expressing the indicated receptors. Cells were stimulated with (a) Aβ1-42 or the (b) control peptide revAβ (10 μM, 5 h) and luciferase activity was measured. (c) Effect of CD36 and CD14 on Aβ1-42-induced expression of an NF-κB luciferase reporter gene in TLR4-TLR6 expressing HEK293 cells. (d) Comparison of the ability of endogenous and microbial ligands of CD36 to activate an NF-κB luciferase reporter gene in HEK293 cells expressing TLR4-TLR6 and TLR2-TLR6 heterodimers. Cells were stimulated with LTA (1 μg/ml), S. aureus (10:1), Aβ1-42 (10 μM) or oxLDL (50 μg/ml) and luciferase activity was measured. Data are the mean ± s.d. of triplicate samples and are representative of an experimental n of 3. *p≤0.05.
TLR4-TLR6 signaling promotes microglial activation
In Alzheimer’s disease, microglia surrounding β-amyloid-containing plaques acquire an activated morphology and secrete inflammatory mediators, including cytokines and reactive oxygen and nitrogen species that promote the neurotoxicity that is pathognomonic of this disease17. To determine whether signaling via CD36-TLR4-TLR6 is involved in the activation of microglia by β-amyloid, we generated microglial cell lines deficient in CD36, TLR2, TLR4 and TLR6. Functional characterization of these microglial cell lines showed that they were similar to primary mouse microglial preparations in morphology, expression of cell surface markers and function, including appropriate TLR responses to microbial ligands (Supplementary Fig. 3 and 18). Consistent with published reports, Aβ1-42 induced secretion of nitric oxide (NO) and ROS by wild-type microglial cells (Fig. 4a-c). Notably, these inflammatory responses were abrogated in microglia deficient in CD36, TLR4 or TLR6, but not TLR2 (Fig. 4a, c). Moreover, Aβ1-42 induced secretion of NO in Md2−/− microglia was comparable to wild-type microglia, whereas these cells exhibited impaired NO production when stimulated with LPS (Fig. 4b). These findings provide functional evidence of the importance of the CD36-TLR4-TLR6 signaling complex in regulating the microglial innate immune response to Aβ1-42.
Fig. 4.

CD36-TLR4-TLR6 signaling induces microglial inflammatory responses that promote neurotoxicity. (a-b) Production of nitric oxide in (a) wild type, Tlr2−/−, Tlr4−/−, Tlr6−/−, Cd36−/− and (b) Md2−/− microglia stimulated with Aβ1-42 (10 μM) or LPS (100ng/ml) for 24 hours. (c) Reactive oxygen species production in microglia of the indicated genotype stimulated with Aβ1-42 (10 μM) for 45 min. Data are the mean ± s.d. of triplicate samples and are representative of an experimental n of 3. *P<0.005. (d) IL-1β and (e) RANTES mRNA measured by QRT-PCR in wild type, Tlr4−/−, Tlr6−/−, Myd88−/− or Trif−/− macrophages stimulated with Aβ1-42 (10 μM, 6h). Data are the mean ± s.d. of triplicate samples and are representative of an experimental n of 2-3. *P<0.05. (f) CAD mouse neuronal cells co-cultured with wild type, Tlr2−/−, Tlr4−/− and Tlr6−/− microglial cells stimulated with Aβ1-42 (10 μM, 72 h) and stained with anti-neuronal class III beta tubulin Ab (green) and DAPI nuclear stain (blue). Quantification of % neuronal survival is shown at right.
