Abstract
Muscle fiber degeneration in sporadic inclusion-body myositis (s-IBM) is characterized by accumulation of multiprotein aggregates, including aggregated amyloid-β-precursor protein 751 (AβPP751), amyloid-β (Aβ), phosphorylated tau (p-tau), and other “Alzheimer-characteristic” proteins. Proteasome inhibition is an important component of the s-IBM pathogenesis. In brains of Alzheimer disease (AD) patients and AD transgenic mouse models, phosphorylation of neuronal AβPP695 (p-AβPP) on Threonine668 (T668) (equivalent to T724 of AβPP751) is considered detrimental because it increases generation of cytotoxic Aβ and induces tau phosphorylation. Activated glycogen synthase kinase3β (GSK3β) is involved in phosphorylation of both AβPP and tau. Lithium, an inhibitor of GSK3β, was reported to reduce levels of both the total AβPP and p-AβPP in AD animal models. In relation to s-IBM, we now show for the first time that: 1. In AβPP-overexpressing cultured human muscle fibers (human muscle culture IBM model: a) proteasome inhibition significantly increases GSK3β activity and AβPP phosphorylation; b) treatment with lithium decreases i) phosphorylated-AβPP; ii) total amount of AβPP, iii) Aβ oligomers, and iv) GSK3β activity; and c) lithium improves proteasome function. 2. In biopsied s-IBM muscle fibers, GSK3β is significantly activated and AβPP is phosphorylated on Thr724. Accordingly, treatment with lithium, or other GSK3β inhibitors, might benefit s-IBM patients.
Keywords: Inclusion-body-myositis, phosphorylated amyloid-β precursor protein, GSK3β, cultured human muscle fibers, lithium chloride, proteasome
INTRODUCTION
Sporadic-inclusion body myositis (s-IBM) is the most common progressive muscle disease associated with aging. Its progressive course gradually leads to pronounced muscle weakness and wasting, resulting in severe disability (Askanas and Engel 2008). The exact pathogenesis of s-IBM is not known, and there is no enduring treatment.
An intriguing aspect of s-IBM is that its muscle-fiber phenotype shares several molecular abnormalities with Alzheimer disease (AD) brain, including accumulation of amyloid-β precursor protein (AβPP) and of its cytotoxic amyloid-β42 (Aβ42) fragment (recently reviewed in Askanas and Engel 2008; Askanas et al. 2009). Both s-IBM and AD affect aging individuals, but they are clinically distinct.
Hallmarks of s-IBM muscle fibers are: 1) vacuolated muscle fibers having intra-myofiber accumulations -- mainly in non-vacuolated cytoplasm -- of congophilic, ubiquitinated, multi-protein aggregates that contain, in addition to Aβ, phosphorylated tau in the form of paired helical filaments, and several other “AD characteristic” proteins; and 2) mononuclear-cell inflammation (Askanas and Engel 2001; Askanas and Engel 2008; Dalakas 2008). Currently there is no consensus regarding whether the degenerative or inflammatory component plays a more significant role in s-IBM pathogenesis. Against a primary role of inflammation is the wide recognition that all types of immunosuppressive treatment fail to reverse, or enduringly halt, disease progression of s-IBM (in contrast to polymyositis). And, there is increasing agreement that Aβ42 is an important pathogenic factor leading to muscle-fiber destruction (Askanas and Engel 2007; Askanas and Engel 2008; Dalakas 2008). This proposed mechanism has been supported by models involving cultured human muscle fibers and transgenic mice. For example, long-term overexpression of wild-type full-length 751AβPP in cultured normal human muscle fibers produced in them several aspects of the s-IBM cellular phenotype, including vacuolization, congophilic amyloid inclusions, cytoplasmic 6–10 nm amyloid-like filaments, nuclear paired helical filaments, mitochondrial cytochrome oxidase deficiency and mitochondrial morphological abnormalities (Askanas et al. 1996; Askanas et al. 1997). Transgenic mouse models based on overexpressing AβPP or its fragment in skeletal muscle produced some aspects of the IBM pathology (Fukuchi et al. 1998; Jin et al., 1998; Kitazawa et al. 2006). In s-IBM muscle fibers, there is increased production (Sarkozi et al. 1993; Guerin et al. 2007) and accumulation of aggregated amyloid-β precursor protein-751 (AβPP751) (Askanas et al. 1993), and preferential accumulation of the cytotoxic Aβ42 form (Vattemi et al. 2009). Accordingly, we postulate that methods reducing muscle-fiber Aβ accumulation may benefit s-IBM patients. Furthermore, probably-pathogenic oxidative and ER stresses, and proteasome inhibition, also have been demonstrated in s-IBM muscle fibers (Vattemi et al. 2004; Fratta et al. 2005; Nogalska et al. 2006; Terracciano et al. 2008).
In AD brain, increased phosphorylation of neuronal AβPP695 on Threonine 668 has been demonstrated (Lee et al. 2003; Shin et al. 2007), and in brains of AD mouse models, it was considered to be detrimental by increasing generation of Aβ and inducing tau phosphorylation (Shin et al. 2007). Active glycogen synthase kinase 3β (GSK3β) was proposed to have an important role in AD pathogenesis because it was shown to modulate phosphorylation of both tau (Anderton et al. 2001) and of AβPP on Threonine668 (Aplin et al. 1996).
Lithium is a reversible inhibitor of GSK3β (Jope 2003, Klein and Melton 1996; Avila and Hernandez 2007). It presumably acts either a) directly, competing with magnesium in forming a complex with ATP, or b) indirectly, by increasing phosphorylation of GSK on Serine 9, which leads to GSK3β inactivation (Jope 2003). In AD and IBM mouse models, inhibition of GSK3β by lithium chloride decreased phosphorylated AβPP and p-tau (Rockenstein et al. 2007; Kitazawa et al. 2008), respectively.
