Summary
The treatment of relapsed acute myeloid leukemia (AML) remains unsatisfactory. We conducted a phase II randomized trial where patients received intermediate-dose cytarabine for four days followed by gemtuzumab ozogamicin on day 5 (Arm A), or combined with liposomal daunorubicin for three days (Arm B), or cytarabine given for five days combined with cyclophosphamide for three days and topotecan by continuous infusion for 5 days (Arm C). Eligible patients had primary refractory AML, a first relapse after a remission of less than one year, or a second or greater relapse. The primary objective of this trial was attainment of a conventional complete remission (CR) or a CR without platelet recovery (CRp) in at least 40% of patients. The CR/CRp rates for the 82 eligible patients were 3/26 (12%) in Arm A, 2/29 (7%) in Arm B, and 1/27 (4%) in Arm C. No patients who had relapsed within six months of initial CR or who had suffered multiple relapses responded. More than 95% of patients subsequently died of AML. No unexpected toxicities were encountered. We conclude that none of these three regimens were effective enough in the treatment of high-risk relapsed or refractory AML to warrant further study. This trial was registered at http://www.clinicaltrials.gov as #NCT00005962.
Keywords: acute myeloid leukemia, relapse, gemtuzumab ozogamicin, liposomal daunorubicin, topotecan
Introduction
Despite remission rates as high as 70–80% for patients with newly diagnosed acute myeloid leukemia (AML), many patients still relapse (Litzow 2004). The median duration of complete remission (CR) is generally less than one year and not more than 40% of younger patients with AML are cured of their leukemia (Shipley and Butera 2009). Patients with poor risk disease features at presentation, such as unfavorable cytogenetics and antecedent hematological disorders or older age, tend to have lower CR rates and lower durations of CR (Grimwade, et al 1998, Slovak, et al 2000). Salvage chemotherapy can induce remissions in up to 50% of patients who relapse or do not respond to initial induction chemotherapy, but their long-term prognosis is poor. Allogeneic and autologous blood and marrow transplantation can provide long-term disease control for some of these patients, but most patients with refractory or relapsed AML will succumb to their disease (Rowe, et al 2005). Clearly, the development of new and more effective agents for the treatment of AML will be important in improving disease control and patient survival.
In recent years, new agents, modified older agents (e.g, liposomal formulations) and new combinations of conventional drugs have been developed and studied. Gemtuzumab ozogamicin (GO) contains a monoclonal antibody directed against CD33. CD33 is a surface antigen that is expressed on early myeloid cells and has been found to be present in more than 90% of cases of AML (Dinndorf, et al 1986, Griffin, et al 1984). This humanized anti-CD33 antibody is conjugated to a potent anti-tumor antibiotic and intercalating agent, calicheamicin, to make up the complete GO molecule (Fenton and Perry 2005). Phase II trials of two doses of GO administered two weeks apart demonstrated CR and CR without platelet recovery (CRp) rates of 16% and 13%, respectively, in relapsed AML (Sievers, et al 2001).
Anthracyclines remain the mainstay of treatment of AML, but are limited by significant toxicity including cardiotoxicity, mucositis, and myelosuppression. Liposomal encapsulation of the anthracyclines offers the opportunity to deliver the drugs with equal or improved clinical efficacy and reduced toxicity. Liposomal daunorubicin has been combined with cytarabine and dose escalated without significant additional toxicity (Cripe, et al 1998).
Topotecan is a topoisomerase-1 inhibitor that has been shown to have significant anti-leukemic activity as a single agent (Kantarjian, et al 1993). It has been combined with cytarabine and demonstrated activity in myelodysplastic syndromes (Beran, et al 1999). Subsequently, cyclophosphamide was combined with topotecan and cytarabine with the rationale that cyclophosphamide causes DNA damage in leukemia cells and topotecan inhibits the repair of that damage (Estey 1998). This combination has been tested in patients with AML and high-grade myelodysplastic syndromes, and CR rates of 67% have been achieved (Estey, et al 1998).
Based on the studies described above, the Eastern Cooperative Oncology Group (ECOG) conducted a randomized phase II trial of GO and of liposomal daunorubicin each combined with cytarabine and of cyclophosphamide, cytarabine, and topotecan regimen in patients with refractory AML or AML beyond first remission.
Patients, Materials and Methods
Study design and patients
Adult patients eligible for this trial were required to have AML of any French-American-British (FAB) classification subtype except patients with AML-M3 were excluded. Patients were stratified into those who had i) relapsed less than 6 months after first CR, ii) relapsed between 6 and 12 months after first CR, iii) were refractory to conventional induction chemotherapy (two courses or less of initial induction or one course of first re-induction) or iv) were in second or greater relapse.
Documentation of CD33 positivity of the patients’ AML blast cells was required for entry into the study. Patients had to have evidence of normal cardiac function and not have received excessive doses of anthracyclines. They could not have received prior treatment with GO, liposomal daunorubicin or topotecan. Adequate organ function and an ECOG performance score of 0–2 were required.
All cases underwent central pathological review and were assigned a FAB subtype whenever possible. Informative immunophenotypes were established centrally by multiparameter flow cytometry using a FACSCalibur (Beckton-Dickinson, Mountainview, CA) and CellQuest software. Antibody panels conformed to those previously reported (Paietta, et al 2003).
Chromosome studies were acceptable for statistical analysis for those patients with ≥ 20 normal bone marrow metaphases or ≥ 5 abnormal blood or bone marrow metaphases. For each patient, we recorded the presence or absence of chromosomally abnormal clones (Heim and Mitelman 1995), percentage of abnormal metaphases, number of chromosome abnormalities, number of subclones and number of clones. Each patient was assigned to a cytogenetic risk category based on cytogenetic anomaly categories according to published Southwestern Oncology Group/ECOG guidelines (Appelbaum, et al 2006, Peniket, et al 2005, Slovak, et al 2000). Chromosome anomalies were considered primary events for well-known translocations, inversions, deletions, or sole numeric anomalies.