TLR4-TLR6 activation primes microglia for IL-1β production
A critical component of the microglial inflammatory response to β-amyloid is the secretion of the inflammatory cytokine IL-1β18. Elevated amounts of this cytokine are detected in the brains and cerebrospinal fluid of individuals affected with Alzheimer’s disease17. We previously established that CD36 is required for Aβ1-42 upregulation of IL-1β mRNA by microglia11, thus we sought to determine whether TLR4-TLR6 signaling is involved in the initiation of this response. Wild-type microglia exposed to Aβ1-42 exhibit a 4-fold increase in IL-1β mRNA (Fig. 4d). Notably, no increase in IL-1β mRNA expression was observed in similarly treated Tlr4−/−, Tlr6−/− and Myd88−/− microglia indicating an essential role for these components in the signaling cascade leading to IL-1β mRNA upregulation (Fig. 4d). In contrast, while TRIF was not required for Aβ1-42 upregulation of IL-1β mRNA (Fig. 4d), it was essential for TLR4-TLR6-dependent induction of the chemokine RANTES by Aβ1-42 (Fig. 4e). Because IL-1β, as well as NO and ROS, have been implicated in the cytotoxic effects of β-amyloid on surrounding neurons18,19, we determined whether Aβ1-42-CD36-TLR4-TLR6 signaling in microglia promotes neuronal dysfunction. As reported previously, incubation of a mouse neuronal cell line with Aβ1-42 alone induced only minimal morphological effects (data not shown)18. In contrast, Aβ1-42 induced widespread neuronal cell death in co-cultures of neurons and wild-type microglial cells (Fig. 4f). Strikingly, no neurotoxic effects of Aβ1-42 were observed in such co-cultures containing Tlr4−/− and Tlr6−/− microglia, consistent with the failure of these microglia to produce IL-1β, NO and ROS (Fig. 4f). However, neurons in co-cultures containing Tlr2−/− microglia were not protected from Aβ1-42-induced cell death. Taken together, these data indicate that TLR4-TLR6 signaling promotes microglial inflammatory responses associated with Alzheimer’s disease pathology and link sterile inflammation in atherosclerosis and Alzheimer’s disease to the activation of a common signalling complex composed of CD36, TLR4 and TLR6.
CD36 regulates TLR4-TLR6 complex formation
To understand how this heterotrimeric signaling complex assembles, we examined the association of CD36, TLR4 and TLR6 in THP-1 monocytes stimulated with oxLDL or Aβ1-42 by immunoprecipitation. Stimulation of THP-1 monocytes with CD36 ligands rapidly induced co-precipitation of CD36 with TLR4 and TLR6 (Fig. 5a, b). To further define the interaction of CD36, TLR4 and TLR6, the localization of these receptors was examined by confocal microscopy in HEK293 cells transfected with these receptors. Minimal co-localization of CD36, TLR4 and TLR6 was seen in resting cells, but upon treatment with oxLDL, CD36, TLR4 and TLR6 co-localized predominantly in intracellular compartments (Fig. 5c). This intracellular co-localization prompted us to test whether endocytosis of oxLDL or Aβ1-42 was required for signaling via this complex. CD36 and TLR4 are both internalized from the plasma membrane via dynamin-dependent endocytosis that can be specifically blocked using the inhibitor ‘Dynasore’20,21. Treatment of macrophages with Dynasore blocked oxLDL uptake and the induction of chemokine expression (Fig. 5d), suggesting that signaling from the CD36-TR4-TLR6 complex also requires dynamin-dependent endocytosis. Moreover, Dynasore treatment of microglia abolished Aβ1-42-upregulation of IL-1β mRNA (Fig. 5e), consistent with the idea that signaling from the CD36-TR4-TLR6 complex is triggered following ligand internalization.
Fig 5.
CD36-ligand induced TLR4-TLR6 complex formation. (a-b) Immunoblot analysis of CD36-precipitated proteins from THP-1 monocytes treated with oxLDL (50 μg/ml) or Aβ1-42 (50 μg/ml) for the indicated times. Precipitated proteins were immunoblotted with antibodies to TLR4, TLR6 and CD36. (c) Fluorescence microscopy of HEK293 cells transfected with CD36 and fluorescently tagged TLR4-YFP and TLR6-CFP. Cells were unstimulated (left) or treated with oxLDL (right) for 30 min, and stained with an anti-CD36 Alexa647 conjugated antibody. In the false-colored merged image triple co-localization of TLR4 (blue), TLR6 (red) and CD36 (green) appears as areas of white (arrowheads). Expression of individual receptors is shown in bottom panels. Scale bar 10 μm. (d-e) Inhibition of dynamin-dependent endocytosis blocks CD36-TLR4-TLR6-signaling in macrophages and microglia. QRT-PCR analysis of gene expression in (d) macrophages stimulated with oxLDL (50 μg/ml, 6h) or (e) microglia stimulated with Aβ1-42 (10 μM, 6h) in the presence and absence of the dynamin inhibitor dynasore (80 μM).