To explore molecular pathogenic mechanisms in s-IBM, we experimentally modified the cellular micro-environment of cultured human muscle fibers (CHMFs) to mimic various aspects of the s-IBM pathogenesis, thereby producing experimental IBM-Cultured-Human-Muscle-Fiber Models. These models have proved very useful in our previous studies (Askanas et al. 1996; Askanas et al. 1997; Fratta et al. 2005; Nogalska et al. 2006, Nogalska et al. in press; Wojcik et al. 2006).
In the present study we asked whether: 1) in s-IBM muscle fibers, AβPP is phosphorylated and GSK3β is activated; and 2) in the established s-IBM model involving overexpression of AβPP in cultured human muscle fibers a) imposed proteasome inhibition or experimental induction of ER stress influences AβPP phosphorylation and GSK3β activity; b) treatment with lithium decreases i) GSK3β activity, ii) total and phosphorylated AβPP, and iii) Aβ oligomers.
EXPERIMENTAL PROCEDURES
Muscle Biopsies
Studies were performed on fresh-frozen diagnostic muscle biopsies obtained (with informed consent) from 12 s-IBM, 2 polymyositis, 1 morphologically-nonspecific myopathy, 2 ALS, 2 peripheral neuropathy, and 12 normal controls age-matched to the s-IBM patients (normal muscle biopsies were of patients who, after all tests were performed, were considered free of muscle disease). s-IBM patients were ages 55–79 years, median age 67; normal control patients were ages 53–84, median age 71. Diagnoses were based on clinical and laboratory investigations, including our routinely-performed 16-reaction diagnostic histochemistry of muscle biopsies.
All s-IBM biopsies met s-IBM diagnostic criteria, as described (Askanas and Engel 2001). Not all studies were performed on all biopsies (details below).
Light-Microscopic Immunocytochemistry
Immunofluorescence was performed as described (Askanas et al.1993; Vattemi et al. 2004; Fratta et al. 2005; Nogalska et al. 2006) on 10-μm-thick transverse sections of 5 s-IBM, 2 age-matched normal, and 7 disease-control muscle biopsies (specified above), using a well-characterized polyclonal antibody specifically recognizing the AβPP751 isoform only when phosphorylated on Threonine at position 724 (p-AβPPT724), equivalent to Threonine668 of the AβPP 695 isoform (Cell Signaling, Danvers, MA), diluted 1:50. This antibody was produced by immunizing rabbits with a synthetic phosphopeptide corresponding to the residues surrounding Threonine 688 of human AβPP695 (corresponding to 724 position on isoform 751) (Ando et al. 1999; Muresan and Muresan 2004). To block non-specific binding of an antibody to Fc receptors, sections were pre-incubated with normal goat serum diluted 1:10, as described (Askanas et al. 1993; Nogalska et al. 2006). Controls for staining specificity were (i) omission of the primary antibody, or (ii) its replacement with non-immune sera or irrelevant antibody. These were always negative.
Combined Immunoprecipitation/Immunoblot Procedure in s-IBM biopsies
To confirm the specificity of the immunocytochemical reaction in s-IBM muscle fibers, a combined immunoprecipitation/immunoblot technique was performed in three s-IBM biopsies, as detailed previously (Vattemi et al. 2004; Fratta et al. 2005). In brief, 100 μg of total muscle protein were immunoprecipitated in precipitation-buffer containing 10 μg of 6E10 antibody (Covance, Princeton, NJ), which on immunoblots recognizes total AβPP and Aβ. The immunoprecipitated complex, containing IgG antibody along with its bound target antigen and all proteins bound to that antigen, was pulled down using Protein G Sepharose 4 Fast Flow (Amersham) during 4 h of incubation at 4°C. The solution was then centrifuged for 5 min (16,000×g at 4°C) and the supernatant removed. The precipitated sepharose immuno-complexes were washed three times with the precipitation buffer by centrifuging 5 min each (16,000×g at 4°C). Immunoprecipitates were then electrophoresed and immunoprobed with the rabbit polyclonal anti-p-AβPPT724 antibody, followed by an appropriate secondary antibody, and developed using the Western Breeze anti-rabbit chemiluminescence kit (Invitrogen, Carlsbad, CA). To confirm specificity of the physical association identified by the immunoprecipitation-immunoblot reaction, primary antibodies were omitted from the immunoprecipitation solution. (Control biopsies were not used for the immunoprecipitation experiments because they do not contain enough AβPP to be demonstrated by this technique.)
Immunoblotting
To evaluate whether GSK3β is activated in s-IBM patients, muscle homogenates of 6 s-IBM and 6 age-matched control biopsies were immunoblotted, as recently detailed (Vattemi et al. 2004; Nogalska et al. 2006; Nogalska et al. 2007; Terracciano et al. 2008). In brief, 20μg of protein were loaded into 4–12% NuPAGE gels (Invitrogen) and electrophoretically separated. After electrophoresis, samples were transferred to a nitrocellulose membrane. To prevent non-specific binding of the antibodies, the nitrocellulose membranes were blocked in Blocking Reagent (Invitrogen). They were then incubated overnight at 4 °C with a primary antibody. Antibodies used were against a) total GSK3β (Santa Cruz Biotechnology, Santa Cruz, Ca), diluted 1:50, b) active-GSK3β phosphorylated at Tyrosine 216 (Novus Biologicals, Littleton, CO), diluted 1:250, and c) inactive-GSK3β phosphorylated at Serine 9 (Cell Signaling), diluted 1:250. Blots were developed using the Western Breeze chemiluminescent kit (Invitrogen), or ECL Western Blotting Analysis System (Amersham Biosciences, Piscataway, NJ) in combination with horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Protein loading was evaluated by the actin band; the results were related to both active and inactive GSK3β and expressed per total GSK3β.
Cultured Human Muscle Fibers (CHMFs) and overexpression of AβPP-gene
Primary cultures of normal human muscle were established, as routinely performed in our laboratory (Askanas and Engel 1992), from archived satellite cells of portions of diagnostic muscle biopsies of patients who, after all tests were performed, were considered free of muscle disease. We established 15 culture sets, each from satellite cells derived from a different muscle biopsy. All experimental and control conditions were studied on sister cultures in the same culture set. Not all studies were performed on every set. Twenty days after myoblast fusion was completed, a 3 Kb 751 AβPP-cDNA was transferred into the well-differentiated myotubes using a replication-deficient adenovirus vector at 0.3×108 pfu/ml culture medium, as detailed (Askanas et al. 1996; Askanas et al. 1997; Wojcik et al. 2006).
