Abstract
Recently a number of randomized trials have shown that patients with advanced colorectal cancer do not benefit from therapies targeting the epidermal growth factor receptor when their tumors harbor mutations in the KRAS, BRAF and PIK3CA genes. We developed two multiplex assays that simultaneously screen 22 nucleotides in the KRAS, NRAS, BRAF and PIK3CA genes for mutations. The assays were validated on 294 tumor DNA samples from patients with advanced colorectal cancer. In these samples 119 KRAS codon 12 and 13 mutations had been identified by sequence analysis, 126 tumors were wild-type for KRAS and the analysis failed in 49 of the 294 samples due to poor DNA quality. The two mutation assays detected 130 KRAS mutations, among which were 3 codon 61 mutations, and in addition 32 PIK3CA, 13 BRAF and 6 NRAS mutations. In 19 tumors a KRAS mutation was found together with a mutation in the PIK3CA gene. One tumor was mutant for both PIK3CA and BRAF. In summary, the mutations assays identified 161 tumors with a mutation, 120 were wild-type and the analysis failed in 13. The material cost of the 2 mutation assays was calculated to be 8-fold lower than the cost of sequencing required to obtain the same data. In addition, the mutation assays are less labor intensive. We conclude that the performance of the two multiplex mutation assays was superior to direct sequencing. In addition, these assays are cheaper and easier to interpret. The assays may also be of use for selection of patients with other tumor types.
Introduction
Recently a number of randomized trials have shown that treatment of patients with advanced colorectal cancer (CRC) do not benefit from therapies targeting the epidermal growth factor receptor (EGFR) when their tumors harbor mutations in the KRAS, BRAF and PIK3CA genes [1], [2], [3]. Consequently, KRAS mutation analysis is a prerequisite for anti-EGFR therapy in metastasized CRC and only patients with tumors that harbor no KRAS mutations receive this therapy (European Medicine Agency – EMEA-H-C-741 and H-C-558 and U.S. Food and Drug Administration - FDA Application No. (BLA) 125084 and No. (BLA) 125147). Recent publications suggest that mutations in BRAF and PIK3CA may also confer resistance to anti-EGFR therapy, although this is not entirely clear for PIK3CA yet [3], [4], [5], [6], [7], [8], [9], [10]. In addition, mutations in KRAS, BRAF and PIK3CA are associated with a worse outcome in patients with colorectal cancer [11], [12]. The protein encoded by the NRAS gene functions in the same pathway as KRAS and mutations in this gene have been found in 3% of CRC (http://www.sanger.ac.uk/genetics/CGP/cosmic/). The NRAS gene is highly expressed in CRC (http://www.oncomine.org), hence it is to be expected that tumors with an NRAS mutations are resistant to EGFR targeted therapy.
The above findings suggest that mutation analysis for the KRAS, NRAS, BRAF and PIK3CA genes should be implemented in molecular diagnostic laboratories. Together these genes harbor 22 possible mutation sites distributed over 7 exons. Mutation analysis by sequencing therefore typically requires 7 individual PCR reactions followed by 14 bi-directional sequence reactions. We have previously developed a multiplex assay for the identification of 11 possible point mutations in the gene for the fibroblast growth factor receptor 3 (FGFR3) [13] and 4 hotspot mutations in PIK3CA [14]. These mutations are a common phenomenon in primary and recurrent urinary bladder carcinomas and various skin lesions [15], [16], [17], [18], [19]. The FGFR3 mutation assay needs little DNA, has a high performance rate on DNA isolated from formalin-fixed paraffin embedded tissue (FFPE DNA) and urine and was found to be highly reproducible. Bearing this in mind we set out to develop similar assays for mutations in the KRAS, NRAS, BRAF and PIK3CA genes. This resulted in two multiplex assays, one for BRAF and KRAS mutations and one for PIK3CA and NRAS. The performance of the assays was tested on 294 CRC samples that had been sequenced for mutations in KRAS exon 2 and was found to be superior to sequencing.