Treatment
Patients were randomized to one of the following regimens. Arm A consisted of intermediate dose cytarabine followed by GO. Arm B consisted of the same dose of cytarabine as in Arm A but it was combined with liposomal daunorubicin. Arm C consisted of cyclophosphamide, mesna, the same dose of cytarabine as noted above and topotecan, hereafter referred to as the CAT regimen. The treatment schedules are summarized in Table 1.
Table 1.
Dose and Schedule of Chemotherapy Regimens
| Arm A: | |
| • | Cytarabine 1 gm/m2/ day IV, days 1–4 |
| • | Gemtuzumab ozogamicin 6 mg/m2 IV, day 5 |
| Arm B: | |
| • | Cytarabine 1 gm/m2/ day IV, days 1–4 |
| • | Liposomal daunorubicin 135 mg/m2/day IV, days 1–3 |
| Arm C: | |
| • | Cyclophosphamide 300 mg/m2 every 12 hours IV, days 1–3 |
| • | Cytarabine 1 gm/m2/ day IV, days 2–6 |
| • | Topotecan 1.5 mg/m2/day CIV, days 2–6 |
| • | Mesna 600 mg/m2/day CIV, days 1–3 |
Abbreviations: IV intravenous infusion, CIV continuous intravenous infusion
A bone marrow biopsy was performed five to seven days after completion of study therapy. Biopsies with leukemic blasts ≥ 10% were interpreted to have persistent leukemia and protocol therapy was discontinued. If leukemic blasts were <10%, weekly bone marrow biopsies were repeated until remission or progression was documented. Supportive care measures were as per standard therapy. Sargramostim ( granulocyte-macrophage colony-stimulating factor, Leukine®) was given intravenously over 4 h at the dose of 250 µg/m2/day following documentation of marrow hypoplasia and was continued until the neutrophil count was > 1.5 ×109/l for three consecutive days.
Patients who achieved a CR were allowed to be taken to alternative therapies including blood and marrow transplantation at the discretion of the investigator or could receive one additional course of the same treatment and duration that was given at induction, except those patients on Arm B who received a consolidation course of liposomal daunorubicin at a reduced dose of 100 mg/m2/day in combination with cytarabine.
This study was conducted by the Leukemia Committee of ECOG. All patients gave written informed consent and the protocol was approved by each participating institution’s Institutional Review Board.
Statistical methods
Patients were randomized using the stratification factor of relapse status as noted above: i) relapse less than 6 months after first CR, ii) relapse between 6 and 12 months after CR, iii) refractory to conventional induction chemotherapy and iv) second or greater relapse.
Treatment assignments for patients at all institutions were assigned from the central randomization desk at the ECOG Coordinating Center. The treatments were assigned using permuted blocks within strata with dynamic balancing within main institution and their affiliate networks.
The primary endpoint of the study was the achievement of a CR (defined as a neutrophil count > 1.0 × 109/l, platelet count of 100 × 109/l, and a cellular bone marrow (> 20%) with ≤ 5% blasts) or a CR without platelet recovery (CRp), which was defined when the criteria for CR were fulfilled except platelet counts were > 50 but < 100 × 109/l. Partial remission was defined when all criteria for complete remission were satisfied except the bone marrow contained between 5 and 25% blasts (Cheson, et al 2003). Relapse upon CR was defined as reappearance of blasts in the blood or the presence of > 5% blasts in the bone marrow not attributable to bone marrow regeneration. Stable disease was defined as patients who were not responders but who had also not progressed.
At least a 40% CR plus CRp rate was required to consider one of these regimens promising for further study. A two-stage design was planned. In the first stage, 26 patients would be accrued to each arm, assuming that 24 would be eligible. If at least seven CR or CRp were seen among the 24 eligible patients, then an additional 29 patients (assuming 26 eligible) would be entered on that arm. If 17 or more responses were seen in 50 eligible patients, this was considered to be evidence of a promising response rate. Using this study design, the chance of concluding that the treatment was active was 0.09, when the true response rate was 25%, and the chance of concluding the treatment was active was 0.81, when the true response rate was 40%; 90% confidence intervals for the observed response rate of 17/50 were (0.24, 0.49), for 20/50 were (0.29, 0.53) and for 23/50 were (0.34, 0.59).
The response rate was calculated and exact confidence intervals for the binomial parameters were provided. The disease-free survival (DFS) was defined as the time from initiation of CR or CRp to the date of progressive disease or death without relapse. As there were few responders, DFS data for each responder was displayed. Survival time was defined as the time from randomization to the date of death or the date last known alive. The survival data were analyzed using the method of Kaplan and Meier (1958) and the significance was tested by log-rank test. P-values were reported for two-sided tests, and were considered significant when ≤ 0.05.
Results
Patient characteristics
Between the dates of July 2000 and May 2002, 85 patients were accrued to the study and their characteristics are shown in Table 2. Three patients were ineligible, two in Arm A and one in Arm C. Therefore, there were 26 patients in Arm A, 29 patients in Arm B, and 27 patients in Arm C, for a total of 82 analyzable cases. The patients in Arm A had a slightly older median age of 60 years compared to 52 and 53 years in Arms B and C, respectively. Status of leukemia at study entry was evenly distributed between groups with two-thirds of the patients being in first relapse. The other third of patients were refractory to initial induction therapy, and only three patients in the entire study, two in Arm B and one in Arm C, were in second or greater relapse.
Table 2.
