Abstract
The expression of C1q, a recognition molecule of the complement system, is upregulated following neuronal injury and is detected early in neurodegenerative disorders such as Alzheimer's disease. This multimeric protein triggers an enhancement of phagocytosis of suboptimally opsonized targets by microglia, the phagocytic cells of the CNS, similar to other phagocytes, enhances the uptake of apoptotic cells in peripheral phagocytes, and suppresses inflammatory cytokine production in human monocytes, macrophages and dendritic cells in the absence of activation of the entire complement cascade. The goal of this study was to determine if C1q could influence the inflammatory response to injury in the CNS, using primary rat microglia and neurons. The data show that microglia preferentially ingest apoptotic cells in comparison to live cells, like other professional phagocytes, that microglial ingestion of apoptotic neurons and neuronal blebs is enhanced by the presence of normal serum and that these enhanced levels of uptake are diminished in serum depleted of C1q. In addition, purified C1q bound to apoptotic neurons and neuronal blebs in a dose dependent manner, and alone triggered a significant enhancement of uptake by microglia. Microglia added to C1q coated wells or fed apoptotic neurons or neuronal blebs coated with C1q suppressed the LPS-induced production of proinflammatory cytokines IL-1α, IL-1β, IL-6 and TNF-α, while the presence of C1q enhanced levels of the chemokine MCP-1/CCL2. The data are consistent with a protective role for C1q in the CNS during early stages of cell death by enhancing microglial clearance of apoptotic cells and suppressing proinflammatory cytokines.
Keywords: Complement, phagocytosis, apoptosis, cytokines, inflammation, microglia
Introduction
The complement protein C1q plays several key roles in the innate immune system. The most well known function is as the recognition component of the macromolecular complex, C1, that initiates the classical complement pathway. Activation of complement leads to a number of downstream effector functions including deposition of opsonin C3b on the surface of target cells, recruitment of phagocytic cells and lysis of many pathogens. While activation of C1 is often via binding of C1q to Fc portions of antibodies in immune complexes, many antibody independent activators of C1 have been discovered and characterized (reviewed in (Fraser and Tenner 2008)).
In Alzheimer's Disease, C1q has been found associated with fibrillar β-amyloid (Aβ) deposits in human tissue and in murine models of AD, providing an in vivo correlate for in vitro demonstration of complement activation by fibrillar Aβ (reviewed in (Tenner and Fonseca 2006)). C1q in the CNS is synthesized by microglia or cells of the monocyte/macrophage lineage (Lynch et al. 2004). In addition, C1q synthesis is induced in neurons in models of development (Stevens et al. 2007) and injury including viral infection, ischemia, aging, chemical injury, and Aβ treatment (reviewed in (Tenner and Pisalyaput 2008)). These data suggest a role for C1q beyond that of the activation of the complement cascade.
A large body of evidence now suggests that C1q is an important factor in the clearance of apoptotic cells (Trouw et al. 2008a). C1q deficiency is associated with excessive inflammation and autoimmunity, as seen in human systemic lupus erythematosus (SLE) and glomerulonephritis and all but one human case of complete genetic deficiency of C1q resulted in lupus-like disease (if the patient lived beyond childhood infections) (Botto and Walport 2002). C1q -/- mice demonstrate increased glomerular apoptotic bodies and have an SLE-like phenotype, although the extent to which to which this occurs is strain dependent, indicating that multiple factors can be involved (Botto et al. 1998). C1q has been shown to bind apoptotic cells in vitro (Korb and Ahearn 1997;Nauta et al. 2002) with greater stable binding of C1q to late apoptotic cells than earlier apoptotic cells (Trouw et al. 2007;Fraser et al. 2009). While not the only factor to mediate apoptotic cell clearance (Ravichandran and Lorenz 2007), C1q has been shown to contribute to apoptotic cell clearance through direct opsonization of cells (Ogden et al. 2001;Fraser et al. 2009) or via binding to natural IgM on late apoptotic cells (Quartier et al. 2005;Chen et al. 2009). In either case, complement activation can occur, leading to C3 deposition and enhanced phagocytosis of apoptotic cells via complement receptor 3 and/or receptors for C1q (Takizawa et al. 1996;Ogden and Elkon 2006). Thus, it has been postulated that C1q plays an important role in preventing autoimmunity and limiting inflammation by facilitating clearance of apoptotic cells.
Chronically activated microglia have been implicated in many neurodegenerative conditions, secreting proinflammatory cytokines, metalloproteinases, nitric oxide, and other neurotoxic substances (Goodwin et al. 1995;Li et al. 2004). However, like macrophages, microglia are capable of taking up dying cells and cellular debris, and thus are likely to play a vital role in clearing dying cells prior to the release of potentially toxic intracellular molecules to the extracellular space (as recently reviewed in (Napoli and Neumann 2009)). Ingestion of apoptotic PC12 cells, a neuronal cell-line, was reported to enhance microglial production of anti-inflammatory and neuroprotective molecules (De Simone et al. 2003). Others observed a decrease in proinflammatory cytokine secretion by microglia that have ingested apoptotic T cells or neurons, while anti-inflammatory cytokine production remains unaffected (Magnus et al. 2004;Takahashi et al. 2005). Evidence has been accumulating that not only the particle being ingested, but also the molecules present during ingestion, will have a profound influence on uptake and on cytokine responses to apoptotic cells by phagocytes in the periphery (reviewed in (Ravichandran and Lorenz 2007)). Since it was previously demonstrated that C1q enhances the phagocytic capacity of microglia in vitro (Webster et al. 2000), and that C1q can modulate inflammatory cytokine production (Fraser et al. 2006;Fraser et al. 2009) and induction of transcription factors in other myeloid cells (Fraser et al. 2007), it was hypothesized that C1q may also modulate inflammation following CNS injury and neuronal damage. Blom and colleagues demonstrated that C4bp, a classical pathway regulator, bound to both plaques and dying/dead cells in Alzheimer's Disease brain thereby limiting downstream complement mediated apoptotic cell lysis and thus “collateral” damage and providing time for facilitated clearance by the early components of the complement cascade (Trouw et al. 2008b). Therefore, we investigated the influence of complement protein C1q, alone and in serum, on the clearance of apoptotic primary neurons by primary microglia in vitro and on the subsequent cytokine responses of microglia that ingested C1q-opsonized apoptotic cells. The data support an important role by C1q in facilitating microglial clearance of apoptotic neurons generated during neuronal injury in the CNS and modulating cytokine production in an environment of competing signals.