To further explore how CD36 regulates TLR4-TLR6 complex formation, mutational analysis of CD36 was performed to identify regulatory sequences essential for TLR4-TLR6 activation. Notably, mutation of the CD36 cytoplasmic C-terminus, a domain postulated to interact with kinases, abrogated oxLDL-induced signaling. While not altering cell surface localization of CD36, selective replacement of residues 460-472 with alanines (CD36Ala) impaired the ability of CD36 to induce NF-κB-dependent luciferase gene expression in HEK293 cells co-expressing TLR4 and TLR6 (Fig. 6a). Furthermore, mutation of Y463 but not the adjacent cysteine (C464) within this domain of CD36, blocked ligand-induced NF-κB-activation (Fig. 6a). Y463 of CD36 was also required for TLR4-TLR6 co-precipitation (Fig. 6b). Whereas oxLDL markedly augmented co-precipitation of TLR4 and TLR6 in HEK293 cells co-expressing wild-type CD36, co-expression of CD36Y463F with these TLRs was insufficient to induce association of TLR4 with TLR6 (Fig. 6b). These data identify Y463 of CD36 as a critical regulator of TLR4-TLR6 dimerization and signaling. We next sought to identify potential interacting partners for this domain using His-tagged peptides corresponding to the native CD36 C-terminus (residues 460-472) or a scrambled version. Immunoblotting of proteins precipitated with these peptides identified three major phosphotyrosine proteins that bound the native CD36 C-terminal peptide but not a scrambled version, including FAK (125 kDa), Paxillin (68 kDa) and a protein of approximately 60 kDa (Supplementary Fig. 4). Using HEK293 cells overexpressing CD36 as a model system, we identified this ≈60 kDa CD36 binding protein as the kinase Lyn. As observed in macrophages12, CD36 immunoprecipitated preferentially with Lyn, but not Src, the prototypic Src kinase (Fig. 6c). A peptide corresponding to the C-terminus of CD36 was sufficient to mediate the interaction with Lyn, and as shown by peptide competition, this binding required the MISY motif (amino acids 460-463) encompassing Y463 (Fig. 6c). To test the functional role of Lyn in CD36-TLR4-TLR6 signaling, the effect of tyrosine kinase inhibitors on NF-κB activation and CD36-TLR4-TLR6 complex formation was assessed. The general tyrosine kinase inhibitor Genistein, but not its inactive analog Daidzen or inhibitors of PI3 kinase (LY294002 and Wortmannin), blocked CD36-TLR4-TLR6-dependent activation of NF-κB-reporter gene expression (Fig. 6d) Notably, treatment with the Lyn kinase inhibitor PP1, but not the inactive analog PP3, replicated these inhibitory effects on CD36-TLR4-TLR6 signaling and blocked the physical association of CD36 with TLR4 and TLR6 in oxLDL-treated monocytes (Fig. 6d, e). Collectively, these data support a role for CD36-Lyn kinase interactions initiated at Tyr463 in facilitating TLR4-TLR6 heterodimerization and signal initiation. Because Lyn is implicated in the phosphorylation of the TLR4 TIR domain and activation of TLR4 signal transduction22, we postulate that CD36 delivery of Lyn during complex formation may promote TLR4-TLR6 signaling in a similar manner.
Fig. 6.