Materials and Methods
Patient Characteristics and Ethics Statement
The samples used in the presented study were taken from a consecutive series of metastasized colon carcinoma cases analyzed for KRAS mutation status in the course of routine molecular pathological identification of applicable patients for anti-EGFR therapy at the Institute of Pathology, Erlangen, Germany. All participants for mutation analysis gave written informed consent via the treating physician. Ethical approval for the retrospective use of paraffin material for the study was given by the ethical committee of the University Erlangen.
DNA Isolation and Sequence Analysis
Tumor tissue was marked on a hematoxylin-eosin-stained tissue section by an experienced surgical pathologist (AH). After deparaffinization and rehydration tumor cells were carefully microdissected manually from serial sections. DNA was isolated using the QIAamp® DNA FFPE Tissue Kit (QIAGEN, Hilden, Germany). Quantity and quality of the DNA were controlled using a spectral photometer (NanoDrop®, peQLab, Erlangen, Germany). Exon 2 of KRAS was amplified using PCR primers published previously [20] and the QIAGEN® Multiplex PCR Kit using 150–200 ng DNA. PCR cycles were as follows: 94°C for 5 min, 35 cycles of 94°C for 1 min, 60°C for 1 min, 72°C for 1 min, followed by an elongation step at 72°C for 10 min. Sequence analysis in both directions was performed using PCR primers and the Big Dye® Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). Products from sequence reaction were purified using the DyeEx® 2.0 Spin Kit (QIAGEN, Hilden, Germany) and analysed by capillary electrophoresis (ABI PRISM® 310 Genetic Analyzer, Applied Biosystems, Foster City, CA, USA). DNA from cell line HCT116 (obtained from ATCC, Middlesex, United Kingdom) containing a heterozygous G13D mutation was used as a control for each analysis.
Mutation Assays
Two multiplex PCRs were designed, the first for BRAF exon 15 and KRAS exons 2 and 3 and the second for PIK3CA exons 9 and 20 and NRAS exons 2 and 3. The primers were chosen in such a way that the single strands of the PCR products contained as little potential secondary structure as possible in order to facilitate efficient annealing of the mutation detection probes. Primers and probes for PIK3CA were derived from Hurst [14]. Primer sequences for multiplex PCR are given in Supplementary Table S1. The multiplex PCR was performed in a total volume of 15 µl, containing 1x PCR buffer, 1.5 mM MgCl2, 0.5 units Go-Taq DNA polymerase (Promega, Madison, WI), 0.17 mM dNTP's (Roche, Basel, Switzerland), 0.3–1 µM primers (Invitrogen, Carlsbad, CA), 5% glycerol (Fluka, Buchs SG, Switzerland) and 5 ng genomic DNA. Thermal cycling conditions were: 5 minutes at 95°C, 35 cycles at 95°C for 45 seconds, 55°C for 45 seconds and 72°C for 45 seconds, followed by 10 minutes at 72°C. The PCR products were treated with 2 units Exonuclease I (ExoI) and 3 units Shrimp Alkaline Phosphatase (SAP) (USB, Cleveland, Ohio USA). This was followed by a single nucleotide probe extension assay using a SNaPshot Multiplex kit (Applied Biosystems, Foster City, CA) and probes designed to anneal to either the forward or the reverse strand of a PCR product adjacent to the mutation site of interest. These probes were fitted with T tails of different length at their 5′ends to allow separation of the extension products by size. The mutation detection reactions were performed in a total volume of 10 µl, containing 1 µl SAP/ExoI treated PCR product, 2.5 µl SNaPshot Multiplex Ready Reaction mix, 1 x Big Dye sequencing buffer and 1 µl probe mix. Thermal cycler conditions were: 35 cycles of 10 seconds at 96°C and 40 seconds at 58.5°C. The products were treated with 1 unit SAP at 37°C for 60 min and 72°C for 15 min and analyzed on an automatic sequencer (ABI PRISM 3130 XL Genetic Analyzer, Applied Biosystems) with the fluorescent label on the incorporated ddNTP indicating the presence or absence of a mutation. For analysis of the data Genescan Analysis Software version 3.7 (Applied Biosystems) was used. Supplementary Table S2 gives an overview of the probes used and indicates the peak color that correlates with each mutation. Contamination may occur when lifting the cover of the PCR plate after PCR and when material from the PCR reaction is transferred to another well for sequencing or for the mutation assay. This risk is the same for sequencing and the mutation assays. Contamination will result in relatively small mutant peaks because only a fraction of the PCR reaction will have been transferred to another well. Because of this, we usually independently verify a mutation when the mutant peak is lower than 10% of the wild type peak.