Inhibition of proteasome or ER-stress induction in cultured human muscle fibers overexpressing AβPP
Three days after AβPP gene-transfer, CHMFs were treated with 1 μM epoxomicin (Biomol Research Laboratories, Plymouth Meeting, PA), an irreversible proteasome inhibitor (Meng et al. 1999), for 24h. For comparison, other AβPP-overexpressing cultures were treated for 24 h with ER-stress inducers, either a) tunicamycin, which inhibits N-glycosylation, 4 μg/ml (Sigma Co, St Louis, MO), or b) thapsigargin, 300 nM (Sigma Co), which inhibits ER calcium-ATPase (Lee, 2005). The doses used were as in our previous experiments (Fratta et al. 2005; Nogalska et al. 2007; Nogalska et al. in press).
Lithium chloride treatment of experimental cultures
Because lithium chloride (LiCl) is known inhibitor of GSK3β (Jope 2003, Klein and Melton 1996; Avila and Hernandez, 2007), AβPP-overexpressing cultures in 12 independent experiments were treated with 5mM LiCl (Sigma Co) for 48 h. Because human muscle material from which those primary cultures are derived is sparse, we were not able to evaluate wide ranges of lithium dosage. In the present study, we chose a 5mM dose, since that dose was shown effective by others (Kim and Thayer 2009; Yeste et al. 2007). In our preliminary experiments, we evaluated doses of 1, 5, 10, 15 and 20 mM, and found that 5mM was effective without causing any obvious adverse reactions (the 1mM dose was not effective).
Immunoblotting of CHMFs
After treatments (detailed above and below), experimental cultures and their sister-control cultures were harvested and processed for immunoblotting, as detailed previously (Nogalska et al. 2007; Wojcik et al. 2006). We evaluated total AβPP, pAβPPT724, and GSK3β with the antibodies described above. 6E10 antibody was used in the concentrations 1:1000 for evaluating total AβPP, and 1:300 to visualize Aβ oligomers. Protein-loading was evaluated by the actin band. Quantification of immunoreactivity was performed by densitometric analysis using NIH Image J-1.310 software.
Measurement of Proteasome Activity
Proteasome activity was measured as described (Fratta et al. 2005). Three main proteasome activities were determined by evaluating the cleavage of specific fluorogenic substrates (Shringarpure et al. 2003). 5 LiCl-treated and 5 non-treated AβPP-overexpressing culture sets were homogenized in 20 mmol/L Tris-HCl, pH 7.2, containing 0.1 mmol/L EDTA and 1 mM of fresh dithiotreitol (Meng et al. 1999, Demasi et al. 2001), centrifuged, the supernatant collected, and protein concentration determined using the Bradford method. Subsequently, 45 μg of protein from cultured muscle were incubated in 100 μmol/L fluorogenic substrates for the three different protease activities: Z-Leu-Leu-Glu-AMC (substrate II) for peptidyl glutamyl-peptide hydrolytic (PGPH) activity (Biomol, Plymouth Meeting, PA); Suc-Leu-Leu-Val-Tyr-AMC (substrate III) for chymotrypsin-like (CTL) activity (Calbiochem, Gibbstown, NJ ); and Z-Ala-Arg-Arg-AMC (substrate VI) for trypsin-like activity (Calbiochem). Fluorescence emission was excited at 355 nm and recorded at 405 nm. Proteasome activities were expressed per 20sβ2 proteasome subunit performed by immunoblotting each culture set using an anti-20sβ2 mouse monoclonal antibody diluted 1:500 (Biomol).
Statistical Analysis
In all experiments these analyses were performed using Student’s t-test. Significance level was set at p<0.05. For all groups data are reported as means ± SEM.
RESULTS
In s-IBM, muscle fibers AβPP was phosphorylated on Threonine 724
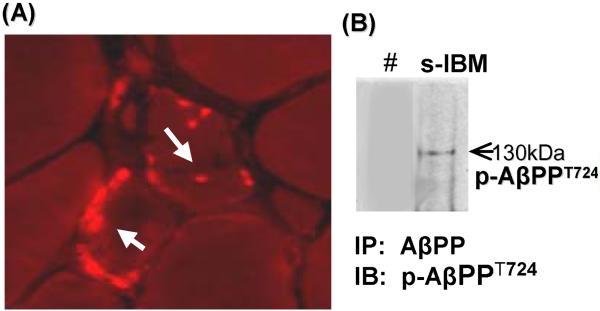
By immunocytochemistry, pAβPPT724 was accumulated in the form of various-sized aggregates located mainly in the non-vacuolated regions of cytoplasm, of approximately 80% of s-IBM-vacuolated muscle fibers in all patients (Fig.1A). None of the normal or disease-control muscle biopsies contained pAβPPT724-immunoreactivity. Endomysial and perivascular mononuclear-cells present in s-IBM and polymyositis muscle biopsies did not contain pAβPPT724 immunoreactivity. Muscle blood vessels did not contain pAβPPT724 immunoreactivity.
Fig. 1.
Phosphorylated AβPP T724 (p-AβPPT724) in sporadic-inclusion body myositis (s-IBM) muscle fibers. (A) Immunofluorescence using the specific phospho-AβPP antibody illustrates pAβPPT724-immunoreactive aggregates in two muscle fibers (arrows). (B). Immunoprecipitation (IP) of p-AβPPT724 in s-IBM muscle biopsy. IP was performed using 6E10 antibody, which recognizes both AβPP and Aβ; immunoblots (IB) were subsequently probed with the specific anti p-AβPPT724 rabbit polyclonal antibody. There is a distinct band corresponding to AβPPT724.