Patient Characteristics at Study Entry for Analyzable Cases (n=82)
| Arm A (n=26) n (%) |
Arm B (n=29) n (%) |
Arm C (n=27) n (%) |
|
|---|---|---|---|
| Age | |||
| Median | 60 | 52 | 53 |
| Range | 27–75 | 27–85 | 25–78 |
| <55 years | 10 (39) | 16 (55) | 14 (52) |
| ≥55 years | 16 (62) | 13 (45) | 13 (48 |
| Gender | |||
| Male | 14 (54) | 11 (38) | 20 (74) |
| Female | 12 (46) | 18 (62) | 7 (26) |
| Race | |||
| White | 22 (85) | 23 (79) | 27 (100) |
| Black | 1 (4) | 4 (14) | 0 (0) |
| Other | 3 (12) | 2 (7) | 0 (0) |
| FAB Classification | |||
| M1 | 8 (31) | 9 (31) | 7 (26) |
| M2 | 2 (8) | 9 (31) | 10 (37) |
| M4 | 6 (23) | 4 (14) | 3 (11) |
| M5 | 0 (0) | 3 (10) | 3 (11) |
| M6 | 2 (8) | 0 (0) | 0 (0) |
| Other+ | 8 (31) | 4 (14) | 4 (15) |
| Immunophenotype | |||
| CD65−, undifferentiated | 9 (29) | 7 (23) | 15 (48) |
| CD11b+ | 6 (32) | 10 (53) | 3 (16) |
| CD 65+, differentiated | 2 (22) | 5 (56) | 2 (22) |
| Megakaryocytic | 5 (56) | 3 (33) | 1 (11) |
| Monocytic (CD14+) | 1 (25) | 1 (25) | 2 (50) |
| Natural killer | 1 (33) | 0 (0) | 2 (67) |
| Erythroid | 1 (100) | 0 (0) | 0 (0) |
| Dendritic cell (CD4+, CD56+) | 0 (0) | 1 (100) | 0 (0) |
| Recurrence Status | |||
| Relapse ≤ 6 months after first CR | 7 (27) | 6 (21) | 6 (22) |
| Relapse > 6–12 months after first CR | 9 (35) | 11 (38) | 11 (41) |
| Refractory | 10 (39) | 10 (35) | 9 (33) |
| Second of greater relapse | 0 (0) | 2 (7) | 1 (4) |
| White Blood Cell count (×109/l) | |||
| Median | 4.9 | 3.3 | 3.4 |
| Range | 0.5–71.6 | 0.5–128.0 | 0.4–113.0 |
| Bone Marrow Blast Cell Count (%) | |||
| Median | 69 (n=25) | 50.0 (n=25) | 53.5 (n=26) |
| Range | 10.0–98.0 | 7.0–94.0 | 12.0–95.0 |
The ECOG Leukemia Translational Studies Laboratory received bone marrow or peripheral blood specimens on 77 of the 82 eligible patients at the time of study entry. The following subdiagnoses were established: CD65 negative, undifferentiated AML (31 cases) (Paietta, et al 2003), CD11bpos AML (19 cases) (Paietta, et al 1998), differentiated AML (9 cases), acute megakaryocytic leukemia (9 cases), acute monocytic leukemias (4 cases), natural killer-cell AML (3 cases) (Paietta, et al 1994) acute erythroleukemia (1 case), and one patient typed as CD4+CD56+ dendritic cell malignancy. A breakdown of each of these immunophenotypes by treatment group (Table 2) demonstrated a predominance of undifferentiated AML in Group C and a high incidence of CD11bpos AML in Group B. Both CD65neg undifferentiated AML (Paietta, et al 2003) and CD11bpos AML (Paietta, et al 1998) have been associated with poor clinical outcome in newly diagnosed AML on previous ECOG trials.
Response to study therapy
Response rates to therapy in the three arms of the study were low. Of the 26 cases in Arm A, only two achieved a standard CR, and one achieved a CRp. Of the other patients, 22 had non-progressive disease, 1 progressed, and 1 was not evaluable because the patient went into anaphylactic shock during treatment with gemtuzumab ozogamicin, and no assessment could be done to establish response. Therefore, the overall response rate in Arm A was 11.5% (95% confidence interval (CI), 2.4, 30.1). In Arm B only two of the 29 patients achieved a CR and there were no CRp. Twenty-five patients had stable disease. Two were not evaluable, one because of early death, and one because there was no bone marrow or peripheral blood cell recovery. Therefore, the response rate in this arm was 6.9% (95% CI, 0.8, 22.8).
In Arm C only one of the 27 cases achieved a CR, 25 had stable disease, and one case was inevaluable due to early death, giving a response rate in this arm of 3.7% (95% CI 0.1, 19). No patients who had relapsed within six months of initial CR or who had suffered multiple relapses responded. All three remissions in Group A plus one CR in Group B were achieved in patients with acute megakaryocytic leukemia; the second CR in Group B was in a case of CD11bpos AML, while the only remitter from Group C had differentiated AML. Disease-free and overall survival durations for the responding patients in Arms A, B and C are summarized in Table 3.
Table 3.
Characteristics of Responders (CR plus CR without Full Platelet Recovery)
| Treatment Arm |
Case No. |
Recurrence Status | Age (years) |
PS | OS (months) |
DFS (months) |
|---|---|---|---|---|---|---|
| A | 49014 | Refractory | 50 | 0 | 20.6 | 17.2 |
| 49017 | Refractory | 44 | 0 | 7.7 | 6.3 | |
| 49022++ | 1st Relapse 6–12 mo. after CR | 60 | 1 | 7.8 | 5.1 | |
| B | 49009 | 1st Relapse 6–12 mo. after CR | 28 | 0 | 49.5+ | 47.9+ |
| 49055 | 1st Relapse 6–12-mo. after CR | 58 | 1 | 26.2 | 24.7 | |
| C | 49041 | Refractory | 32 | 0 | 12.3 | 10.8 |
Represents censored OS or DFS.
Represents CR without full platelet recovery.
