Abstract
Human Vγ9Vδ2 T lymphocytes recognize phosphorylated alkyl antigens. Isopentenyl pyrophosphate (IPP) was previously proposed as the main antigen responsible for Vγ9Vδ2 T cell activation by cancer cells. However ApppI, a metabolite in which the isopentenyl moiety is linked to ATP, was reported in cells activated with aminobisphosphonates. The contribution of this compound to tumor stimulatory activity was thus examined. ApppI induces selective expansion of Vγ9Vδ2 T cells from PBMCs. In the absence of APCs however, ApppI has little stimulatory activity on Vγ9Vδ2 T cells and optimal activation with ApppI requires addition of a nucleotide pyrophosphatase releasing IPP + AMP. Thus ApppI has no intrinsic stimulatory activity. Nevertheless, stimulation by ApppI is strengthened by the presence of APCs. Moreover, in contrast to IPP, ApppI can be efficiently pulsed on dendritic cells as well as on non-professional APCs. Pulsed APCs display stable and phosphatase-resistant stimulatory activity, indicative of antigen modification. HPLC analysis of tumor cell extracts indicates that latent phosphoantigenic activity is stored intracellularly in the Vγ9Vδ2 cell-sensitive tumor Daudi and can be activated by a nucleotide pyrophosphatase activity. The presence of ApppI in Daudi cell extracts was demonstrated by mass spectrometry. Nucleotidic antigens such as ApppI are thus a storage form of phosphoantigen which may represent a major source of phosphoantigenic activity in tumor cells. The unique properties of ApppI may be important for the design of antigens used in anti-cancer immunotherapeutic protocols using Vγ9Vδ2 cells.
Keywords: Adenosine Monophosphate; Adenosine Triphosphate; analogs & derivatives; immunology; Antigen-Presenting Cells; immunology; Cell Line, Tumor; Cells, Cultured; Diphosphonates; Hemiterpenes; immunology; Humans; Lymphoma, B-Cell; immunology; pathology; Organophosphorus Compounds; immunology; Receptors, Antigen, T-Cell, gamma-delta; immunology; T-Lymphocytes; immunology
Keywords: Gamma-delta T cells, phosphoantigens, nucleotide antigens, antigen presentation
Introduction
The majority of human peripheral T cells of the γδ type express a T cell receptor (TCR) comprising Vγ9 and Vδ2 variable regions. These usually represent 1 to 10% of circulating T lymphocytes, expand and release inflammatory cytokines in response to diverse infectious agents such as mycobacteria, bacteria and parasites(1). They also display cytotoxicity towards diverse tumor cells including laboratory tumor lines (Daudi Burkitt’s lymphoma, RPMI-8226 myeloma) and multiple cancer lines from patients with, among others, lung, prostate, liver or kidney cancers(2–4).
Metabolites which stimulate Vγ9Vδ2 T cells in a TCR-dependent manner have been purified from mycobacteria and called phosphoantigens because they consist in a short alkyl chain and a terminal pyrophosphate group(5–7). The most powerful natural antigen reported so far is hydroxyl dimethylallyl pyrophosphate (HDMAPP), a metabolite produced by microbial organisms through the deoxylulose pathway, an alternative bacterial route to the isoprenoid precursor isopentenyl pyrophosphate (IPP)(8–10). Pyrophosphorylated antigens can directly activate Vγ9Vδ2 T cells and do not absolutely require to be presented by specialized antigen presenting cells nor to be displayed on MHC or CD1 antigens(7, 11). Nucleotidic derivatives of phosphoantigens have been purified from mycobacteria and have been found to stimulate Vγ9Vδ2 cells(5, 6). Multiple synthetic antigens analogous to pyrophosphorylated compounds as well as nucleotidic variants have been studied and some of them proved to be powerful stimulants of Vγ9Vδ2 T cells(12–14).
The precise mechanism underlying phosphoantigen recognition is still unclear. Structure-activity correlation studies of synthetic phosphoantigens indicated that, although short alkyls such as ethyl pyrophosphate display some activity, optimal structures contain five carbon atoms and one double bond(15). Belmant et al. reported that the β-phospho-ester bond in pyrophosphorylated antigens must be cleavable(16) and a recent study by Boedec et al. reported the strong stereospecificity of structural alkyl variants(17). Whether this reflects constraints for binding to the TCR or to a putative presentation molecule is unknown.
TCR gene transfer experiments indicate that phosphoantigen recognition by Vγ9Vδ2 T cells is TCR-dependent(11). Nevertheless, a direct interaction between the TCR and phosphoantigens is only indirectly supported by structure-function correlations(18–20) and molecular interaction models(21). In these studies, aminoacids in the gamma chain complementarity determining region-3 were found essential for phosphoantigen recognition.
The presence of APCs improves direct Vγ9Vδ2 T cell responses to phosphoantigens(22) but their exact contribution is only partially characterized. Analyses with strong pyrophosphorylated agonist antigens such as bromohydrin pyrophosphate, or HDMAPP(22–24) have shown that to some extent these antigens can be captured by APCs and confer stable stimulatory activity, indicating that they can be somehow displayed on the cell surface as on stimulatory tumors. A mechanism for phosphoantigen presentation is however supported by recent experiments showing binding of soluble Vγ9Vδ2 tetramers on APCs pulsed with HDMAPP. This revealed a direct and antigen-specific recognition of the membrane of pulsed cells which was dependent on a trypsin-sensitive structure(24, 25).
Tumor cells such as Daudi Burkitt’s lymphoma produce weak phosphoantigens through the mevalonate pathway. The major stimulatory compound, IPP, is overproduced in some tumors following hyper-activation of the mevalonate pathway(26, 27). IPP can also accumulate in cells after treatment with aminobisphosphonates through inhibition of the mevalonate pathway enzyme farnesyl pyrophosphate synthase, resulting in an increased sensitivity to Vγ9Vδ2 T cell cytolytic activity. Aminobisphosphonates also induce intracellular accumulation of ApppI, an adenine nucleotide derivative of IPP in macrophages(28). As the structure of this compound is reminiscent of bacterial phosphoantigens such as TubAg3/4 and other analogous synthetic nucleotide derivatives with stimulatory activity, we have studied its biological activity on Vγ9Vδ2 T cells(6). This revealed unique properties for this nucleotidic phosphoantigen, making this type of compound of particular interest for in vivo administration. We show evidence that nucleotidic antigens are naturally produced in tumor cells and can play a major role in their stimulatory activity towards Vγ9Vδ2 lymphocytes.