In # the primary antibody was omitted from the immunoprecipitation reaction to determine the specificity of the reaction.
To confirm the specificity of the immunocytochemical staining in s-IBM, we immunoprecipitated three s-IBM muscle-biopsy homogenates with antibody 6E10 (which on immunoblots recognizes total AβPP as well as Aβ oligomers), and we immunoprobed the blots with the specific anti-pAβPPT724 antibody. Immunoprecipation/immunoblotting confirmed the existence of AβPP phosphorylated on Threonine724 in s-IBM muscle (Fig.1B).
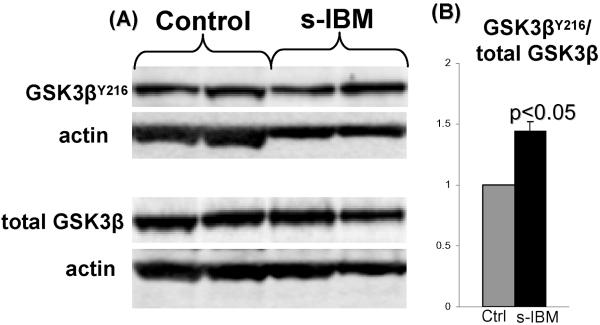
Active form of GSK3β was increased in s-IBM muscle fibers
GSK3β activity is regulated mainly by phosphorylation on Tyrosine 216 (Y216) which increases its activity, and on Serine 9 (Ser9) which inhibits its activity (Bhat et al. 2000; Lochhead et al. 2006).
In 6 s-IBM and 6 age-matched normal-control muscle biopsies, we evaluated GSK3β on immunoblots, using antibodies specifically recognizing total GSK3β, p-GSK3βY216, and GSK3βSer9 (studied in duplicate samples) after their normalization to actin. In s-IBM muscle biopsies, total GSK3β was not changed as compared to normal controls, whereas the amount of active p-GSK3βY216 expressed per total GSK3β was increased 1.5 fold ( p<0.05) (Fig 2). (Inactive p-GSK3βSer9 was decreased 1.6 fold in s-IBM muscle biopsies, but this did not reach p-value significance [data not shown].)
Fig. 2.
GSK3β is activated in s-IBM biopsied muscle. (A) Representative immunoblots of normal control and s-IBM muscle biopsies. B) The ratio of GSK3βY216 to total GSK3β, obtained by densitometric analysis of protein bands, shows that in s-IBM, as compared to controls, GSK3βY216 is significantly increased (p< 0.05, 2 tail Student’s t-test). ± SEM.
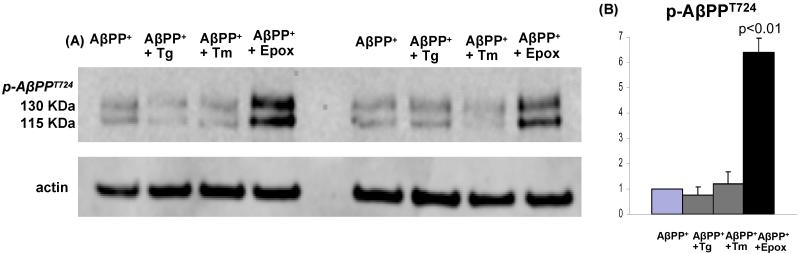
Proteasome inhibition increased p-AβPP724 and induced activation of GSK3β in our AβPP-overexpressing cultures (human-muscle-culture IBM model)
To explore possible mechanisms involved in AβPP phosphorylation and GSK3β activation in s-IBM muscle fibers, we utilized our experimental model consisting of primary cultures of normal human muscle fibers, with experimental modifications of the intracellular micro-environment to mimic aspects of the s-IBM muscle-fiber milieu. These manipulations involved: overexpressing the AβPP-gene, inhibiting the 26S proteasome, and inducing endoplasmic reticulum (ER) stress.
In 12 independent experiments, immunoblots of cultured human muscle-fiber homogenates, using the phospho-specific antibody recognizing p-AβPPT724, showed that: a) exposure of the AβPP-overexpressing (AβPP+) cultured human muscle fibers to the proteasome inhibitor epoxomicin caused 6.5 fold (p< 0.005) increase of p-AβPPT724, while b) ER stress inducers tunicamycin and thapsigargin had no effect (Fig.3). (Proteasome-inhibition increased total AβPP 3 fold (< 0.01) [not shown]). As in our previous studies, AβPP from our AβPP+-Culture IBM model muscle-fibers migrates as a “mature” 130kDa band and an “immature” 115 kDa band.
Fig. 3.
p-AβPPT724 in i) proteasome-inhibited, and ii) ER-stress-induced human-muscle-culture AβPP+ IBM model. (A) Representative immunoblots of densitometric analysis in (B).
(B) Densitometric analysis based on 12 independent experiments of the pAβPPT724 protein bands relative to the actin bands, expressed in arbitrary units, shows that after treatment with Epoxomicin (Epox) pAβPPT724 is significantly increased (6.5 fold, p<0.01, 2 tail Student’s t-test), while the ER stress inducers (Thapsigargin [Tg] and Tunicamycin [Tm]) did not exert this effect. ± SEM.
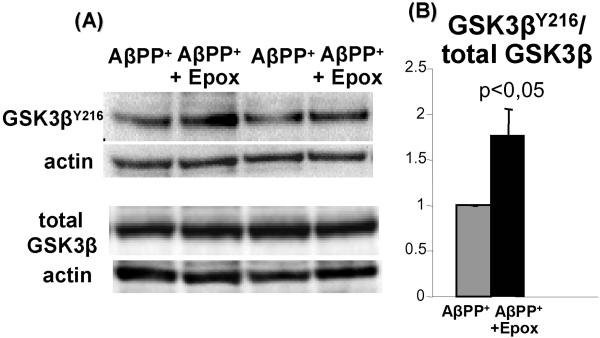
To determine whether the proteasome-inhibition-induced increase of p-AβPPT724 is associated with GSK3β activation, we performed immunoblots on 6 independent tissue-culture experiments using the same 3 anti-GSK3β antibodies as described above for human muscle biopsies. We found that after proteasome-inhibition active p-GSK3βY216 was increased 1.7 fold (p<0.05) (Fig. 4), while the total GSK3β and GSK3βSer9 were not affected (data not shown). Since the ER stress did not influence AβPP phosphorylation, we did not evaluate its influence on GSK3β activation.