PS, ECOG performance score; OS, overall survival; DFS, disease-free survival
None of the responding patients received a second cycle of protocol therapy, but three patients, two with stable disease (one on Arm A and one on Arm C) and one with progressive disease (Arm A) did receive a second cycle of their respective protocol therapy, contrary to protocol guidelines. Because the primary endpoint of this trial was complete remission, subsequent non-protocol therapy was not tracked in study patients.
Survival
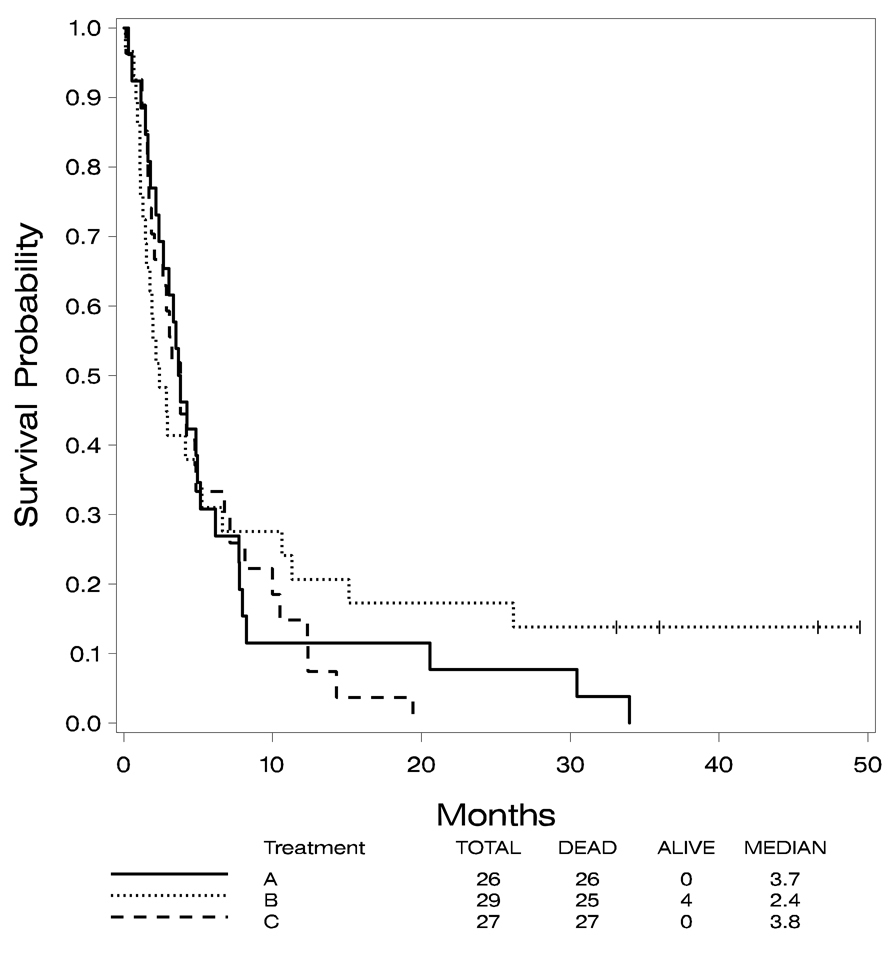
All of the patients in Arms A and C and 25 of the 29 cases in Arm B have died. The median survival for the 82 analyzable cases was 3.4 months (95% CI 2.6, 4.8). The median survivals in the three arms were 3.7, 2.4, and 3.8 months, respectively. No significant difference was noted in these survival distributions (p=0.7).
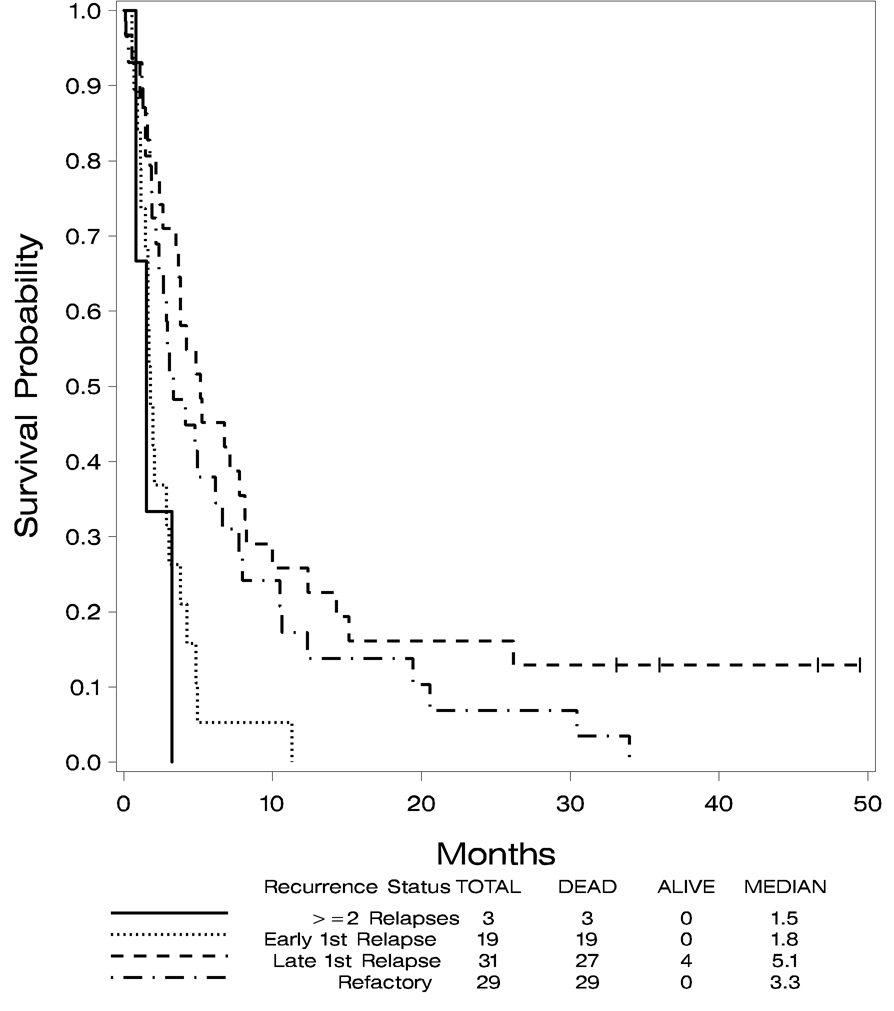
The four patients remaining alive in Arm B have survived between 36 and 50 months. Since the primary endpoint of this study was achievement of a CR, data on treatment patients received after induction therapy is, unfortunately, not available. Overall survival is shown in Figure 1 by treatment arm and Figure 2 demonstrates overall survival by recurrence status. Univariate log-rank tests were done comparing survival to various baseline patient characteristics: age ≤ 55 years vs. age > 55 years, treatment arm, FAB classification, recurrence status, AML prognostic category determined by cytogenetics data and WBC count. Survival data combining all three treatment groups were used. These tests identified age ≤ 55 years vs. age > 55 years (p=0.012) and recurrence status (p=0.001) as statistically significant predictors of survival. Cytogenetic prognostic category was not significant with a p value of 0.08. The other patient characteristics were not significantly associated with survival. The median survival for patients 55 years of age or younger was 4.5 months (95% CI 2.86, 774), while the median survival for those > 55 years was 2.75 months (95% CI 1.80, 3.80). The median survival for those who relapsed within six months after their first CR was 1.8 months (95% CI 1.4, 3.05) and for those who relapsed between 6 and 12 months after first CR was 5.5 months (95% CI 3.7, 8.1). For those who were refractory, the median survival was 3.3 months (95% CI 2.4, 6.6). For those who had two or more relapses, the median survival was 1.5 months (95% CI 0.8, 3.25).
Figure 1.
Overall survival by treatment arm.
Figure 2.
Overall survival by recurrence status at study entry.
Toxicity
The toxicity analysis was based on 28 patients treated in Arm A, 29 patients in Arm B, and 27 patients in Arm C and was regardless of eligibility for the study. Hematological toxicities were excluded in calculation of the worst degree as it is difficult to distinguish between treatment-related nadirs from the cytopenias and those due to disease. There was no significant difference in distributions of grade 3 or higher non-hematological worst degree toxicities between the three treatment arms. There were four cases of treatment-related lethal toxicities in Arm B and one case in Arm C. In Arm B, three of the four patients died of infection and one of hemorrhage. In Arm C, one patient died of infection.