TLR4-TLR6 activation is triggered by a membrane proximal signaling event initiated by CD36. (a) Effect of wild type or C-terminal domain mutants of CD36 (CD36Ala, CD36Y463F, CD36C464S) on oxLDL-induced expression of an NF-κB luciferase reporter gene in TLR4-TLR6 expressing HEK293 cells. (b) Immunoblot analysis of TLR4-precipitated proteins from HEK293 cells transfected with TLR4-tagged with a fluorescent protein (YFP), TLR6 and wild type CD36 or CD36Y463F. Cells were untreated or treated with oxLDL (50 μg/ml) for 15 min and TLR4-precipitated proteins were immunoblotted with antibody to TLR6 or TLR4 (α-GFP). (c) CD36 immunoblot of Src- or Lyn-precipitated proteins from empty vector or CD36 transfected HEK293 cells (upper panel). Lyn immunoblot of proteins precipitated by a His-tagged CD36 C-terminal peptide in the presence or absence of soluble peptide competitors corresponding to the terminal 6, 9, or 13 amino acids of CD36 (460-472). (d) Effect of kinase inhibition on oxLDL-induced expression of an NF-κB luciferase reporter gene in CD36-TLR4-TLR6 expressing HEK293 cells. Cells were treated with oxLDL (50 μg/ml, 5 h) in the presence of kinase inhibitors, [Genistein (5 μM); PP1 (10 μM); LY294002 (LY, 5 μM)]; Wortmannin (10 nM) or inactive analogs [Daidzen (5 μM); PP3 (10 μM)] and cellular luciferase levels were measured. (e) Effect of kinase inhibition on co-precipitation of CD36 with TLR4 and TLR6 in THP-1 monocytes. Immunoblot analysis of CD36-precipitated proteins from THP-1 monocytes treated with oxLDL (50 μg/ml) in the absence or presence of kinase inhibitors described in (d). Data in all experiments are representative of three separate experiments. Data in a, d are the mean ± s.d. of triplicate samples (**p≤0.005).
DISCUSSION
The discovery of mammalian TLRs and their critical role in detecting pathogen associated molecular patterns has greatly expanded our understanding of the innate immune response to infection. By contrast, the molecular mechanisms underlying sterile inflammatory conditions remain less well understood. Atherosclerosis and Alzheimer’s disease are characterized by a protracted sterile inflammatory response in which cells of the monocyte lineage accumulate prominently in plaques that underlie the pathology of these diseases14,17. A common characteristic of these age-related diseases is the anomalous deposition of modified self-components in affected tissues: oxidized LDL and β-amyloid peptide accumulate in plaques of the artery wall and brain respectively, and are believed to fuel the recruitment and activation of mononuclear cells. Our work identifies a previously undescribed TLR heterodimer composed of TLR4 and TLR6 that senses these endogenous ligands and initiates innate immune activation of macrophages and microglia. Notably, this pathway is triggered through binding of atherogenic lipids and β-amyloid to a shared receptor, CD36, that provides the proximal signalling event required for TLR4-TLR6 activation. Together, this heterotrimeric signalling complex regulates the expression of proinflammatory mediators directly implicated in the deleterious effects of these modified self-components in vivo, including chemokines and reactive oxygen and nitrogen species. Additionally, CD36-TLR4-TLR6 signaling in macrophages and microglia results in pro-IL1β transcription, priming these cells for inflammasome activation and IL-1β secretion. This work thus provides the molecular mechanism of how these endogenous ligands can act as TLR mimetics and identifies a common means for chronic mononuclear cell activation in two important age-related chronic inflammatory diseases.
Targeted deletion of the TLR adaptor MyD88 in a mouse model of atherosclerosis causes a dramatic reduction in atherosclerotic lesion size, attributable in part to decreased chemokine expression in the aorta15. Upstream activation of TLR4 or TLR2 has been implicated in this MyD88-dependent inflammation of the artery wall16,23,24, however the mechanism by which these microbial sensing pathways are triggered and the ligands responsible are not known. Notably, we find that oxLDL induced CD36-TLR4-TLR6 signaling in macrophages triggers a MyD88-dependent chemokine signature similar to that observed in vivo, and additionally elicits expression of the TRIF-dependent chemokine RANTES. Consistent with these findings, expression of these chemokines is reduced in the aortae of hyperlipidemic Apoe−/− mice lacking CD36, emphasizing the important role of this co-receptor in facilitating TLR4-TLR6 signaling. In addition to oxLDL-induced chemokine expression, MyD88-dependent signaling initiated by CD36-TLR4-TLR6 activates the macrophage respiratory burst and production of reactive oxygen species. This innate defense function of macrophages amplifies oxidative stress in the artery wall, thereby promoting the generation of oxidized lipoprotein ligands and chronic macrophage activation. Our identification of a CD36-TLR4-TLR6 complex that regulates these inflammatory responses to oxidized LDL thus provides mechanistic insight into how MyD88-dependent signaling is triggered during atherogenesis.