Results
Mutation Detection Assays
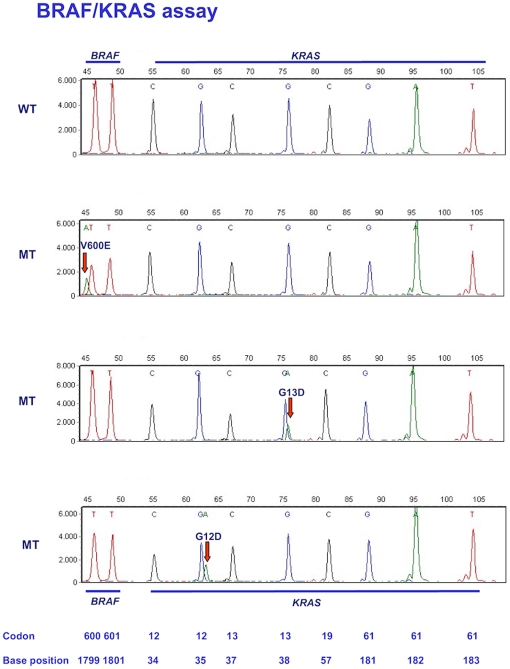
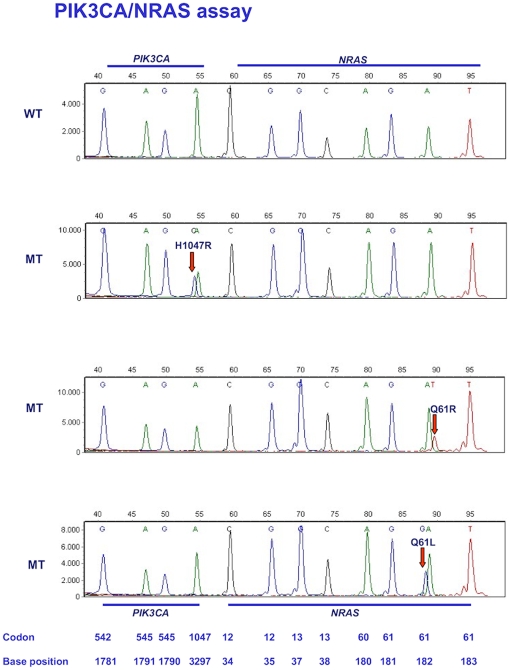
The BRAF/KRAS assay is depicted in Figure 1 with the interrogated codons and nucleotides shown at the bottom. The colors of the peaks indicate the nature of the specific dideoxynucleotide that was added to the mutation detection probe. The top panel is a wild type control and the three other panels show examples of mutations. When a mutation is present a different dideoxynucleotide is incorporated resulting in a peak of a different color. Because the type of fluorescent label influences separation through the polymer, mutant and wild type extension products usually migrate to slightly different positions, further facilitating identification of mutations. The BRAS/KRAS assay simultaneously interrogates 10 nucleotides in 3 exons for 22 possible point mutations. Figure 2 depicts the PIK3CA/NRAS assay for wild type control DNA and 3 samples containing mutations. This assay is able to detect 25 possible mutations in 12 nucleotides in 4 exons.
Figure 1. Assay for BRAF and KRAS mutations.
Panel A: wild-type control. Panels B–D: examples of BRAF and KRAS mutations. Positions of codons and nucleotides are indicated at the bottom of the figure.
Figure 2. Assay for PIK3CA and NRAS mutations.
Panel A: wild-type control. Panels B–D: examples of PIK3CA and NRAS mutations. Positions of codons and nucleotides are indicated at the bottom of the figure.