Materials and methods
Antibodies and other reagents
Anti-TCRVδ2-FITC (IMMU360) and CD3-PE (UCHT1) were from Beckman-Coulter, anti-IFNγ-PE (25273) from R&D systems, and anti-CD107a-PE (H4A3) from BD- Pharmingen. Apyrase, Crotalus adamanteus venom nucleotide pyrophosphatase (NPP) and other reagents were from Sigma Aldrich except Isopentenyl Pyrophosphate (Isoprenoids LC, Tampa, Florida, USA) and ApppI (see below). Apyrase and NPP were used in cellular and biochemical assays at the concentration of 0.2U/ml and 0.02U/ml respectively.
Synthesis of ApppI
The procedure was adapted from Ryu et al.(29). Briefly, 110 mg (0.2 mmol) of ATP (sodium salt) were converted into the corresponding tetrabutylammonium salt then dissolved in dry acetonitrile (2 ml). To this mixture a solution of isopentenyl tosylate (1 eq., 48 mg) was added in dry acetonitrile (2 ml). The reaction mixture was stirred at room temperature overnight and concentrated. The oily residue was purified on a DEAE sephadex column using a gradient of triethylammonium bicarbonate buffer (TEAB, pH 6.9, 0.2 to 1M). The fractions of interest were evaporated under vacuum, and passed through an ion-exchange Dowex (Na+ form) column to give ApppI (1-(adenosin-5′-yl) 3-(3-methylbut-3-enyl) triphosphoric diester) as sodium salt with a final yield of 45%. ApppI was stored at −20°C. Aliquots were reconstituted in molecular biology grade water and stored at −80°C. In some preparations, HPLC analysis of reconstituted product revealed the presence of 5–10% ADP (presumably from spontaneous degradation). ADP does not affect responses of Vγ9Vδ2 cells in stimulations. This was however checked additionally in control experiments in which this contaminating product was removed by apyrase treatment. To this end, ApppI was pre-treated for 1h with 0.2U/ml apyrase and the enzyme was then inactivated by heating at 100°C for 10 minutes.
Cell culture and pulsing with phosphoantigens
Cell culture reagents were from Invitrogen except human serum (PAA Laboratories, Dusseldorf, Germany) and IL-2 (Sanofi-Synthelabo, Toulouse, France). Tumor cell lines were obtained from ATCC and grown in RPMI 1640 Glutamax supplemented with 10% heat-inactivated FCS, sodium pyruvate and penicillin/streptomycin, or in Hybridoma SFM medium supplemented with sodium pyruvate, penicillin/streptomycin and L-glutamine (Complete SFM) for pulsing experiments since pulsing was less efficient in presence of FCS. In this case, cells were washed and incubated with the indicated concentration of IPP or ApppI for 2h or 16h, washed again 4 times, pelleted by centrifugation and used in T cell stimulation assays.
For polyclonal Vγ9Vδ2 T cell lines establishment, PBMCs isolated from buffy coats from healthy donors (Etablissement Français du Sang, Toulouse, France) were stimulated with 5μM phosphoantigen (IPP or ApppI) in RPMI 1640-glutamax medium supplemented with 10% heat-inactivated human serum, sodium pyruvate and penicillin/streptomycin. 24h after stimulation, IL-2 was added at a final concentration of 200U/ml. On day 5, IL-2 concentration was raised to 400U/ml. Cells were passed every 2–3 days, maintained at a concentration of 6.105/ml and frozen after 22–24 days of culture. Cell lines contained >94% Vδ2+ cells. Upon thawing, lymphocytes were left to recover overnight in culture medium without IL-2 before assessment of antigen responses.
Generation of DCs
PBMCs from healthy donors were sorted for CD14+ cells using a MACS column (Miltenyi Biotec). DCs were obtained by culturing CD14+ cells in six-well plates (3×106 cells/ ml) for 7 days in AIM-V serum-free medium (Invitrogen) supplemented with GM-CSF (100 ng /ml; Novartis) and interleukin-13 (IL-13, 50 ng /ml). Fresh IL-13 was added again after 4 days of culture. The phenotype of iDCs was monitored as described previously (30) and was as follows: CD1a+, MHC-I+, MHC-II+, CD64−, CD83−, CD80low, CD86low. For induction of maturation, immature DCs were cultured for 48h in presence of 100ng/ml LPS and checked for maturation marker (CD83 and CD86). In some experiments, DCs were either pulsed with pamidronate / IPP / ApppI (10μM) for 14–16h or kept untreated. After pulsing cells were washed three times with RPMI and used as antigen presenting cells in Vγ9Vδ2 T cell activation assay using a T cell:APC ratio of 1:1.
Proliferation assay
PBMCs were stimulated as described for polyclonal Vγ9Vδ2 T cell lines establishment, collected at the indicated times and stained for 30min at 4°C with anti-TCRVδ2-FITC and anti-CD3-PE antibodies (1/25 and 1/50 final dilutions, respectively) in PBS 5% FCS (FACS medium). Data were acquired using a FACScan cytometer (Becton Dickinson).
Intracellular cytokine staining
Polyclonal Vγ9Vδ2 T cell lines were stimulated for 5h in 96 U-bottom well plates with the indicated antigens after a brief centrifugation to increase cell-cell contact. Brefeldin A (10μg/ml) was added 1h after the initiation of culture. Cells were then washed and stained with anti-TCRVδ2-FITC antibody (1/25 final dilution) in FACS medium, fixed for 15min with PBS containing 2% paraformaldehyde (w/v) at room temperature and then permeabilized with saponin buffer (PBS, 10mM HEPES, 5% FCS, 0.1% saponin) for 15min at room temperature. Cells were then stained with anti-IFNγ-PE antibody (1/100 final dilution) in saponin buffer for 30min at 4°C and washed twice in FACS medium. Data were acquired and analyzed as for proliferation assays. Results are expressed as the mean of triplicate microwell cultures +/−SD.