The inflammatory cytokine IL-1β is found prominently in atherosclerotic and Alzheimer’s disease plaques14,17, and targeted inhibition of this cytokine reduces markers of disease18,25,26. The secretion of IL-1β is a two step process requiring both transcriptional upregulation of pro-IL-1β via NF-κB and activation of a cytosolic multiprotein complex called the inflammasome that processes pro-IL-1β protein to its mature form27. The recent identification of β-amyloid peptide18 and cholesterol crystals (E. Latz, personal communication) as activators of the NALP-3 inflammasome has emphasized the importance in understanding the regulation of pro-IL-1β expression in atherosclerosis and Alzheimer’s disease. Monocytes deficient in CD36 show impaired IL-1β expression in response to oxLDL and β-amyloid10,11. Our work now identifies TLR4-TLR6 as critical components of the signaling pathway that primes macrophages and microglia for pro-IL-1β expression. These data identify signaling via this novel CD36-TLR4-TLR6 complex as the “first hit” in the induction of this highly regulated cytokine and we show that disruption of TLR4-TLR6 signaling in microglia abrogates IL-1β, nitric oxide, and reactive oxygen production and protects surrounding neurons from β-amyloid-induced death. TLR4 expression in the brain is increased in a mouse model of Alzheimer’s disease28, and consistent with our model, targeted deletion of TLR4 blocks the upregulation of cerebral IL-1β amounts and microglial production of nitric oxide28,29. Although the role of IL-1β and neuroinflammation in Alzheimer’s disease remains controversial30, it is notable that a TLR4 polymorphism that attenuates receptor signalling (Asp299Gly) is associated with decreased risk of atherosclerosis, acute coronary events and Alzheimer’s disease31-33.
Although we focus on two endogenous ligands involved in sterile inflammatory processes, this TLR heterodimer may also recognize as yet unidentified pathogen associated molecular patterns as several microbial ligands for CD36 have been identified4,5. Moreover, this work has also revealed some generally applicable insights into TLR recognition. First, our work identifies a novel TLR heterodimer composed of TLR4-TLR6. We find that atherogenic lipids and β-amyloid initiate signalling that requires co-expression of both TLR4 and TLR6, and is independent of known components of the TLR4 homodimeric complex, such as MD-2 and CD14. This signaling leads to the expression of both MyD88- and TRIF-dependent genes, indicating that TLR4-TLR6 engages both of these adaptor pathways. Second, our data suggest a new model of TLR heterodimerization in which dimer assembly and activation is regulated by signaling initiated from a cell surface co-receptor, CD36. Co-immunoprecipitation assays showed a ligand-dependent physical association of TLR4 and TLR6, requiring expression of functional CD36. The early description of TLR2-TLR1 and TLR2-TLR6 heterodimers led to the speculation that heterodimerization might further diversify the TLR repertoire3. However, it is notable that no other heterodimers with ascribed ligands have since been described. Our identification of a TLR4-TLR6 heterodimer was only made possible because of coexpression with the relevant coreceptor, CD36, which was required for its activation. While several TLR co-receptors, such as CD14 and the vitronectin receptor, have been suggested to concentrate microbial molecules and deliver them to pattern recognition complexes2,6, our data indicate a more active role for CD36 in modulating TLR4-TLR6 signal transduction. The signals that induce TLR4-TLR6 heterodimerization occur from the C-terminus of CD36 within the cell, leading us to compare this to the “inside-out” signaling described for integrins34. Our data are consistent with TLR4-TLR6 signaling being triggered by a proximal membrane event initiated by CD36 interaction with Lyn kinase. This model is supported by the following observations: first, mutation of tyrosine 463 of CD36 blocks its ability to induce TLR4-TLR6 heterodimerization and NF-κB activation; second, tyrosine 463 of CD36 regulates its interaction with Lyn kinase; third, chemical inhibitors of Lyn kinase block CD36-ligand induced TLR4-TLR6 association and activation of NF-κB and fourth, Lyn-deficient macrophages show impaired responses to β-amyloid, including the production of ROS12. Because tyrosine phosphorylation of the TIR domain of TLR4 is required for LPS-signaling22, we suggest that through recruitment of Lyn kinase, CD36 may similarly promote TLR4 and/or TLR6 phosphorylation and activation. This model highlights the importance of co-receptor function in triggering TLR4-TLR6 signaling and suggests that other TLR heterodimers may be identified if, as we demonstrate herein, the TLRs are co-expressed with their relevant co-receptors.