Validation of the Assays
To validate the assays we analyzed DNA samples isolated from 294 CRCs that had already been analyzed for mutations in exon 2 of the KRAS gene. In 281/294 (96%) of the samples the two mutation assays were successful in establishing a mutant or wild-type outcome. Mutations were identified in 161/281 (57%) of the cases and120 samples were wild-type (43%). We then we performed a second independent mutation analysis on all samples and observed that the results of the BRAF/KRAS and PIK3CA/NRAS assays were completely reproducible. We found 130 KRAS, 32 PIK3CA, 13 BRAF and 6 NRAS mutations. Details of the mutations are given in table 1. Of the 32 PIK3CA mutations 12 were single mutations, 19 occurred together with a KRAS mutation and 1 with a BRAF mutation. The mutation assays were performed in a blinded fashion and the results were compared afterwards with the results of the sequence analysis. There were 14 samples with discrepant results between sequence analysis for KRAS exon 2 (covering codons 12 and 13) and the BRAF/KRAS mutation assay. As the latter had already been confirmed by an independent assay, the 14 samples were resequenced. In 9 cases, the sequence outcome now appeared identical to the mutation assay result and in 4 cases sequencing was unsuccessful due to poor DNA quality. In the 1 remaining sample (no. 289) sequencing suggested wild-type whereas the mutation assay detected a G12V mutation with the mutant peak being 6% of the wild type peak, suggesting that the tumor was heterogeneous. Figure 3 depicts a concise overview of sequence and mutation assay results. A detailed overview of the results of sequencing and mutation assays is given in Supplementary Table S3. In this table we also included the relative peak height of the mutant peaks compared to the wild type peak as observed in the mutation assays. Note that this is at best semi-quantitative because of the different absorbances of the fluorescent labels, however, it gives an indication of the relative proportions of mutant and wild-type genes. Wild-type peaks in the assays have a height between 2000–8000. Peaks (mutant) with a peak height of 100 are always visible. Based on this we estimate that sensitivity is between 1–5%. This correlates well with the sensitivity calculated from dilution experiments in a similar assay as published previously [13]. Note that in some samples the mutant KRAS peaks were much higher than the peaks representing the wild type allele. We presume that this indicates loss of the wild-type allele.
Table 1. Mutations identified with the BRAF/KRAS and PIK3CA/NRAS assays.
| Gene | Codon | No. | Total |
| KRAS | |||
| G12A | 5 | ||
| G12C | 11 | ||
| G12D | 48 | ||
| G12S | 6 | ||
| G12V | 35 | ||
| G13D | 21 | ||
| G13R | 1 | ||
| Q61L | 2 | ||
| Q61H | 1 | 130 | |
| PIK3CA | |||
| E542K | 5 | ||
| E545G | 1 | ||
| E545K | 13 | ||
| H1047R | 13 | 32 | |
| BRAF | |||
| V600E | 13 | 13 | |
| NRAS | |||
| G12V | 2 | ||
| Q61L | 1 | ||
| Q61R | 3 | 6 | |
Figure 3. Overview of the resultsobtained by the mutation assays (A) and by sequence analysis (B) in 294 tumor DNA samples from patients with advanced colorectal cancer.
Costs Comparison
Next we compared the costs of both approaches. We observed mutations in all 4 genes, hence we assume that future mutation analysis, be it by sequencing or any other technique, will include the PIK3CA, NRAS and BRAF genes. We calculated the costs for all materials and reagents including those associated with the running of samples on the ABI 3130 XL sequencer. These amounted to € 7.03 for the two mutation assays together and € 59.54 for bi-directional sequencing of the 7 exons. Details are given in Supplementary Table S4. Personnel costs are also lower for the mutation assays compared to sequencing. This is because only 2 PCR reactions have to be performed compared to 7 for sequencing and only 2 electrophoresis runs need to be performed compared to 14 for sequence analysis. There is no need to buy other equipment than that required for sequence analysis. Any laboratory with PCR machines and a capillary sequencer can perform these assays. The cost comparison thus indicates that the mutation assays are less expensive than sequencing.