CD107 expression assay for cytolytic function
Experiments were performed as previously described (31) with minor modifications. Briefly, polyclonal Vγ9Vδ2 T cell lines (105 cells) were stimulated in 96-well U-bottom plates with the indicated antigens, antibody-coated beads or tumor cell lines (1:1 ratio) for 5h after a brief centrifugation, in presence of the anti-CD107a-PE antibody (1/25 final dilution) in a final volume of 100 μL. Brefeldin A was omitted. Cells were washed twice in FACS medium and stained for TCR-Vδ2. Data were acquired and analyzed as for proliferation assays. Results are expressed as the mean of triplicate microwell cultures +/−SD.
Confocal microscopy analysis of TCR aggregation
K562 were either pulsed with ApppI (20μM) or kept untreated for 16–18h, washed twice, stained with hexidium iodide (Molecular Probes, 5μM) and mixed with Vγ9Vδ2 T cells (1:1 ratio), centrifuged at 1200rpm for 1 min and incubated at 37°C for 30min (APCs). Cells were then resuspended gently and layered onto poly-L lysine-coated 8-well slides. After 10 min, 0.02% NaN3 was added and slides were kept at room temperature for further 15 min. The wells were washed once with PBS containing 5% FBS and 0.02% NaN3. Finally cells were stained with anti-δ2-FITC, the slides were mounted and observed under a Zeiss LSM 510 (Zeiss) confocal microscope with a 63 Plan-Apochromat objective (1.4 oil), electronic zoom 3.
HPLC analysis of nucleotidic compounds
For NPP and apyrase sensitivity assays, reaction mixes containing nucleotidic compounds were adjusted to 200μL with water and products were separated by HPLC (System Gold, Beckman Coulter) on a PL-SAX anion exchange column (1000Å, 5μm, 50×4.6mm from Polymer Labs Varian, Marseille, France) using water (A) and 1M (NH4)2HPO4, pH 3.5 (B) and the following elution gradient: 0–1 min: 0% B; 1–11 min: 0% to 30% B; 11–13 min: 100% B; 13–20min: 100% A. Eluted nucleotides were detected by monitoring UV absorbance at 260nm. To evaluate phosphatase sensitivity, ApppI and ATP (100μM final concentration) were incubated for 1h at 37°C in 20μl RPMI in the presence of 0.2U/ml apyrase or 0.02U/ml nucleotide pyrophosphatase before analysis.
For the assessment of intracellular antigens, Daudi or K562 cells (3×108 in 250ml) were washed in PBS, pelleted and resuspended in 600μl of ice-cold H2O + 600μl of ice-cold acetonitrile. Cell lysis was completed using a FastPrep-24 tissue homogenizer and lysing matrix D ceramic spheres (MP Biochemicals, Selom, OH) set at 4m/s, with 4 pulses of 15s. The cell lysate was ultracentrifuged (2×105g, 15min) and the supernatant (cytosolic fraction) was lyophilized and redissolved in 200μl of H2O. This was loaded on an anion exchange cationic column (Amersham Life Sciences, type Q) and bound material was eluted (2ml/min) using H20 (A) and ammonium acetate 1M, pH6.5 (B), with the following gradient: 0 to 1min: 0% B, 1 to 17min: 0 to 100% B, 17 to 20min: 100% B, 20 to 25min: 0% B. Fractions were collected every 0.5min, lyophilized and redissolved in 100μl of H2O. 10 μl of each fraction was then tested for stimulatory activity on Vγ9Vδ2 T cell microcultures.
Mass Spectrometry
Mass spectrometry was performed using an LCQ ion-trap mass spectrometer (Finnigan MAT, San Jose, CA). Nano electrospray analysis was performed using a nanospray ESI source (The Protein Analysis Co., Odense, Denmark). Palladium and gold-coated glass capillaries (Proxeon Biosystems, Odense, Denmark) were filled with 3 μL of analyte solution and positioned using a stereomicroscope at a distance of 1 mm to the opening of the heated transfer capillary kept at a temperature of 150°C. A nebulizer gas was not necessary in this spray mode. The nanospray needle voltage was set to 700 V. Samples were analyzed in the negative ion mode. ApppI (m/z 574) was fragmented using the following parameters: parent ion isolation width, 3 m/z; collision energy, 30%; activation Qz, 0.25; activation time, 30 ms. MS3 analysis of the ApppI fragment at m/z 408 was performed using the following parameters: parent ion isolation width, 3 m/z; collision energy, 33%; activation Qz, 0.25. Analysis was performed with the Xcalibur 1.2 software (Thermo Electron, San Jose, CA).
Results
Direct stimulation of γδ T cells with nucleotidic phosphoantigens
The adenylated derivative of IPP, namely triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester, will be referred to as ApppI. In this compound the isopentenyl moiety is linked to the γ-phosphate of ATP instead of being linked to a pyrophosphate group (Fig. 1). When added in a PBMC culture, ApppI induced the selective expansion of Vγ9Vδ2 T cells and the culture reached homogeneity (~94%) in 3 weeks, as it is the case for most phosphoantigens (Fig. 2A). Although IPP and ApppI both induce proliferation and intracellular IFN-γ production, ApppI required a 5 to 10-fold higher molar concentration for a similar effect on Vγ9Vδ2 T cell expansion from PBMCs (Fig. 2B). The adenylated derivative was also less bioactive than IPP for the stimulation of lymphokine production by Vγ9Vδ2 T cell, with half maximum responses obtained at ~30μM instead of ~1μM for IPP (Fig. 2C). Both compounds induced the cytotoxicity machinery as evidenced by the surface expression of the lysosomal marker CD107a although IPP induced again a stronger stimulation (Fig. 2D). Addition of ApppI to IPP slightly increased stimulation, indicating that ApppI does not compete with IPP and has no toxic activity on responder cells (Fig. 2E).
Figure 1. Structure of ApppI and IPP.

NPP and Apyr indicate the cleavage sites by nucleotide pyrophosphatase and apyrase.
Figure 2. Activation of Vγ9Vδ2 T cells by ApppI.

(A,B) PBMCs were stimulated with phosphoantigens and IL-2 before staining for CD3 and TCR Vδ2 expression. (A) PBMC staining before and after in vitro culture (19 days) with ApppI (5μM). Numbers indicate the percentage of cells in each quadrant. (B) Comparative outgrowth of Vδ2 T cell 1 week after PBMC stimulation with IPP or ApppI plus IL-2. (C) Intracellular IFN-γ staining of Vδ2+ cells following PBMC stimulation with IPP and ApppI. (D) A Vγ9Vδ2 T cell line was stimulated with the indicated antigens in the presence of anti-CD107a-PE antibody to measure the activation of the cytolysis machinery. Cells were then washed and stained for TCRVδ2. In dot plots, numbers indicate the percentage of CD107a+ cells among Vδ2+ cells after stimulation with antigens at 100μM. The dose-response curve is shown on the right. Data are representative ≥10 experiments. (E) PBMCs were stimulated in the presence of IPP (10μM) plus ApppI and stained as in C. (C,E) Results are means of triplicate cultures +/− SD.