Fig.4.
GSK3β is activated in the epoxomicin-inhibited proteasome in the human-muscle-culture IBM model. (A) Representative immunoblots of densitometric analysis in (B). (B) The ratio of GSK3βY216 to total GSK3β obtained by densitometric analysis of protein bands in 6 independent experiments shows that in proteasome-inhibited cultures, as compared to controls, GSK3βY216 is significantly increased (1.7 fold, p< 0.05, 2 tail Student’s t-test) ± SEM.
Treatment with Lithium inhibited GSK3β, and decreased total AβPP, p-AβPPT724 and Aβ-oligomers in our culture IBM model muscle-fibers
Lithium is known to inhibit GSK3β by increasing its inactive p-GSK3βSer9 form. When in 12 independent experiments we treated AβPP-overexpressing cultured human muscle-fibers with 5mM LiCl, the inactive p-GSK3βSer9 form of GSK3β was increased 2.2 fold (p<0.01) (Fig.5). That lithium treatment decreased total AβPP 30% (p<0.005), p-AβPPT724 50% (p<0.0001), and Aβ oligomers (8–25kDa) 25% (p<0.05) (Fig.6). Because in different experiments LiCl decreased Aβ oligomers of different molecular weights, we calculated all oligomers together.
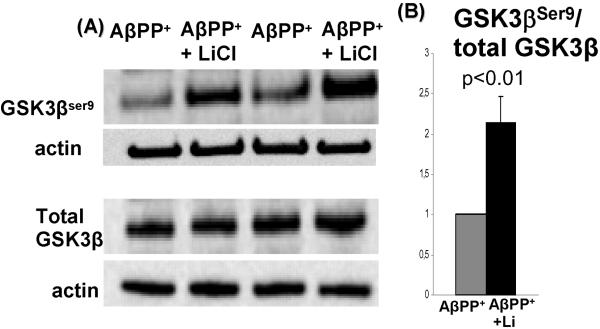
Fig.5.
Inactive form of GSK3β is increased in LiCl-treated culture-IBM-model. (A) Representative immunoblot of GSK3βSer9 in LiCl-treated and sister-control untreated culture AβPP+ IBM model. (B) Densitometric analysis of GSK3βSer9 bands relative to the actin bands, expressed in arbitrary units, shows that after lithium treatment GSK3βSer9 is significantly increased (2.2, p<0.01, 2 tail, Student’s t-test).
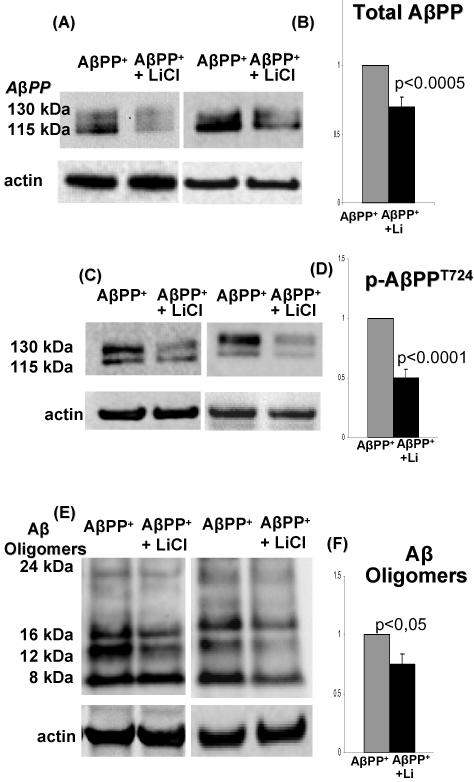
Fig. 6.
Lithium decreases the amount of total AβPP, p-AβPPT724, and Aβ-oligomers.
(A) Representative immunoblots of total AβPP in LiCl-treated and control untreated culture AβPP+-IBM-model. (B) Densitometric analysis of the total AβPP bands (130 and115 kDa) relative to the actin bands, expressed in arbitrary units, shows that after lithium treatment AβPP is decreased 30% (p<0.0005, 2 tail); (C) Representative immunoblots of p-AβPPT724 in LiCl-treated and untreated control culture AβPP+ -IBM-model. (D) Densitometric analysis of p-AβPPT724 bands relative to the actin bands, expressed in arbitrary units, shows that after lithium treatment p-AβPPT724 is decreased 50% ( p<0.0001, 2 tail). (E) Representative immunoblots of Aβ oligomers (8 kDa, 12kDa, 16 kDa, 25 kDa bands) in LiCl-treated and control untreated AβPP+-IBM-culture-model. (F) Densitometric analysis of Aβ oligomers bands relative to the actin bands, expressed in arbitrary units, shows that after lithium treatment all Aβ bands calculated together were decreased 25% ( p<0.05, 2 tail Student’s t-test).
Lithium induced proteasome activity
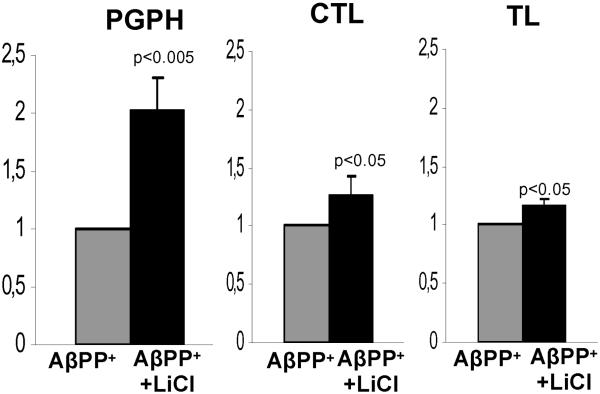
In view of the results obtained in our IBM-culture model indicating that proteasome inhibition increased both p-AβPPT724 and total AβPP, we asked whether our observed decrease of them by lithium might be, at least partially, influenced by a lithium enhancement of proteasome activity. Therefore, we studied three main proteasome activities in 5 independent sets of the IBM-culture models. We found that in lithium-treated cultures, all three proteasome enzymatic activities were increased; PGPH activity 2 fold (p<0.005), CTL activity 1.3 fold (p<0.05), and TL activity 1.2 fold (p<0.05), as compared to the sister-non-treated controls, (Fig.7).