Discussion
The EGF receptor signals through the RAS-MAPK and PIK3 kinase pathways. Activating mutations in proteins that function downstream of the EGFR in these signal transduction pathways renders the cell independent of EGFR signaling. Hence inhibition of the receptor has no effect. Most other receptor tyrosine kinases employ the same signaling pathways and therefore the same mechanism is likely to apply to trastuzumab resistant breast cancer or gefitinib or erlotinib resistant NSCLC [21]. KRAS mutation analysis is now standard for selection of patients for EGFR targeted therapy. Recently, it has been shown that mutations in the PIK3CA and BRAF genes may also confer resistance to anti-EGFR therapy although patient numbers are still small [3], [4], [6], [7], [8], [22], [23]. It is likely that patients with tumors harboring NRAS mutations will also not benefit from anti-EGFR therapy, however, this remains to be proven which is also the case for mutations in the NRAS gene. Once this has been established, mutation analysis for the selection of patients for whom anti-EGFR therapy will be beneficial presumably has to be extended to KRAS exon 3, PIK3CA, BRAF and NRAS. Here we presented two simple assays that together screen 22 mutation sites in the KRAS, BRAF, PIK3CA and NRAS oncogenes. When we dismiss the failed samples, KRAS exon 2 mutations were found by sequence analysis in 119/245 (49%) of the patients, the mutation assays detected 161/281 (57%) patients with mutant tumors. This suggests that when both sequencing and mutation analysis are 100% successful, 8% of patients (57 minus 49) would receive anti-EGFR therapy in vain when only exon 2 of KRAS would have been assayed. In practice, patients with tumors in which the analysis failed will receive anti-EGFR therapy, suggesting that efficient mutation detection is important.
Identification of the mutations in the assays presented here is more straight forward than with sequencing and the results were completely reproducible. Sequence analysis failed in 17% of the samples. This was 4% for the KRAS mutation assay. The DNA samples used in our analyses were FFPE derived. It is known that FFPE derived DNA is not always of good quality and this was indeed the reason for the failed samples. In principle sequencing and the mutation assays are similar techniques. That the mutation assay is able to give a result in more samples than sequencing is probably because the fluorescent signals are distributed over fewer peaks. In cases with poor DNA quality, this is also immediately apparent from much lower or even missing peaks. Although not used in this work, it is possible to repeat the mutation assay with a single probe when one doubts the result, for instance when the presumed mutant peak is very small.
For analysis of all the mutations by sequencing one would have to perform independent PCRs for the 7 exons, followed by 14 bi-directional sequencing reactions. In the mutation assays two multiplex PCRs for 3 and 4 exons, respectively, are followed by two multiplex detection assays and two electrophoresis runs on the sequencer. Besides being considerably cheaper (€7 compared to €60), less DNA is needed and the assays are less labor intensive.
We conclude that these assays provide a simple and inexpensive companion diagnostic for the selection of CRC patients for anti-EGFR therapy. The assays may also be of use for selection of patients with ERBB2 positive breast cancer or non-small cell lung cancer carrying EGFR mutations.
Supporting Information
Primer sequences for multiplex PCR.
(0.02 MB XLS)
Overview of the probes used and indication of the peak color that correlates with each mutation.
(0.02 MB XLS)
Detailed overview of the results of sequencing and mutation assays.
(0.06 MB XLS)
Costs: Mutation Assays vs. Sequencing (Bi-directional).