Effect of phosphatases on ApppI responses
The phosphate groups of IPP are sensitive to terminal phosphatases such as alkaline phosphatase (5, 14) or apyrase. Conversely ApppI is expected to be resistant to degradation by the same enzymes. Indeed, treatment of ApppI with apyrase did not change its HPLC profile whereas ATP used as a control for enzyme activity is completely hydrolyzed in similar conditions (Fig 3A). Strikingly, when added in stimulation assays, apyrase abolished the response to ApppI in the CD107 expression assay (Fig. 3B) as well as in cytokine production assays (data not shown). A toxic effect of apyrase on lymphocyte responses is excluded as this enzyme does not influence the responses induced by anti-CD3 stimulation. This suggests that ApppI has no stimulatory activity per se and requires to be converted into a compound in which the phosphate is terminal, most likely IPP.
Figure 3. Effect of apyrase on ApppI and ApppI-induced lymphocyte response.

(A) Individual nucleotides or nucleotide mixtures were either untreated or treated with apyrase before analysis by anion exchange chromatography to assess their sensitivity to the enzyme. (B) Vγ9Vδ2 T cells were stimulated with ApppI, anti-CD3 antibody or control IgG1-coated beads, or control medium in the presence or absence of apyrase, and the expression of CD107a was monitored as in Fig. 2D. Data are means of triplicate stimulation experiments +/− SD.
The structure of ApppI indicates that it could be hydrolysed to give IPP + AMP through cleavage of the α-β phosphoester bond by a nucleotide pyrophosphatase activity(32). ApppI was thus treated with Crotalus adamanteus venom nucleotide pyrophosphatase (NPP) and subsequently analyzed by HPLC. The profile of NPP-treated ApppI indicates the release of a nucleotidic moiety which elutes like AMP but not like ADP and is compatible with the expected cleavage of ApppI between the α and β phosphates relative to the adenosinyl group(Fig. 4A). The release of IPP following NPP treatment was further checked by mass spectrometry detection of IPP in a fraction eluting with the same retention time as ADP (data not shown). We then evaluated the effect of NPP addition in the culture medium of ApppI-stimulated Vγ9Vδ2 T cells. NPP addition to the culture medium strongly increased the stimulatory activity of ApppI. As the enzyme was deprived of any effect in the absence of antigen, this was not due to the presence of nucleotidic antigens in the cultures or to a non-specific stimulatory activity of the enzyme (Fig. 4B). NPPs are sensitive to the pyrophosphatase inhibitor pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS(33)). PPADS completely abolished the weak spontaneous response to ApppI in the absence of exogenous NPP, confirming that this response is due to partial conversion of ApppI into IPP in the culture medium. PPADS had no effect on the CD3-mediated stimulation, excluding a non-specific toxicity of this compound (Fig. 4C). Altogether, these data indicate that ApppI has no significant intrinsic stimulatory activity and requires conversion into IPP for the stimulation of Vγ9Vδ2 T cells in the absence of APCs.
Figure 4. Effect of nucleotide pyrophosphatase on ApppI and ApppI-induced lymphocyte response.

(A) Individual nucleotides or nucleotide mixtures were either untreated or treated with NPP before HPLC analysis as in Fig. 3A. (B) Vγ9Vδ2 T cells were stimulated with ApppI in the presence or absence of NPP and the expression of CD107a was monitored as in Fig. 2D. (C) The effect of NPP and its inhibitor PPADS was tested on lymphocyte stimulations by ApppI or anti-CD3 and monitored as in B. Data (B,C) are means of triplicate stimulation experiments +/− SD. *: p≤0.001; NS: non-significant (2-tailed Student test).
Effect of tumoral APCs on phosphoantigen-mediated stimulation
It is largely documented that Vγ9Vδ2 T cell stimulation is increased by the presence of bystander APCs(22). However, although strong agonist pyrophosphorylated antigens can be pulsed on antigen presenting cells(23, 24), this is generally considered as poorly efficient even on dendritic cells(34, 35). When RPMI-8226 cells, which are week stimulatory tumors were added in cultures of Vγ9Vδ2 T cells and phosphoantigens, we observed a synergistic activation which was more pronounced with ApppI than with IPP (Fig. 5A). This prompted us to compare the pulsing efficiency of these two antigens on different APCs. We first compared the ability of IPP and ApppI to be pulsed on tumor lines which are not professional APCs. K562 and RPMI-8226 myeloma cell lines have weak spontaneous stimulatory activity, whereas Daudi has a strong spontaneous stimulatory activity for Vγ9Vδ2 T cells. All three cell lines were incubated overnight with both antigens, extensively washed and used for stimulation. Although pulsing with both antigens increased the stimulatory activity of tumors, pulsing with ApppI was again more efficient than with IPP, and ApppI-pulsed tumors were stronger stimulators than cells pulsed with the same molar concentration of IPP (Fig. 5B).
Figure 5. Effect of antigen presentation on ApppI response.
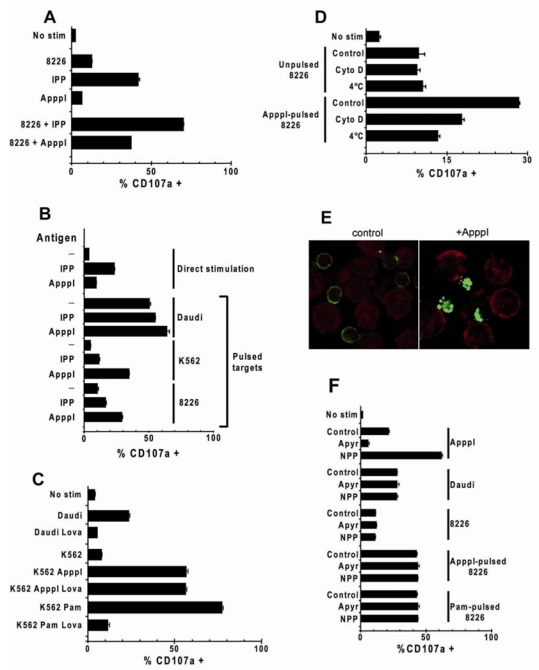
Vγ9Vδ2 T cells were stimulated as indicated and CD107a expression was monitored as in Fig. 2D. Data are means of triplicate stimulation experiments +/− SD.