Cytogenetics
Cytogenetic information was available for 62 of the 82 patients (76%) (Table 4). The cytogenetic risk category was favorable for only 5 (8%) patients. In comparison, the cytogenetic risk category was intermediate for 24 (39%) patients and unfavorable for 21 (34%) patients. Twelve patients (19%) had chromosome abnormalities for which the prognostic category has not been established (indeterminate). This group included 3 patients with del(11q)(q23) as the sole anomaly.
Table 4.
Distribution by Cytogenetic Risk Groups (n=62)
| Risk Group | # of Patients (% Total) |
|
|---|---|---|
| Favourable | 5 (8%) | |
| inv(16)/del(16)(q13q22) | 2 | |
| t(8;21)(q22;q22) and variants | 3 | |
| Intermediate | 24 (39%) | |
| normal (at least 20 bone marrow metaphases) | 18 (29%) | |
| trisomy 8 | 3 | |
| del(9q) [alone] | 1 | |
| +8, del(9q) | 1 | |
| del(12)(p11.2p12) | 1 | |
| Unfavourable | 21 (34%) | |
| complex with 1 or more unfavorable anomaly | 14 (23%) | |
| complex only | 2 | |
| t(9;11) | 2 | |
| trisomy 13 [alone] | 1 | |
| trisomy 4 [alone] | 1 | |
| t(9;22)(q34;q11.2) | 1 | |
| Indeterminate | 12 (19%) | |
| del(11q)(q23) [alone] | 3 | |
| other anomalies | 9 |
Of the 6 patients that achieved a CR at the time of study entry, 3 had a normal karyotype, 1 had only 15 normal metaphases, 1 had t(9;11)(p22;q23), and 1 had no cytogenetic data.
Follow-up studies at the time of clinical remission were available for only one of the remitters; in this instance the patient initially had a clone with t(9;11), but a bone marrow collected six weeks post study entry had a complete cytogenetic response.
Discussion
The CR rates of 12% or less in all three arms of the study in this phase II randomized trial of new regimens are disappointing. On the other hand, the data demonstrated a paucity of cases with favorable cytogenetics, as would be expected from a study in relapsed/refractory disease. Patients with intermediate risk and unfavorable karyotypes each accounted for 30–40% of the study population; 66% of patients with unfavorable cytogenetics had complex aberrations. Additionally, ECOG has previously established that both CD65neg, undifferentiated AML and CD11bpos AML are immunodiagnoses associated with inferior outcome (Paietta, et al 1998, Paietta, et al 2003); it is not surprising that the current study population presented predominantly with these immunodiagnoses with only a few cases of differentiated AML.
Further, those in first relapse enrolled on this study had relapsed within 12 months of initial remission, constituting another a higher risk group of patients. This point highlights the difficulty in interpreting and comparing trials in relapsed and refractory AML because of the heterogeneity of patients described in these studies. Estey et al (1997) highlighted this heterogeneity by attempting to stratify patients with relapsed AML treated at their center into four groups . The majority of patients treated in our study had a first CR duration of less than 12 months and were receiving first salvage treatment; therefore they would fit into Estey’s Group 3, where only a 14% complete remission rate was seen. Our results are certainly comparable to those reported by Estey and colleagues.