Supplementary Material
ACKNOWLEDGEMENTS
We thank S. Akira (Osaka University) for provision of knock-out mice. This work was supported by the National Institutes of Health (R01AG20255 to K.J.M.; R01NS059005 to J.E.K.; R01 AG032349 to K.J.M./J.E.K.), the Ellison Medical Foundation (K.J.M.), the American Health Assistance Foundation (A2008-130 to K.J.M.) and the Wellcome Trust (068089/Z/02/Z, L.M.S.).
Appendix
METHODS
Mice
C57BL/6 mice were from Jackson Laboratories. Tlr2−/−, Tlr4−/−, Tlr6−/−, Myd88−/− and TRIF−/− mice were provided by Dr. S. Akira (University of Osaka, Japan). Md2−/− mice were from Dr. K. Miyake and Japan Science and Technology Agency. Cd14−/−, Cd36−/−, Apoe−/− and Cd36−/−Apoe−/− mice were generated in our laboratory35-37. Six week old female Apoe−/− and Cd36−/−Apoe−/− mice were fed a western diet (Teklad Adjusted Calories 88137; 21% [w/w] fat; 0.15% [w/w] cholesterol) for 12 weeks and aortas were isolated as described37. All mice were housed and bred under pathogen-free conditions. Animal care and use for all procedures was done in accordance with the USDA Animal Welfare Act and the Public Health Survey Policy for the Humane Care and Use of Laboratory Animals.
Ligands, Reagents and Plasmids
Human LDL from Biomedical Technologies Inc was oxidized as described35. Aβ1-42 and reverse Aβ42-1 peptides from American Peptide Company were prepared as described38. S. aureus-derived LTA was from Sigma-Aldrich. S. aureus and zymosan were prepared as described5,7. pcDNA3.1 vectors containing TLR2-YFP, TLR4-YFP, TLR6-CFP, TLR9-CFP and NF-κB-luciferase reporter (Addgene.org; plasmids 13016, 13018, 13021, 13029, 13642), wild type CD36 and C-terminal mutants of CD36 (CD36Ala, CD36Y463F, CD36C464S) have been described5.
Mononuclear cell culture
The THP-1 cell line was from American Type Culture Collection. Primary peritoneal macrophages and microglia were isolated as described35. Immortalized microglial cell lines were generated from primary mixed glial cultures using J2 recombinant retrovirus as described39,40. Microglial cells were primed with 100 U/ml IFNγ 1h and stimulated in serum free medium. To inhibit dynamin, cells were pretreated with 80 uM Dynasore (Sigma-Aldrich) or dimethyl sulfoxide (control) for 30 min20.
Analysis of chemokine and cytokine expression
Five μg of total RNA was reverse transcribed using MultiScribe™ reverse transcriptase (Applied Biosystems). 1 μl of complementary DNA (1:10 dilution) was used with 0.3 μM of target (Cxcl1, Cxcl2, Ccl9, Ccl5, Il1b) or control (GAPDH) oligonucleotide primers. QRT-PCR was performed using an iQ Real-Time PCR Detection System (Bio-Rad). Relative mRNA expression was calculated using the comparative cycle method (ΔΔCt).