(0.02 MB XLS)
Acknowledgments
We thank Ms. Claudia Knoll and Ms. Andrea Kosel for excellent technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a research grant by the German Research Foundation (DFG) to AH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 2.Raponi M, Winkler H, Dracopoli NC. KRAS mutations predict response to EGFR inhibitors. Curr Opin Pharmacol. 2008;8:413–418. doi: 10.1016/j.coph.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101:1308–1324. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–1857. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 5.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–2648. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 6.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 7.Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, Molinari F, De Dosso S, et al. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One. 2009;4:e7287. doi: 10.1371/journal.pone.0007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhawer M, Goel S, Wilson AJ, Montagna C, Ling YH, et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008;68:1953–1961. doi: 10.1158/0008-5472.CAN-07-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prenen H, De Schutter J, Jacobs B, De Roock W, Biesmans B, et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res. 2009;15:3184–3188. doi: 10.1158/1078-0432.CCR-08-2961. [DOI] [PubMed] [Google Scholar]
- 10.Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–5930. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- 11.Barault L, Veyrie N, Jooste V, Lecorre D, Chapusot C, et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer. 2008;122:2255–2259. doi: 10.1002/ijc.23388. [DOI] [PubMed] [Google Scholar]
- 12.Ogino S, Nosho K, Kirkner GJ, Shima K, Irahara N, et al. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol. 2009;27:1477–1484. doi: 10.1200/JCO.2008.18.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Oers JM, Lurkin I, van Exsel AJ, Nijsen Y, van Rhijn BW, et al. A simple and fast method for the simultaneous detection of nine fibroblast growth factor receptor 3 mutations in bladder cancer and voided urine. Clin Cancer Res. 2005;11:7743–7748. doi: 10.1158/1078-0432.CCR-05-1045. [DOI] [PubMed] [Google Scholar]
- 14.Hurst CD, Zuiverloon TC, Hafner C, Zwarthoff EC, Knowles MA. A SNaPshot assay for the rapid and simple detection of four common hotspot codon mutations in the PIK3CA gene. BMC Res Notes. 2009;2:66. doi: 10.1186/1756-0500-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger M, van der Aa MN, van Oers JM, Brinkmann A, van der Kwast TH, et al. Prediction of progression of non-muscle-invasive bladder cancer by WHO 1973 and 2004 grading and by FGFR3 mutation status: a prospective study. Eur Urol. 2008;54:835–843. doi: 10.1016/j.eururo.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Hafner C, van Oers JM, Vogt T, Landthaler M, Stoehr R, et al. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201–2207. doi: 10.1172/JCI28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junker K, van Oers JM, Zwarthoff EC, Kania I, Schubert J, et al. Fibroblast growth factor receptor 3 mutations in bladder tumors correlate with low frequency of chromosome alterations. Neoplasia. 2008;10:1–7. doi: 10.1593/neo.07178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Oers JM, Zwarthoff EC, Rehman I, Azzouzi AR, Cussenot O, et al. FGFR3 Mutations Indicate Better Survival in Invasive Upper Urinary Tract and Bladder Tumours. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Kompier LC, van der Aa MN, Lurkin I, Vermeij M, Kirkels WJ, et al. The development of multiple bladder tumour recurrences in relation to the FGFR3 mutation status of the primary tumour. J Pathol. 2009;218:104–112. doi: 10.1002/path.2507. [DOI] [PubMed] [Google Scholar]
- 20.Feng YZ, Shiozawa T, Miyamoto T, Kashima H, Kurai M, et al. BRAF mutation in endometrial carcinoma and hyperplasia: correlation with KRAS and p53 mutations and mismatch repair protein expression. Clin Cancer Res. 2005;11:6133–6138. doi: 10.1158/1078-0432.CCR-04-2670. [DOI] [PubMed] [Google Scholar]
- 21.Hynes NE, Macdonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009 doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Perrone F, Lampis A, Orsenigo M, Di Bartolomeo M, Gevorgyan A, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol. 2009;20:84–90. doi: 10.1093/annonc/mdn541. [DOI] [PubMed] [Google Scholar]
- 23.Souglakos J, Philips J, Wang R, Marwah S, Silver M, et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer. 2009;101:465–472. doi: 10.1038/sj.bjc.6605164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences for multiplex PCR.
(0.02 MB XLS)
Overview of the probes used and indication of the peak color that correlates with each mutation.
(0.02 MB XLS)
Detailed overview of the results of sequencing and mutation assays.
(0.06 MB XLS)
Costs: Mutation Assays vs. Sequencing (Bi-directional).
(0.02 MB XLS)