(A) Stimulation with IPP, ApppI, RPMI-8226 cells (1:1 ratio) or RPMI-8226 plus phosphoantigens. (B) Indicated tumor cell lines were pulsed overnight with the indicated antigens before co-culture with Vγ9Vδ2 cells. (C) Daudi and K562 cells were treated overnight with the indicated compounds, washed and used as stimulators for Vγ9Vδ2 T cells. Lova: lovastatin (20μM); ApppI: 20μM; Pam: pamidronate (50μM). (D) RPMI-8226 cells were pulsed with ApppI (2h, 10μM) either at 37°C (Control), 4°C, or at 37°C after a 2h-pre-treatment with cytochalasin D (10μM), and washed extensively before addition of Vγ9Vδ2 T cells. (E) Vγ9Vδ2 T cells (surface-stained with anti-d2-FITC, green) were kept in contact for 20min at 37°C with K562 cells loaded with hexidium iodide (red) and loaded or not with ApppI. TCR aggregation was analyzed by confocal microscopy. (F) Tumor cells were pulsed overnight with pamidronate (50μM) or ApppI (20μM) or left untreated, washed, and polyclonal Vγ9Vδ2 T cells were added along with the indicated enzymes (Apyr: Apyrase, 0.2U/ml, NPP: Nucleotide Pyrophosphatase, 0.02U/ml).
These results could be explained by an effect of ApppI similar to aminobisphosphonate blockade of farnesyl pyrophosphate synthase. If this was the case, ApppI effect would be abolished by simultaneous treatment of tumors with lovastatin, preventing accumulation of endogenous IPP(26). Fig. 5C shows that lovastatin treatment abolishes Daudi spontaneous stimulatory potential and K562 sensitization with pamidronate but has no effect on K562 sensitization with ApppI. Thus ApppI does not act as an inhibitor of farnesyl pyrophosphate synthase.
In order to determine if the acquisition of stimulatory activity by tumor cells required an active process, we studied the effect of temperature and cytoskeleton integrity on pulse efficiency. We used RPMI-8226 cells and short pulsing conditions (2h) which confer stable stimulatory activity without cell alteration when varying the temperature of pulse or with drug addition during pulsing. Pulsing was abrogated when performed at 4°C or on cells which were previously treated with the actin polymerization inhibitor cytochalasin D (Fig. 5D). This indicates that pulsing is not simply due to passive adsorption, requires an active mechanism and involves cytoskeletal components, indicative of an active process. Finally, a specific TCR engagement following ApppI recognition on K562 cells is substantiated by a massive TCR aggregation on the T cell surface (Fig. 5E) as well as subsequent TCR downmodulation by FACS analysis (not shown).
ApppI-pulsed cells behave as classical Vγ9Vδ2 target cell lines
As the direct stimulation of Vγ9Vδ2 T cells by ApppI can be modulated by addition of NPP and apyrase, it was important to check the effect of such treatments on the stimulation by pulsed cells. These enzymes were thus added in T cell stimulation assays using either Daudi cells or RPMI-8226 cells pulsed with pamidronate or ApppI (Fig. 5F). In all cases, stimulation with tumor cells was completely resistant to treatment with apyrase and NPP whereas direct simulation was sensitive to phosphatases. Thus the antigen was not recognized in soluble form, was somehow processed to become resistant to these enzymes and was stably presented on the surface of tumors. This also indicates that the Vγ9Vδ2 T cell stimulation by antigen-pulsed tumors and their direct stimulation by soluble IPP or ApppI result from different mechanisms and different antigenic forms.
Pulsing of professional APCs with ApppI
Dendritic cells have previously been shown to potentiate phosphoantigen responses(36). Based on above observations we compared the pulsing efficiency of IPP and ApppI on monocyte-derived dendritic cells (DC). As immature DC (iDC) are more prone to capture exogenous antigens than mature DCs (mDC) we also analyzed the ability of DC to be pulsed with IPP and ApppI and to stimulate Vγ9Vδ2 cells following maturation by 24-h LPS treatment (Fig. 6). Maturation of DC was confirmed by the increase of CD83 and CD86 surface expression. After incubation with IPP or ApppI (10μM) and extensive washing to eliminate passively adsorbed antigens, both i-DC and m-DC populations were able to stimulate Vγ9Vδ2 lymphocytes. ApppI was however more efficiently pulsed than IPP and the stimulatory activity of ApppI-pulsed DC reached that of the same population treated with pamidronate (50μM) instead of phosphoantigen. The use of mature DC was associated with a much lower Vγ9Vδ2 lymphocyte response, suggesting that antigens are captured less efficiently after DC maturation. Thus ApppI is similarly captured with higher efficiency than IPP by professional and non-professional APCs.
Figure 6. Pulsing of phosphoantigens on dendritic cells.

(A) DC were derived from CD14+ PBMCs (iDC) and maturation was induced by 48h LPS stimulation (mDC). They were subsequently pulsed with IPP or ApppI, extensively washed and co-cultured with Vγ9Vδ2 cells. T cell activation was monitored by CD107a expression. in Vδ2+ cells. (B) Representative pattern of CD83 and CD86 expression on DC populations before and after LPS treatment.
Daudi cells naturally produce nucleotidic antigens
Above results indicate that ApppI-pulsed cells are quite similar to spontaneously stimulating tumors and that ApppI is more prone than IPP to be captured and displayed in a stimulatory form by tumors. This suggests that nucleotidic derivatives of phosphoantigens could be produced intracellularly by tumors as well as IPP. To check for the presence of nucleotidic stimulatory compounds in Daudi cells, cytosolic extracts were prepared and separated by ion exchange HPLC. After elution of bound material, fractions were collected, treated or not with apyrase to remove non-nucleotidic phosphoantigens, and tested for their stimulatory activity on Vγ9Vδ2 cells in the presence or absence of NPP. In the absence of enzyme treatment, the major peak of activity eluted at 9.75 min, corresponding to the elution time of purified IPP (not shown). This activity was severely decreased following apyrase treatment, in agreement with the presence of antigens with terminal phosphates in this fraction. After apyrase treatment, stimulatory activity was barely detectable in the absence of NPP. However, in the presence of this enzyme, several fractions were active, suggesting the presence of nucleotidic phosphoantigens. A major peak of activity eluted with a retention time close to that of purified ApppI (10.5 to 11min) and the resulting activity exceeded that of the apyrase-sensitive fraction (Fig. 7). This fraction was subsequently analysed by electrospray ionization ion trap mass spectrometry (ESI-ITMS MS).