HEK293 cell assays
HEK293 cells were cultured in 6 well plates (6 × 105 cells/well) for 24 h prior to transfection in medium lacking penicillin and streptomycin. For expression of CD36, CD14 and TLRs cells were transfected with pcDNA3.1 vectors contaning the cDNAs overnight in OptiMEM containing Lipofectamine 2000 as described5. After 24 h, the media was changed to DMEM containing penicillin (100 units/ml) and streptomycin (100 μg/ml) and 0-2% FBS. Equivalent cell surface expression of wild type and C-terminal mutants of CD36 (CD36Ala, CD36Y463F, CD36C464S) was confirmed by flow cytometry as described5. For NF-κB assays, cells were co-transfected with an NF-κB-luciferase reporter gene. After stimulation with ligand for 5 hours, cells were lysed and reporter gene activity was measured using the Dual Luciferase Assay Reporter System (Promega)5. Secreted IL-8 was measured in cell culture supernatants by ELISA (R & D Systems). In some experiments, soluble MD-2 from cells stably secreting MD-2 was provided41. For immunofluorescence, HEK293T cells were plated on glass coverslips and transfected with TLR4-YFP, TLR6-CFP and CD36 plasmids as described, and stimulated with 50 μg/ml oxLDL for 30 min. Cells were fixed with ice-cold methanol, treated with 0.5% saponin and stained with Alexa fluor 647 anti-CD36 antibody (1:100 dilution, Biolegend). Immunofluorescence was visualized with a Nikon Exlipse Ti microscope with Ultraview Spinning disc (CSU-X1, Perkin Elmer) with a Plan-apo 60x/1.4 oil objective and appropriate filters. Image acquisition and processing (deconvolution) was performed with Velocity 4.4.1 software.
ROS and NO Production
Reactive oxygen production was measured by nitroblue tetrazolium (NBT) reduction assay and nitric oxide was measured by Griess reaction as described35,18.
Neurotoxicity assay
For assessment of microglia-induced neurotoxicity, CAD mouse neuronal cells42 were seeded on poly-L-lysine coated glass cover slips in F12/DMEM containing ciprofloxacin (Cellgro) and 10% FCS. Neuronal differentiation was induced by serum withdrawal (48 h) and wild type or knock-out (Tlr2−/−, Tlr4−/−, Tlr6−/−) microglia (3 × 104) were seeded into Transwell inserts (5 μm polycarbonate membrane) on top of neuronal cultures. 72 h after stimulation with Aβ1-42 or vehicle, neurons were fixed and stained with mouse anti-neuronal class III beta tubulin antibody (Clone Tuj1) followed by Alexa Fluor® 488 goat anti mouse IgG (Invitrogen) and Hoechst nuclear stain (Invitrogen). Neuronal cells were imaged with a Leica TCS SP2 AOBS laser scanning confocal microscope and cell viability was assessed by quantifying number of nuclei per field in 15 fields per condition under blinded conditions. The percent neuronal survival was calculated by comparing the number of neuronal nuclei in Aβ1-42 and vehicle treated wells.
Immunoprecipitation
Cells were lysed in radioimmune precipitation buffer containing protease and phosphatase inhibitors and 1 mg of protein lysate was incubated with 4 μg of anti-human CD36 (FA6, Cell Sciences), anti-GFP (Roche Applied Science) or control IgG (Sigma) overnight at 4°C, then for 2h with agarose-conjugated secondary antibodies as described.35 Immune complexes were washed three times in RIPA buffer and eluted with Laemmli SDS sample buffer and Western blotted with antibodies against CD36 (H300, Santa Cruz), TLR4 or TLR6 (e-Bioscience), Lyn or Src (Santa Cruz) as described.35 Signal was recorded using ECL Plus reagent and Hyperfilm (Amersham Biosciences). For peptide binding experiments, peptides corresponding to the cytoplasmic C-terminal domain of CD36 (native, His6MISYCACRSKTIK; scrambled, His6CKITSRICAYMKS; and soluble peptides, MISYCACRSKTIK, CACRSKTIK, RSKTIK) were synthesized by the Washington University Protein and Nucleic Acid Chemistry Laboratory. His6-tagged peptides were coupled to Ni-NTA resin (Qiagen) and incubated with cell lysates (1 mg/ml) at 4°C for 1.5 h. The His6peptide Ni-NTA resin was washed sequentially with RIPA or 150 mM imidazole in RIPA. Bound proteins eluted with Laemmli SDS sample buffer and Western blotted for Lyn as described above. In peptide competition assays, cell lysates were pre-incubated with soluble peptides MISYCACRSKTIK, CACRSKTIK or RSKTIK before addition of the His6peptide Ni-NTA resin.