The phase II trials of single agent GO enrolled patients in first untreated relapse following a first CR of more than three months duration. Approximately 30% of patients achieved complete remissions, with approximately half of these being CR and half CRp (Larson, et al 2005, Sievers, et al 2001). GO in combination with chemotherapy for newly diagnosed AML patients demonstrated that significant dose reductions of GO are required but that it can be safely administered with achievement of respectable responses (Kell, et al 2003). It is possible that the addition of an anthracycline and filgrastim to the combination of cytarabine and GO may have improved the outcome compared to what was seen in this trial with cytarabine and GO alone (Kell, et al 2003, Yavuz, et al 2006).
Liposomal daunorubicin has been utilized as a single agent for the treatment of acute leukemia in four reported trials (Bieker, et al 2003, Cortes, et al 1999, Ermacora, et al 2000, Fassas, et al 2002). In a phase I dose escalation study, Cortes et al (1999) did not observe significant cardiac events and mucositis was the dose-limiting toxicity at 450 mg/m2. Two CRs in 24 patients were seen including one patient with blast phase of chronic myeloid leukemia (CML) , and a second patient who had acute promyelocytic leukemia and second relapse (Cortes, et al 1999). Similar results with single agent liposomal daunorubicin have been obtained by others (Bieker, et al 2003, Ermacora, et al 2000, Fassas and Anagnostopoulos 2005, Fassas, et al 2002, Fassas, et al 2003).
Cortes et al (2001) subsequently added cytarabine 1 g/m2 for four days with escalating doses of liposomal daunorubicin of 75–135 mg/m2 for three days in 62 patients (Cortes, et al 2001), a schedule similar to that utilized in the study reported herein. They reported an overall response rate of 40% including 18 patients with CR and seven patients with CRp but 4 patients had cardiac failure and again dose-limiting toxicity (135 mg/m2) was mucositis. Similar, but somewhat higher CR rates have been seen with the combination of liposomal daunorubicin and cytarabine in children with relapsed or refractory AML (Reinhardt, et al 2002). Liposomal daunorubicin with cytarabine has also been combined with topotecan, thalidomide, or cyclosporine and GO in relapse and refractory patients or in newly diagnosed patients with poor risk cytogenetics but has either been found to be inactive or excessively toxic (Apostolidou, et al 2003, Cortes, et al 2003). In contrast, our study reported a CR and CRp rate of only 7% with liposomal daunorubicin and cytarabine, probably on the basis of patient heterogeneity.
Topotecan as a single agent can be given at maximum tolerated doses of 2.0–2.1 mg/m2/day in relapsed or refractory acute leukemia or blast crisis of CML, with dose-limiting toxicity being oral and peri-anal mucositis (Kantarjian, et al 1993, Rowinsky, et al 1994). Combinations of topotecan with several other chemotherapeutic agents have been studied in patients with refractory leukemia (Rowinsky and Kaufmann 1997). Seiter et al (1997) postulated that leukemic cells escaping the cytarabine-induced S-phase block would be more susceptible to topotecan and gave sequential cytarabine 1 g/m2/day for five consecutive days followed by topotecan 2.5–7.75 mg/m2 12 h after the cytarabine completion to 53 patients. Dose-limiting toxicity was oropharyngeal mucositis and marrow aplasia was achieved in 28/53 induction courses. Four of 39 patients with AML achieved a CR, including two patients with t(8;21) who had complete cytogenetic responses. Two of six patients with ALL had a CR (Seiter, et al 1997). M.D. Anderson Cancer Center investigators, in step-wise fashion, have combined topotecan with cytarabine, and then added cyclophosphamide (CAT regimen used in this protocol) reasoning that cyclophosphamide causes DNA damage while topotecan inhibits the repair of that damage (Beran, et al 1999, Estey 1998). They treated 39 newly diagnosed AML (refractory anaemia with excess blasts [RAEB] or RAEB in transformation [RAEB-T]) patients and reported a 67% CR rate with only 5% of patients dying during the four weeks of therapy (Estey, et al 1998). Subsequently they reported CAT therapy for refractory or relapsed acute leukemia in 66 patients with different doses of drugs including cyclophosphamide 500 mg/m2, topotecan 1.25 mg/m2 and cytarabine 2 g/m2 in the same schedule as we used; they noted a CR rate of only 17% with two cases of hematological improvement resulting in an overall response rate of 20% (Cortes, et al 2000). In the study reported here, the response rate with this regimen was even poorer, at only 4%.
Despite the incorporation of a new agent (GO), modification of an established agent (liposomal daunorubicin) and utilization of a new combination of agents (CAT regimen), this trial did not show an increased benefit for these regimens in the high-risk relapsed patient population tested. Progress in the treatment of AML will require systematic approaches to identify high-risk patients earlier in their course, including the identification of new molecular markers and assessing the quality of remissions achieved through careful assessment of cytogenetic as well as morphological response (Balleisen, et al 2009, Schlenk, et al 2008). Early incorporation of allogeneic transplant for high-risk patients will also improve outcome. The identification of new targets within the leukemia cells and the development of agents that inhibit these targets may also bring a hope of improved outcomes. A successor trial to the one described here has recently been activated in the Eastern Cooperative Oncology Group and will attempt to target new pathways in the pathogenesis of AML including the mammalian target of rapamycin and serine/threonine cyclin-dependent kinases by combining sirolimus and flavopiridol with conventional chemotherapy.
Acknowledgments
The authors thank Mrs. Denise Chase for her careful preparation of the manuscript.
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA13650, CA49883, CA16116, CA17145, CA14548, CA11083, CA14958 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Larry D. Cripe has received clinical trial research support from Wyeth and GlaxoSmithKline. All other authors declare no competing financial interests.