Figure 7. Daudi cells produce endogenous nucleotidic phosphoantigens.
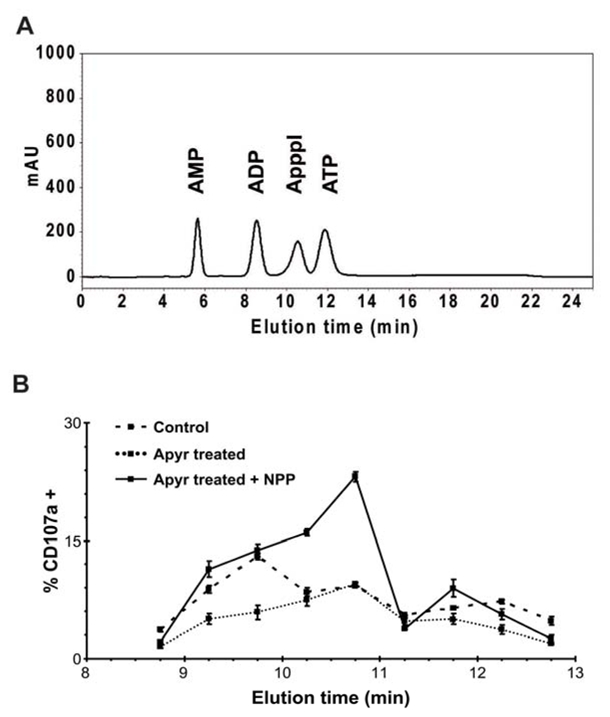
(A) Elution profile of AMP, ADP, ATP and ApppI. (B) Fractions eluted from Daudi cytosolic extracts were either untreated (control) or treated with apyrase. This was then denatured by heating and samples were assayed for Vγ9Vδ2 T cell stimulation with or without adding exogenous NPP. Data are means of triplicate stimulations +/− SD.
Synthetic ApppI gives a m/z 574 pseudo molecular ion ([M-H]−). As previously described(28), its fragmentation produces major ions at m/z 408 and m/z 227). To increase the MS signature of ApppI, MS3 analysis was performed on the major ion with m/z 408 which could be further fragmented and produced an ion with m/z 273 and the pyrophosphate-related ion with m/z 159 (Fig 8A). The HPLC fraction containing the putative natural nucleotidic phosphoantigen was then analyzed using identical MS parameters. An ion with m/z 574 was present at trace levels (not shown). After fragmentation this gave the characteristic ions of ApppI in both the MS2 and the MS3 spectra (Fig. 8B). Thus, although other stimulatory compounds may also be present in cell extracts, ApppI is produced intracellularly by Daudi cells. In our experimental conditions, IPP could not be unequivocally identified in the corresponding elution fraction by mass spectrometry (not shown).
Figure 8. Detection of ApppI in Daudi cell extracts by mass spectrometry.

(A) Nano ESI-ITMS analysis of synthetic ApppI. Top panel: full scan MS, showing the pseudo molecular ion signal of ApppI corresponding to ([M-H]−). Middle panel: MS2 spectrum of m/z 574. Bottom panel: MS3 spectrum resulting from the fragmentation of the m/z 408 ion. (B) Nano ESI-ITMS analysis of biological extract corresponding to HPLC fraction eluting between 10.5 and 11 min (cf. Fig. 7A). Top Panel: MS2 spectrum of an m/z 574 ion from full scan, showing the characteristic m/z 408 ion. Bottom: spectrum resulting from the fragmentation of the m/z 408 ion showing a pattern similar to that of synthetic ApppI.
A similar experiment was performed on extracts from the K562 cell line which does not efficiently activate Vγ9Vδ2 cells. An ion with the corresponding m/z 574 was detected and fragmented to identify ion fragments characteristic of ApppI. The m/z 227 ion could not be detected. An ion with a mass of m/z 408 was detected at trace level. However, its fragmentation released an unidentified compound (m/z 326) and no pyrophosphate (m/z 179). Thus, if present, ApppI content is below the detection level in K562 cells. As a control, a similar analysis of pamidronate-stimulated K562 extracts unequivocally revealed the presence of ApppI (Suppl. fig. S1).
Discussion
When studying the inhibitory effect of aminobisphosphonates, Monkkonnen et al. found that these compounds induced the intracellular accumulation of ApppI which presents pro-apoptotic properties on macrophages and osteoclasts. They also provided evidence that this nucleotidic metabolite arose in cells following farnesyl pyrophosphate synthase inhibition and IPP accumulation and a tRNA synthase activity was proposed to be responsible for IPP to ApppI conversion(28, 37). The possibility that ApppI is somehow involved in the stimulatory effect of tumors on Vγ9Vδ2 lymphocytes was however never investigated before, and although many nucleotidic derivatives of phosphoantigens have been studied, ApppI has never been tested.
We show here that ApppI efficiently stimulates Vγ9Vδ2 T cells when added in PBMC cultures and is thus comparable to other phosphoantigens. However ApppI has no intrinsic stimulatory activity on Vγ9Vδ2 T cells in the absence of APCs, requiring cleavage of the α-β phosphoester bond and IPP release for activity. This is apparently at odds with previous reports indicating that nucleotide addition had little effect on phosphoantigen activity. However, although it is not excluded that isopentenyl pyrophosphate derivatives display specific properties, in previous reports, the activity of nucleotidic antigens was evaluated in the presence of APCs(12, 14).