Kinase inhibitors
Cells were pre-treated with genistein, daidzen, PP1, PP3 (EMD Biosciences), wortmannin or LY294002 (Cell Signaling Technology Inc) for 1 h prior to stimulation.
Statistical Analysis
The difference between two groups was statistically analyzed by Student’s t-test or an analysis of variance (one-way ANOVA) for groups of three or more with post-hoc testing using Tukey HSD. A P value of <0.05 was considered significant.
REFERENCES
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 3.Ozinsky A, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoebe K, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–7. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 5.Stuart LM, et al. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005;170:477–85. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerold G, et al. A Toll-like receptor 2-integrin beta3 complex senses bacterial lipopeptides via vitronectin. Nat Immunol. 2008;9:761–8. doi: 10.1038/ni.1618. [DOI] [PubMed] [Google Scholar]
- 7.Ip WK, Takahashi K, Moore KJ, Stuart LM, Ezekowitz RA. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med. 2008;205:169–81. doi: 10.1084/jem.20071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–11. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 10.Janabi M, et al. Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterioscler Thromb Vasc Biol. 2000;20:1953–60. doi: 10.1161/01.atv.20.8.1953. [DOI] [PubMed] [Google Scholar]
- 11.El Khoury JB, et al. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–66. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore KJ, et al. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–9. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 13.Rahaman SO, et al. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–21. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 15.Bjorkbacka H, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–21. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 16.Michelsen KS, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aslan M, Ozben T. Reactive oxygen and nitrogen species in Alzheimer’s disease. Curr Alzheimer Res. 2004;1:111–9. doi: 10.2174/1567205043332162. [DOI] [PubMed] [Google Scholar]
- 20.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–8. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun B, et al. Distinct mechanisms for OxLDL uptake and cellular trafficking by class B scavenger receptors CD36 and SR-BI. J Lipid Res. 2007;48:2560–70. doi: 10.1194/jlr.M700163-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Medvedev AE, et al. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–53. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005 doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullick AE, et al. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–83. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–85. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 26.Kirii H, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–60. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 27.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–22. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Walter S, et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem. 2007;20:947–56. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- 29.Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–15. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 31.Ameziane N, et al. Association of the Toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler Thromb Vasc Biol. 2003;23:e61–4. doi: 10.1161/01.ATV.0000101191.92392.1D. [DOI] [PubMed] [Google Scholar]
- 32.Minoretti P, et al. Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer’s disease. Neurosci Lett. 2006;391:147–9. doi: 10.1016/j.neulet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 33.Kiechl S, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 34.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–59. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 35.Kunjathoor VV, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–8. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 36.Moore KJ, et al. Divergent response to LPS and bacteria in CD14-deficient murine macrophages. J Immunol. 2000;165:4272–80. doi: 10.4049/jimmunol.165.8.4272. [DOI] [PubMed] [Google Scholar]
- 37.Moore KJ, et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coraci IS, et al. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to b-amyloid fibrils. Am J Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberson SM, Walker WS. Immortalization of cloned mouse splenic macrophages with a retrovirus containing the v-raf/mil and v-myc oncogenes. Cell Immunol. 1988;116:341–51. doi: 10.1016/0008-8749(88)90236-5. [DOI] [PubMed] [Google Scholar]
- 40.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–37. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 41.Visintin A, Mazzoni A, Spitzer JA, Segal DM. Secreted MD-2 is a large polymeric protein that efficiently confers lipopolysaccharide sensitivity to Toll-like receptor 4. Proc Natl Acad Sci U S A. 2001;98:12156–61. doi: 10.1073/pnas.211445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi Y, Wang JK, McMillian M, Chikaraishi DM. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J Neurosci. 1997;17:1217–25. doi: 10.1523/JNEUROSCI.17-04-01217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.