Fig.7.
Three main proteasome enzyme activities -- peptidyl-glutamyl-peptide hydrolytic (PGPH), chymotrypsin-like (CTL) and trypsin-like (TL) -- in LiCl treated and sister-control untreated culture AβPP+ IBM model. Proteasome activities are expressed per β2 20S proteasome subunit in each culture. After lithium treatment, PGPH activity was increased 2 fold (p<0.005, 2 tail Student’s t-test), CTL activity was increased 1.3 fold (p<0.05, 1 tail Student’s t-test), and TL activity was increased 1.2 fold (p<0.05, 1 tail Student’s t-test).
DISCUSSION
While AβPP695 is specifically present in neuronal cells (da Cruz e Silva and da Cruz e Silva 2003), the AβPP751 isoform is most abundant in peripheral tissues (Tanaka et al.1989). In s-IBM muscle fibers, AβPP751 is the isoform overproduced and accumulated as aggregates (Askanas et al.1993; Sarkozi et al. 1993; Guerin et al. 2008). Phosphorylation of AβPP is considered a regulatory mechanism of AβPP metabolism (da Cruz e Silva and da Cruz e Silva 2003). Phosphorylation on Threonine 668 of the neuronal isoform AβPP695 (equivalent to threonine at position 724 of AβPP751) was reported a) to be associated with increased Aβ production (Lee et al. 2003; Ando et al. 2001), and b) to mediate pathological interaction between Aβ and tau (Shin et al. 2007). There were reduced levels of Aβ in neuronal cells treated with T688 kinase inhibitors or overexpressing mutant p-AβPPT688 (Lee et al., 2003).
Active p-GSK3βY216 has been shown increased in the frontal cortex of AD patients (Leroy et al. 2007), and proposed to be a component of Alzheimer disease pathogenesis (Jope 2003; Jope and Johnson 2004). Active p-GSK3βY216 was shown to phosphorylate both AβPP on Threonine 688 (Aplin et al. 1996) and tau protein (Anderton et al. 2001).
GSK3β is inactivated by being phosphorylated on Serine 9 (Bhat et al. 2000; Shaw et al. 1997). In AD transgenic mice, overexpression of a dominant negative GSK3β, as well as treatment with lithium or other pharmacological inhibitors, was reported to result in GSK3β inhibition and a subsequent reduction of Aβ, which was associated with improved cognitive performance (Rockenstein et al. 2007; Ryder et al. 2003; Su et al. 2004). GSK3β activity was also found increased in muscle fibers of the transgenic-mouse IBM model (Kitazawa et al. 2006); in this model, a decrease of GSK3β activity by lithium correlated with decreased tau phosphorylation (Kitazawa et al. 2008). In the present study, we demonstrated for the first time that a) in biopsied s-IBM muscle fibers and in our AβPP+ cultured human muscle-fibers (ABPP+ human muscle culture IBM model), AβPP is phosphorylated on Threonine724, and b) in s-IBM patients, active GSK3β is significantly increased as compared to the normal aged-matched-control muscle biopsies. In addition, we demonstrated in our IBM culture model that proteasome inhibition significantly enhanced the increase of active GSK3β, which corresponded to the increase of phosphorylated AβPP. Accordingly, we postulate that in s-IBM, phosphorylation of AβPP is influenced by proteasome inhibition, possibly via activation of GSK3β. The increase of total AβPP after proteasome inhibition also suggests that in this model the ubiquitin-proteasome system is involved in the degradation of AβPP.
Our results showed that lithium increased activities of all three proteasome enzymes studied, to various degrees. Lithium is known to have various effects on cells (Phiel and Klein 2001), but to our knowledge it has not been reported to improve proteasome function. However, since AβPP was previously shown to inhibit proteasome function in this culture model (Fratta et al. 2005), it is presently not known whether the increase of proteasome function by lithium that we observed in the current study represents its direct influence on the ubiquitin-proteasome system, or it is related to the overall reduction of the AβPP level.
In conclusion, we have demonstrated, apparently for the first time, that in s-IBM muscle fibers GSK3β activity is increased and AβPP is phosphorylated. Our experimental data also strongly suggest that AβPP phosphorylation is increased by GSK3β activation, which is increased by proteasome inhibition.
And, we have shown that in our culture IBM model, lithium treatment significantly decreased the levels of both total and phosphorylated AβPP as well as Aβ oligomers, accompanied by increased proteasome function and decreased GSK3β activation. This is in agreement with the transgenic-mouse IBM model in which lithium treatment decreased both GSK3β activation and tau phosphorylation (Kitazawa et al. 2008). Accordingly, treatment with lithium, or other GSK3β inhibitors, might be beneficial for s-IBM patients.
Acknowledgements
Supported by grants (to VA) from the National Institutes of Health (AG 16768 Merit Award), the Muscular Dystrophy Association, and The Myositis Association. AN was supported in part by The Newman’s Own Foundation. AN is on leave from Department of Biochemistry, Medical University of Gdansk, Gdansk, Poland. CT was on leave from Department of Neuroscience, University of Tor Vergata and Fondazione S. Lucia, Rome, Italy.
Abbreviations used
- Aβ
amyloid-β
- AβPP
amyloid-β precursor protein
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- CHMFs
cultured human muscle fibers
- CTL
chymotrypsin-like
- GSK-3β
glycogen synthase kinase 3β [E.C. 2.7.11.36]
- Epox
epoxomicin
- ER
endoplasmic reticulum
- PGPH
peptidyl -glutamyl peptide hydrolyzing
- S (Ser)
Serine
- s-IBM
sporadic inclusion-body myositis
- T (Thr)
Threonine
- TL
trypsin-like
- Tm
tunicamycin
- Tg
thapsigargin
- Y (Tyr)
Tyrosine
Footnotes
None of the authors has conflict of interest.