ApppI can be captured by professional or non-professional APCs and confers them a stimulatory activity. Strikingly, this is more efficient than pulsing with an equivalent molar concentration of IPP. Once captured by cells, the phosphoantigenic activity is stably “expressed” on the cell surface, and this display is strongly impaired when cells are kept at 4°C or when cytoskeletal components are altered by cytochalasin D. After exposition to ApppI, cells behave as spontaneously stimulatory tumors or aminobisphosphonate-treated cells and their stimulatory activity is not only stable on the cell surface but also resistant to phosphatases which normally degrade pyrophosphorylated antigens (such as apyrase) or nucleotidic derivatives (such as NPP), indicating antigen modification or protection. Altogether, these data suggest the existence of a specific receptor and transport mechanism for nucleotide derivatives. Is tumor cell stimulatory activity due to pyrophosphates or nucleotidic antigens? HPLC analysis of cytosolic extracts reveals that nucleotidic antigens are present in stimulatory tumors. Their characterization is based upon the fact that the stimulatory activity requires NPP addition for activation of Vγ9Vδ2 cells in the absence of APCs.
Another peak of activity was detected in the same biological extracts in the absence of apyrase or NPP treatment. As this was sensitive to apyrase, it is probably related to the presence of stimulatory pyrophosphate antigens including IPP. This is in agreement with the data of Gober et al (26) although we could not undoubtedly identify IPP in our samples. This may be due to differences in the sample preparation or sensitivity of MS equipment.
Thus, both nucleotidic and non-nucleotidic antigens may be responsible for tumor stimulatory activity. ApppI ultimately requires re-hydrolysis into IPP and the latter might be the only biologically relevant antigenic form for Vγ9Vδ2 T cell stimulation. However, we propose that IPP to ApppI conversion has two biologically important consequences. 1) ApppI represents an inactive “storage” form of phosphoantigen which may bind to carrier proteins and may be protected from degradation. Thus storage of antigens as nucleotidic derivatives might prolong their half-life and immunogenicity or allow there intracellular transportation. Moreover, the activity revealed by NPP treatment of cell extracts is higher than that of apyrase-sensitive fractions indicating that this antigenic material is not marginal. 2) Even if ApppI does not present any direct stimulatory activity, it is more readily captured and displayed as a stimulatory antigen by cells. After sensitization with ApppI, tumors acquire a stimulatory activity which is resistant to phosphatases and in this respect are similar to naturally stimulating tumors.
Based on these observations, we propose that tumoral phosphoantigens are in fact transported and displayed on the cell surface of tumors in the form of nucleotidic derivatives and are stably associated with a presentation structure. Such presentation mechanism is supported by classical demonstrations of cell-cell contact requirement (34, 38) and the recent demonstration that soluble recombinant TCR constructs bind the tumor cell surface after exposition of cells to HDMAPP(24). Although the antigen used in the latter quoted experiments were not nucleotidic derivatives, it is quite possible that part of the antigen has been converted into a nucleotidic derivative and exposed in this form on the cell surface, either covalently bound or strongly associated to a presentation structure.
It remains that ApppI is not active directly and requires final conversion to IPP to activate T cells. However, this process could conceivably be achieved by cell-surface associated and tightly regulated NPPs(39). Thus, nucleotidic derivatives of phosphoantigens may represent important intermediates in a possible phosphoantigen processing mechanism, although direct evidence of phosphoantigens on cell membranes is still actively searched for.
Supplementary Material
Acknowledgments
We thank F. L’Faqihi, V. Duplan-Eche (technical platform of cytometry of IFR150) for their technical assistance in cytometry.
Financial support
This work was financially supported by the French Association pour la Recherche sur le Cancer (PV and contracts #3711-3913-4847) and Ligue Nationale contre le Cancer (#R07002BBA). LM and JMB are supported by Inserm (Avenir) and FRM respectively.
Abbreviations
- ApppI
triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester
- HDMAPP
hydroxyl dimethylallyl pyrophosphate
- IPP
isopentenyl pyrophosphate
- Nano ESI-ITMS
nano electrospray ion trap mass spectrometry
- NPP
nucleotide pyrophosphatase
References
- 1.Chen ZW, Letvin NL. Vgamma2Vdelta2+ T cells and anti-microbial immune responses. Microbes Infect. 2003;5:491–498. doi: 10.1016/s1286-4579(03)00074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 3.Kabelitz D, Wesch D, Pitters E, Zoller M. Potential of human gammadelta T lymphocytes for immunotherapy of cancer. Int J Cancer. 2004;112:727–732. doi: 10.1002/ijc.20445. [DOI] [PubMed] [Google Scholar]
- 4.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 6.Poquet Y, Constant P, Halary F, Peyrat MA, Gilleron M, Davodeau F, Bonneville M, Fournie JJ. A novel nucleotide-containing antigen for human blood gamma delta T lymphocytes. Eur J Immunol. 1996;26:2344–2349. doi: 10.1002/eji.1830261011. [DOI] [PubMed] [Google Scholar]
- 7.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 8.Altincicek B, Moll J, Campos N, Foerster G, Beck E, Hoeffler JF, Grosdemange-Billiard C, Rodriguez-Concepcion M, Rohmer M, Boronat A, Eberl M, Jomaa H. Cutting edge: human gamma delta T cells are activated by intermediates of the 2-C-methyl-D-erythritol 4-phosphate pathway of isoprenoid biosynthesis. J Immunol. 2001;166:3655–3658. doi: 10.4049/jimmunol.166.6.3655. [DOI] [PubMed] [Google Scholar]
- 9.Puan KJ, Jin C, Wang H, Sarikonda G, Raker AM, Lee HK, Samuelson MI, Marker-Hermann E, Pasa-Tolic L, Nieves E, Giner JL, Kuzuyama T, Morita CT. Preferential recognition of a microbial metabolite by human V{gamma}2V{delta}2 T cells. Int Immunol. 2007 doi: 10.1093/intimm/dxm031. [DOI] [PubMed] [Google Scholar]