References
- Apostolidou E, Cortes J, Tsimberidou A, Estey E, Kantarjian H, Giles FJ. Pilot study of gemtuzumab ozogamicin, liposomal daunorubicin, cytarabine and cyclosporine regimen in patients with refractory acute myelogenous leukemia. Leuk Res. 2003;27:887–891. doi: 10.1016/s0145-2126(03)00021-3. [DOI] [PubMed] [Google Scholar]
- Appelbaum FR, Kopecky KJ, Tallman MS, Slovak ML, Gundacker HM, Kim HT, Dewald GW, Kantarjian HM, Pierce SR, Estey EH. The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. Br J Haematol. 2006;135:165–173. doi: 10.1111/j.1365-2141.2006.06276.x. [DOI] [PubMed] [Google Scholar]
- Balleisen S, Kuendgen A, Hildebrandt B, Haas R, Germing U. Prognostic relevance of achieving cytogenetic remission in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome following induction chemotherapy. Leuk Res. 2009;33:1189–1193. doi: 10.1016/j.leukres.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Beran M, Estey E, O'Brien S, Cortes J, Koller CA, Giles FJ, Kornblau S, Andreeff M, Vey N, Pierce SR, Hayes K, Wong GC, Keating M, Kantarjian H. Topotecan and cytarabine is an active combination regimen in myelodysplastic syndromes and chronic myelomonocytic leukemia. J Clin Oncol. 1999;17:2819–2830. doi: 10.1200/JCO.1999.17.9.2819. [DOI] [PubMed] [Google Scholar]
- Bieker R, Lerchenmuller C, Wehmeyer J, Serve HL, Mesters RM, Buchner T, Berdel WE. Phase I study of liposomal daunorubicin in relapsed and refractory acute myeloid leukemia. Oncol Rep. 2003;10:915–920. [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- Cortes J, O'Brien S, Estey E, Giles F, Keating M, Kantarjian H. Phase I study of liposomal daunorubicin in patients with acute leukemia. Invest New Drugs. 1999;17:81–87. doi: 10.1023/a:1006216001681. [DOI] [PubMed] [Google Scholar]
- Cortes J, Estey E, Beran M, O'Brien S, Giles F, Koller C, Keating M, Kantarjian H. Cyclophosphamide, ara-C and topotecan (CAT) for patients with refractory or relapsed acute leukemia. Leuk Lymphoma. 2000;36:479–484. doi: 10.3109/10428190009148395. [DOI] [PubMed] [Google Scholar]
- Cortes J, Estey E, O'Brien S, Giles F, Shen Y, Koller C, Beran M, Thomas D, Keating M, Kantarjian H. High-dose liposomal daunorubicin and high-dose cytarabine combination in patients with refractory or relapsed acute myelogenous leukemia. Cancer. 2001;92:7–14. doi: 10.1002/1097-0142(20010701)92:1<7::aid-cncr1285>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cortes J, Kantarjian H, Albitar M, Thomas D, Faderl S, Koller C, Garcia-Manero G, Giles F, Andreeff M, O'Brien S, Keating M, Estey E. A randomized trial of liposomal daunorubicin and cytarabine versus liposomal daunorubicin and topotecan with or without thalidomide as initial therapy for patients with poor prognosis acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2003;97:1234–1241. doi: 10.1002/cncr.11180. [DOI] [PubMed] [Google Scholar]
- Cripe LD, Kneebone P, Roberts L. A phase I trial of liposomal daunorubicin (Daunoxome) administered with high-dose cytarabine to patients with relapsed acute leukemia. Blood. 1998;92:233a. [Google Scholar]
- Dinndorf PA, Andrews RG, Benjamin D, Ridgway D, Wolff L, Bernstein ID. Expression of normal myeloid-associated antigens by acute leukemia cells. Blood. 1986;67:1048–1053. [PubMed] [Google Scholar]
- Ermacora A, Michieli M, Pea F, Visani G, Bucalossi A, Russo D. Liposome encapsulated daunorubicin (daunoxome) for acute leukemia. Haematologica. 2000;85:324–325. [PubMed] [Google Scholar]
- Estey EH. New agents for the treatment of acute myelogenous leukemia: focus on topotecan and retinoids. Leukemia. 1998;12 Suppl 1:S13–S15. [PubMed] [Google Scholar]
- Estey E, Thall P, David C. Design and analysis of trials of salvage therapy in acute myelogenous leukemia. Cancer Chemother Pharmacol. 1997;40 Suppl:S9–S12. doi: 10.1007/s002800051054. [DOI] [PubMed] [Google Scholar]
- Estey E, Kantarjian H, Giles FJ, Beran M. Treatment of newly diagnosed AML and MDS with cyclophosphamide, Ara-C, topotecan. Blood. 1998;92:232a. [Google Scholar]
- Fassas A, Anagnostopoulos A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk Lymphoma. 2005;46:795–802. doi: 10.1080/10428190500052438. [DOI] [PubMed] [Google Scholar]
- Fassas A, Buffels R, Anagnostopoulos A, Gacos E, Vadikolia C, Haloudis P, Kaloyannidis P. Safety and early efficacy assessment of liposomal daunorubicin (DaunoXome) in adults with refractory or relapsed acute myeloblastic leukaemia: a phase I–II study. Br J Haematol. 2002;116:308–315. [PubMed] [Google Scholar]
- Fassas A, Buffels R, Kaloyannidis P, Anagnostopoulos A. Safety of high-dose liposomal daunorubicin (daunoxome) for refractory or relapsed acute myeloblastic leukaemia. Br J Haematol. 2003;122:161–163. doi: 10.1046/j.1365-2141.2003.04395_3.x. [DOI] [PubMed] [Google Scholar]
- Fenton C, Perry CM. Gemtuzumab ozogamicin: a review of its use in acute myeloid leukaemia. Drugs. 2005;65:2405–2427. doi: 10.2165/00003495-200565160-00014. [DOI] [PubMed] [Google Scholar]
- Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res. 1984;8:521–534. doi: 10.1016/0145-2126(84)90001-8. [DOI] [PubMed] [Google Scholar]
- Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, Goldstone A. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- Heim S, Mitelman F. Cancer cytogenetics. New York: Wiley-Liss; 1995. [Google Scholar]
- Kantarjian HM, Beran M, Ellis A, Zwelling L, O'Brien S, Cazenave L, Koller C, Rios MB, Plunkett W, Keating MJ. Phase I study of Topotecan, a new topoisomerase I inhibitor, in patients with refractory or relapsed acute leukemia. Blood. 1993;81:1146–1151. [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Kell WJ, Burnett AK, Chopra R, Yin JA, Clark RE, Rohatiner A, Culligan D, Hunter A, Prentice AG, Milligan DW. A feasibility study of simultaneous administration of gemtuzumab ozogamicin with intensive chemotherapy in induction and consolidation in younger patients with acute myeloid leukemia. Blood. 2003;102:4277–4283. doi: 10.1182/blood-2003-05-1620. [DOI] [PubMed] [Google Scholar]
- Larson RA, Sievers EL, Stadtmauer EA, Lowenberg B, Estey EH, Dombret H, Theobald M, Voliotis D, Bennett JM, Richie M, Leopold LH, Berger MS, Sherman ML, Loken MR, van Dongen JJ, Bernstein ID, Appelbaum FR. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104:1442–1452. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- Litzow MR. The therapy of relapsed acute leukaemia in adults. Blood Rev. 2004;18:39–63. doi: 10.1016/s0268-960x(03)00036-5. [DOI] [PubMed] [Google Scholar]
- Paietta E, Gallagher RE, Wiernik PH. Myeloid/natural killer cell acute leukemia: a previously unrecognized form of acute leukemia potentially misdiagnosed as FAB-M3 acute myeloid leukemia. Blood. 1994;84:2824–2825. [PubMed] [Google Scholar]
- Paietta E, Andersen J, Yunis J, Rowe JM, Cassileth PA, Tallman MS, Bennett JM, Wiernik PH Eastern Cooperative Oncology Group. Acute myeloid leukaemia expressing the leucocyte integrin CD11b-a new leukaemic syndrome with poor prognosis: result of an ECOG database analysis. Br J Haematol. 1998;100:265–272. doi: 10.1046/j.1365-2141.1998.00561.x. [DOI] [PubMed] [Google Scholar]
- Paietta E, Neuberg D, Bennett JM, Dewald G, Rowe JM, Cassileth PA, Cripe L, Tallman MS, Wiernik PH. Low expression of the myeloid differentiation antigen CD65s, a feature of poorly differentiated AML in older adults: study of 711 patients enrolled in ECOG trials. Leukemia. 2003;17:1544–1550. doi: 10.1038/sj.leu.2402999. [DOI] [PubMed] [Google Scholar]
- Peniket A, Wainscoat J, Side L, Daly S, Kusec R, Buck G, Wheatley K, Walker H, Chatters S, Harrison C, Boultwood J, Goldstone A, Burnett A. Del (9q) AML: clinical and cytological characteristics and prognostic implications. Br J Haematol. 2005;129:210–220. doi: 10.1111/j.1365-2141.2005.05445.x. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Hempel G, Fleischhack G, Schulz A, Boos J, Creutzig U. [Liposomal daunorubicine combined with cytarabine in the treatment of relapsed/refractory acute myeloid leukemia in children] Klin Padiatr. 2002;214:188–194. doi: 10.1055/s-2002-33185. [DOI] [PubMed] [Google Scholar]
- Rowe JM, Li X, Cassileth PA, Appelbaum FR, Schiffer CA, Wiernik PH, Litzow MR, Cripe LD, Lazarus HM, Paietta E, Dewald GW, Weinstein HJ, Ogden AK, Woods WG, Shepherd L, Feusner JH, Bloomfield CD, Tallman MS. Very poor survival of patients with AML who relapse after achieving a first complete remission: The Eastern Cooperative Oncology Group Experience (Abstract 546) Blood. 2005;106:162a. [Google Scholar]
- Rowinsky EK, Adjei A, Donehower RC, Gore SD, Jones RJ, Burke PJ, Cheng YC, Grochow LB, Kaufmann SH. Phase I and pharmacodynamic study of the topoisomerase I-inhibitor topotecan in patients with refractory acute leukemia. J Clin Oncol. 1994;12:2193–2203. doi: 10.1200/JCO.1994.12.10.2193. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Kaufmann SH. Topotecan in combination chemotherapy. Semin Oncol. 1997;24:S20-11–S20-26. [PubMed] [Google Scholar]
- Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, Habdank M, Spath D, Morgan M, Benner A, Schlegelberger B, Heil G, Ganser A, Dohner H. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- Seiter K, Feldman EJ, Halicka HD, Traganos F, Darzynkiewicz Z, Lake D, Ahmed T. Phase I clinical and laboratory evaluation of topotecan and cytarabine in patients with acute leukemia. J Clin Oncol. 1997;15:44–51. doi: 10.1200/JCO.1997.15.1.44. [DOI] [PubMed] [Google Scholar]
- Shipley JL, Butera JN. Acute myelogenous leukemia. Exp Hematol. 2009;37:649–658. doi: 10.1016/j.exphem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, Karanes C, Theobald M, Bennett JM, Sherman ML, Berger MS, Eten CB, Loken MR, van Dongen JJ, Bernstein ID, Appelbaum FR. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- Yavuz S, Paydas S, Disel U, Sahin B. IDA-FLAG regimen for the therapy of primary refractory and relapse acute leukemia: a single-center experience. Am J Ther. 2006;13:389–393. doi: 10.1097/01.mjt.0000181690.21601.09. [DOI] [PubMed] [Google Scholar]