REFERENCES
- Anderton BH, Betts J, Blackstock WP, et al. Sites of phosphorylation in tau and factors affecting their regulation. Biochem Soc Symp. 2001;67:73–80. doi: 10.1042/bss0670073. [DOI] [PubMed] [Google Scholar]
- Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J. Biol. Chem. 2001;276:40353–40361. doi: 10.1074/jbc.M104059200. [DOI] [PubMed] [Google Scholar]
- Ando K, Oishi M, Takeda S, Iijima K, Isohara T, Nairn AC, Kirino Y, Greengard P, Suzuki T. Role of phosphorylation of Alzheimer’s amyloid precursor protein during neuronal differentiation. J. Neurosci. 1999;19:4421–4427. doi: 10.1523/JNEUROSCI.19-11-04421.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin AE, Gibb GM, Jacobsen JS, Gallo JM, Anderton BH. In vitro phosphorylation of the cytoplasmic domain of the amyloid precursor protein by glycogen synthase kinase-3beta. J. Neurochem. 1996;67:699–707. doi: 10.1046/j.1471-4159.1996.67020699.x. [DOI] [PubMed] [Google Scholar]
- Askanas V, Engel WK. Cultured normal and genetically abnormal human muscle. In: Rowland LP, Di Mauro S, editors. The Handbook of Clinical Neurology, Myopathies. Vol. 18. North Holland; Amsterdam: 1992. pp. 85–116. [Google Scholar]
- Askanas V, Alvarez RB, Engel WK. Beta-amyloid precursor epitopes in muscle fibers of inclusion body myositis. Ann. Neurol. 1993;34:551–560. doi: 10.1002/ana.410340408. [DOI] [PubMed] [Google Scholar]
- Askanas V, McFerrin J, Baque S, Alvarez RB, Sarkozi E, Engel WK. Transfer of beta-amyloid precursor protein gene using adenovirus vector causes mitochondrial abnormalities in cultured normal human muscle. Proc. Natl Acad. Sci. USA. 1996;93:1314–1319. doi: 10.1073/pnas.93.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askanas V, McFerrin J, Alvarez RB, Baque S, Engel WK. Beta APP gene transfer into cultured human muscle induces inclusion-body myositis aspects. Neuroreport. 1997;8:2155–2158. doi: 10.1097/00001756-199707070-00012. [DOI] [PubMed] [Google Scholar]
- Askanas V, Engel WK. Inclusion-body myositis: newest concepts of pathogenesis and relation to aging and Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60:1–14. doi: 10.1093/jnen/60.1.1. [DOI] [PubMed] [Google Scholar]
- Askanas V, Engel WK. Inclusion-body myositis, a multifactorial muscle disease associated with aging: current concepts of pathogenesis. Curr. Opin. Rheumatol. 2007;19:550–559. doi: 10.1097/BOR.0b013e3282efdc7c. [DOI] [PubMed] [Google Scholar]
- Askanas V, Engel WK. Inclusion-body myositis: muscle-fiber molecular pathology and possible pathogenic significance of its similarity to Alzheimer’s and Parkinson’s disease brains. Acta Neuropathol. 2008;116:583–595. doi: 10.1007/s00401-008-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askanas V, Engel WK, Nogalska A. Inclusion body myositis: a degenerative muscle disease associated with intra-muscle fiber multi-protein aggregates, proteasome inhibition, endoplasmic reticulum stress and decreased lysosomal degradation. Brain Pathol. 2009;19:493–506. doi: 10.1111/j.1750-3639.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J, Hernández F. GSK-3 inhibitors for Alzheimer’s disease. Expert Rev. Neurother. 2007;7:1527–1533. doi: 10.1586/14737175.7.11.1527. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, Lee CM. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc. Natl Acad. Sci. U S A. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz e Silva EF, da Cruz e Silva OA. Protein phosphorylation and APP metabolism. Neurochem. Res. 2003;28:1553–1561. doi: 10.1023/a:1025630627319. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Interplay between inflammation and degeneration: using inclusion body myositis to study “neuroinflammation”. Ann. Neurol. 2008;64:1–3. doi: 10.1002/ana.21452. [DOI] [PubMed] [Google Scholar]
- Demasi M, Shringarpure R, Davies KJ. Glutathiolation of the proteasome is enhanced by proteolytic inhibitors. Arch. Biochem. Biophys. 2001;389:254–263. doi: 10.1006/abbi.2001.2332. [DOI] [PubMed] [Google Scholar]
- Fukuchi K, Pham D, Hart M, Li L, Lindsey JR. Amyloid-beta deposition in skeletal muscle of transgenic mice: possible model of inclusion body myopathy. Am. J. Pathol. 1998;153:1687–1693. doi: 10.1016/s0002-9440(10)65682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratta P, Engel WK, McFerrin J, Davies KJ, Lin SW, Askanas V. Proteasome inhibition and aggresome formation in sporadic inclusion-body myositis and in amyloid-beta precursor protein-overexpressing cultured human muscle fibers. Am. J. Pathol. 2005;67:517–526. doi: 10.1016/s0002-9440(10)62994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin C, Sinnreich M, Karpati G. Transcriptional Dysregulation in Muscle Fibres in Sporadic Inclusion Body Myositis (sIBM) Neurology. 2008;7(Suppl3):A304. [Google Scholar]