- 10.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/s0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 11.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. V gamma 2V delta 2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 12.Morita CT, Lee HK, Wang H, Li H, Mariuzza RA, Tanaka Y. Structural features of nonpeptide prenyl pyrophosphates that determine their antigenicity for human gamma delta T cells. J Immunol. 2001;167:36–41. doi: 10.4049/jimmunol.167.1.36. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci U S A. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Kobayashi H, Terasaki T, Toma H, Aruga A, Uchiyama T, Mizutani K, Mikami B, Morita CT, Minato N. Synthesis of Pyrophosphate-Containing Compounds that Stimulate Vgamma2Vdelta2 T Cells: Application to Cancer Immunotherapy. Med Chem. 2007;3:85–99. doi: 10.2174/157340607779317544. [DOI] [PubMed] [Google Scholar]
- 16.Belmant C, Espinosa E, Halary F, Tang Y, Peyrat MA, Sicard H, Kozikowski A, Buelow R, Poupot R, Bonneville M, Fournie JJ. A chemical basis for selective recognition of nonpeptide antigens by human delta T cells. Faseb J. 2000;14:1669–1670. doi: 10.1096/fj.99-0909fje. [DOI] [PubMed] [Google Scholar]
- 17.Boedec A, Sicard H, Dessolin J, Herbette G, Ingoure S, Raymond C, Belmant C, Kraus JL. Synthesis and Biological Activity of Phosphonate Analogues and Geometric Isomers of the Highly Potent Phosphoantigen (E)-1-Hydroxy-2-methylbut-2-enyl 4-Diphosphate. J Med Chem. 2008 doi: 10.1021/jm701101g. [DOI] [PubMed] [Google Scholar]
- 18.Davodeau F, Peyrat MA, Hallet MM, Gaschet J, Houde I, Vivien R, Vie H, Bonneville M. Close correlation between Daudi and mycobacterial antigen recognition by human gamma delta T cells and expression of V9JPC1 gamma/V2DJC delta-encoded T cell receptors. J Immunol. 1993;151:1214–1223. [PubMed] [Google Scholar]
- 19.Casorati G, De Libero G, Lanzavecchia A, Migone N. Molecular analysis of human gamma/delta+ clones from thymus and peripheral blood. J Exp Med. 1989;170:1521–1535. doi: 10.1084/jem.170.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita S, Tanaka Y, Harazaki M, Mikami B, Minato N. Recognition mechanism of non-peptide antigens by human {gamma}{delta} T cells. Int Immunol. 2003;15:1301–1307. doi: 10.1093/intimm/dxg129. [DOI] [PubMed] [Google Scholar]
- 21.Allison TJ, Garboczi DN. Structure of gammadelta T cell receptors and their recognition of non-peptide antigens. Mol Immunol. 2002;38:1051–1061. doi: 10.1016/s0161-5890(02)00034-2. [DOI] [PubMed] [Google Scholar]
- 22.Rojas RE, Torres M, Fournie JJ, Harding CV, Boom WH. Phosphoantigen presentation by macrophages to mycobacterium tuberculosis--reactive Vgamma9Vdelta2+ T cells: modulation by chloroquine. Infect Immun. 2002;70:4019–4027. doi: 10.1128/IAI.70.8.4019-4027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato Y, Tanaka Y, Hayashi M, Okawa K, Minato N. Involvement of CD166 in the Activation of Human {gamma}{delta}T Cells by Tumor Cells Sensitized with Nonpeptide Antigens. J Immunol. 2006;177:877–884. doi: 10.4049/jimmunol.177.2.877. [DOI] [PubMed] [Google Scholar]
- 24.Wei H, Huang D, Lai X, Chen M, Zhong W, Wang R, Chen ZW. Definition of APC presentation of phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate to Vgamma2Vdelta2 TCR. J Immunol. 2008;181:4798–4806. doi: 10.4049/jimmunol.181.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarikonda G, Wang H, Puan KJ, Liu XH, Lee HK, Song Y, Distefano MD, Oldfield E, Prestwich GD, Morita CT. Photoaffinity Antigens for Human {gamma}{delta} T Cells. J Immunol. 2008;181:7738–7750. doi: 10.4049/jimmunol.181.11.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced gamma, delta-T-cell proliferation and activation in vitro. J Bone Miner Res. 2004;19:278–288. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- 28.Monkkonen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsalainen J, Monkkonen J. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol. 2006;147:437–445. doi: 10.1038/sj.bjp.0706628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryu Y, Scott AI. Efficient One-Step Syntheses of Isoprenoid Conjugates of Nucleoside 5′-Diphosphates. Org Lett. 2003;5:4713–4715. doi: 10.1021/ol035880b. [DOI] [PubMed] [Google Scholar]
- 30.Arrode G, Boccaccio C, Abastado JP, Davrinche C. Cross-presentation of human cytomegalovirus pp65 (UL83) to CD8+ T cells is regulated by virus-induced, soluble-mediator-dependent maturation of dendritic cells. J Virol. 2002;76:142–150. doi: 10.1128/JVI.76.1.142-150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 32.Bollen M, Gijsbers R, Ceulemans H, Stalmans W, Stefan C. Nucleotide pyrophosphatases/phosphodiesterases on the move. Critical reviews in biochemistry and molecular biology. 2000;35:393–432. doi: 10.1080/10409230091169249. [DOI] [PubMed] [Google Scholar]
- 33.Grobben B, Anciaux K, Roymans D, Stefan C, Bollen M, Esmans EL, Slegers H. An ecto-nucleotide pyrophosphatase is one of the main enzymes involved in the extracellular metabolism of ATP in rat C6 glioma. J Neurochem. 1999;72:826–834. doi: 10.1046/j.1471-4159.1999.0720826.x. [DOI] [PubMed] [Google Scholar]
- 34.Lang F, Peyrat MA, Constant P, Davodeau F, David-Ameline J, Poquet Y, Vie H, Fournie JJ, Bonneville M. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–5994. [PubMed] [Google Scholar]
- 35.Spencer CT, Abate G, Blazevic A, Hoft DF. Only a subset of phosphoantigen-responsive gamma9delta2 T cells mediate protective tuberculosis immunity. J Immunol. 2008;181:4471–4484. doi: 10.4049/jimmunol.181.7.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devilder MC, Maillet S, Bouyge-Moreau I, Donnadieu E, Bonneville M, Scotet E. Potentiation of Antigen-Stimulated V{gamma}9V{delta}2 T Cell Cytokine Production by Immature Dendritic Cells (DC) and Reciprocal Effect on DC Maturation. J Immunol. 2006;176:1386–1393. doi: 10.4049/jimmunol.176.3.1386. [DOI] [PubMed] [Google Scholar]
- 37.Monkkonen H, Ottewell PD, Kuokkanen J, Monkkonen J, Auriola S, Holen I. Zoledronic acid-induced IPP/ApppI production in vivo. Life Sci. 2007 doi: 10.1016/j.lfs.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 39.Goding JW, Grobben B, Slegers H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochimica et biophysica acta. 2003;1638:1–19. doi: 10.1016/s0925-4439(03)00058-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