- Jin LW, Hearn MG, Ogburn CE, Dang N, Nochlin D, Ladiges WC, Martin GM. Transgenic mice over-expressing the C-99 fragment of betaPP with an alpha-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am. J. Pathol. 1998;153:1679–1686. doi: 10.1016/s0002-9440(10)65681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Thayer SA. Lithium increases synapse formation between hippocampal neurons by depleting phosphoinositides. Mol. Pharmacol. 2009;75:1021–1030. doi: 10.1124/mol.108.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Green KN, Cacciamo A, LaFerla FM. Genetically augmenting Abeta42 levels in skeletal muscle exacerbates inclusion body myositis-like pathology and motor deficits in transgenic mice. Am. J. Pathol. 2006;168:1986–1997. doi: 10.2353/ajpath.2006.051232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Vasilevko V, Cribbs DH, LaFerla FM. Immunization with amyloid-beta attenuates inclusion body myositis-like myopathology and motor impairment in a transgenic mouse model. J. Neurosci. 2008;29:6132–6141. doi: 10.1523/JNEUROSCI.1150-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc. Natl Acad. Sci. U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, Neve R, Ahlijanian MK, Tsai LH. APP processing is regulated by cytoplasmic phosphorylation. J. Cell. Biol. 2003;163:83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy K, Yilmaz Z, Brion JP. Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 2007;33:43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- Lochhead PA, Kinstrie R, Sibbet G, Rawjee T, Morrice N, Cleghon V. A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol. Cell. 2006;24:627–633. doi: 10.1016/j.molcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo anti-inflammatory activity. Proc. Natl Acad. Sci. U S A. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan Z, Muresan V. A phosphorylated, carboxy-terminal fragment of β-amyloid precursor protein localizes to the splicing factor compartment. Hum.Mol.Gen. 2004;13:475–488. doi: 10.1093/hmg/ddh054. [DOI] [PubMed] [Google Scholar]
- Nogalska A, Engel WK, McFerrin J, Kokame K, Komano H, Askanas V. Homocysteine-induced endoplasmic reticulum protein (Herp) is up-regulated in sporadic inclusion-body myositis and in endoplasmic reticulum stress-induced cultured human muscle fibers. J. Neurochem. 2006;96:1491–1499. doi: 10.1111/j.1471-4159.2006.03668.x. [DOI] [PubMed] [Google Scholar]
- Nogalska A, Wojcik S, Engel WK, McFerrin J, Askanas V. Endoplasmic reticulum stress induces myostatin precursor protein and NF-kappaB in cultured human muscle fibers: relevance to inclusion body myositis. Exp. Neurol. 2007;204:610–618. doi: 10.1016/j.expneurol.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogalska A, D’Agostino C, Engel WK, Davies KJ, Askanas V. Decreased SIRT1 deacetylase activity in sporadic inclusion-body myositis muscle fibers. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.021. in press, doi:10.1016/j.neurobiolaging.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Klein PS. Molecular targets of lithium action. Annu. Rev. Pharmacol. Toxicol. 2001;41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB, Crews L, Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci. 2007;27:1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder J, Su Y, Liu F, Li B, Zhou Y, Ni B. Divergent roles of GSK3 and CDK5 in APP processing. Biochem. Biophys. Res. Commun. 2003;312:922–924. doi: 10.1016/j.bbrc.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Sarkozi E, Askanas V, Johnson SA, Engel WK, Alvarez RB. beta-Amyloid precursor protein mRNA is increased in inclusion-body myositis muscle. Neuroreport. 1993;4:815–818. doi: 10.1097/00001756-199306000-00055. [DOI] [PubMed] [Google Scholar]
- Shaw M, Cohen P, Alessi DR. Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett. 1997;416:307–311. doi: 10.1016/s0014-5793(97)01235-0. [DOI] [PubMed] [Google Scholar]
- Shin RW, Ogino K, Shimabuku A, Taki T, Nakashima H, Ishihara T, Kitamoto T. Amyloid precursor protein cytoplasmic domain with phospho-Thr668 accumulates in Alzheimer’s disease and its transgenic models: a role to mediate interaction of Abeta and tau. Acta Neuropathol. 2007;113:627–636. doi: 10.1007/s00401-007-0211-z. [DOI] [PubMed] [Google Scholar]
- Shringarpure R, Grune T, Mehlhase J, Davies KJ. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J. Biol. Chem. 2003;278:311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- Su Y, Ryder J, Li B, et al. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry. 2004;43:6899–6908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Shiojiri S, Takahashi Y, Kitaguch N, Ito H, Kameyama M, Kimura J, Nakamura S, Ueda K. Tissue-specific expression of three types of β-protein precursor mRNA; enhancement of protease inhibitor-harboring types in Alzheimer’s disease brain. Biochem. Biophys. Res. Comm. 1989;165:1406–1414. doi: 10.1016/0006-291x(89)92760-5. [DOI] [PubMed] [Google Scholar]
- Terracciano C, Nogalska A, Engel WK, Wojcik S, Askanas V. In inclusion-body myositis muscle fibers Parkinson-associated DJ-1 is increased and oxidized. Free Radic. Biol. Med. 2008;45:773–779. doi: 10.1016/j.freeradbiomed.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattemi G, Engel WK, McFerrin J, Askanas V. Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am. J. Pathol. 2004;164:1–7. doi: 10.1016/S0002-9440(10)63089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattemi G, Nogalska A, Engel WK, D’Agostino C, Checler F, Askanas V. Amyloid-beta42 is preferentially accumulated in muscle fibers of patients with sporadic inclusion-body myositis. Acta Neuropathol. 2009;117:569–574. doi: 10.1007/s00401-009-0511-6. [DOI] [PubMed] [Google Scholar]
- Wojcik S, Engel WK, McFerrin J, Paciello O, Askanas V. AbetaPP-overexpression and proteasome inhibition increase alphaB-crystallin in cultured human muscle: relevance to inclusion-body myositis. Neuromuscul. Disord. 2006;16:839–844. doi: 10.1016/j.nmd.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeste M, Alvira D, Verdaguer E, Tajes M, Folch J, Rimbau V, Pallàs M, Camins A. Evaluation of acute antiapoptotic effects of Li+ in neuronal cell cultures. J. Neural. Transm. 2007;114:405–416. doi: 10.1007/s00702-006-0557-8. [DOI] [PubMed] [Google Scholar]