Materials and Methods
Media and Reagents
RPMI-1640, penicillin/streptomycin, DMEM, trypsin-EDTA, CFSE and N2 supplement were purchased from Invitrogen (Grand Island, NY). Serum-free neurobasal (NB) medium, fetal calf serum (FCS), L-glutamine and B27 supplements were obtained from Gibco Life Technologies (Grand Island, NY, USA). HL-1 medium (serum free) was purchased from BioWhittaker (Walkersville, MD). Bovine serum albumin, ovalbumin, poly-L-lysine, etoposide and lipopolysaccharide (LPS; from Escherichia coli 0127:B8; Cat. #L-3129) were obtained from Sigma-Aldrich (St. Louis, MO). C3- and properdin-depleted NHS and purified properdin and C3 were purchased from CompTech (Tyler, TX). C1q, and serum depleted of C1q (C1qD), were isolated from plasma-derived normal human serum (NHS) as described in (Fraser et al. 2009).
Microglia cell culture
Glial cells were isolated from 2-4 day old Sprague-Dawley rat pups (Charles River, Wilmington, MA) as previously described (Van Muiswinkel et al. 1996). Briefly, cortices were obtained from rat pups and exposed to 0.25% trypsin (Gibco BRL, NY) for 10 min. Trypsin digests were pelleted at 540g for 7 min., resuspended in DMEM-10% fetal calf serum, triturated with flame polished glass pipettes and pelleted at 540 g for 7 min prior to being added to poly-L-lysine (20 μg/ml) coated flasks. Media was replaced with fresh media at 2 and 7 days after plating. On day 7 or day 10, microglia were isolated from the glial culture by shaking flasks for 90-120 min. at 160 rpm, 37°C. Microglia were washed 3 times with HBSS++ and plated at 1×106 cells/mL in phagocytosis buffer (RPMI-no serum, 5 mM MgCl2).
Neuron cell culture and isolation of neuronal blebs
Neurons were isolated from d18 Sprague-Dawley rat embryos (Charles River Laboratories, Inc., Wilmington, MA, USA) as previously described (Li et al. 2004). Neurons were grown on poly-L-lysine (10 μg/ml) coated 6-well plates (Costar, Cambridge, MA, USA); cells were plated at a density of 1 × 106 cells per well in 2 ml in neurobasal medium (NB) supplemented B27 (NB/B27) and grown for 3-4 days in vitro before use.
For induction of apoptosis, cells were treated with 1μM etoposide for 16 hours. Cell culture media containing dying neurons was collected and spun at 1000 rpm (Sorvall RT-6000) at room temperature for 10 minutes to pellet whole cells. Neuronal blebs were purified as described in (Nauta et al. 2002). Blebs were washed once with PBS and resuspended in the appropriate assay buffer (as described below).
C1q/C3 Binding Assay
Live or apoptotic neurons or neuronal blebs were resuspended at 5 × 106/mL in PBS/1% OVA. Purified human C1q was added to a final concentration of 0-300μg/mL as indicated, or NHS, C1qD or C1qD reconstituted with 140μg/mL - 700μg/mL purified human C1q (which corresponds to 2× to 10× serum concentration of C1q) were added to a final concentration of 10% in each sample volume. After 30 min of incubation at 37°C, cells were washed twice with FACS buffer (HBSS++/0.2% Sodium azide/0.2% BSA). Binding of C1q or C3 was detected using monoclonal antibodies to C1q or C3d (Quidel, San Diego, CA), with secondary detection using fluorochrome-conjugated secondary antibodies (Jackson ImmunoResearch, Inc., West Grove, PA). Samples were analyzed by standard flow cytometry on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ).
Uptake of Apoptotic Neurons and Neuronal Blebs
Live neurons in 6-well culture dishes were labeled with CFSE as follows. NB-B27 media was removed and stored at 37°C until later. Cells were washed twice and media was replaced with 2mL/well HBSS++ containing 1 μM CFSE at 37°C for 30 min. Wells were washed with NB-N2 media, and replaced with 2mL original NB-B27 media prior to induction of apoptosis. CFSE-labeled live or apoptotic neurons or neuronal blebs were added to microglial cultures in phagocytosis buffer (RPMI-5mM MgCl2) in LabTek chamber slides at a ratio of 3:1 (neuron:microglia) or 10:1 (neuronal blebs isolated from 10 neurons: 1 microglia). Phagocytosis buffer (no serum control) or purified human C1q at 14 - 70μg/mL were added to wells, or NHS, C1qD, or C1qD reconstituted with 140 - 700μg/mL human C1q were then added to wells at a final volume of 10%. Cells in LabTek chambers were centrifuged at 70g for 3 min. and incubated at 37°C, 5 % CO2 for 60 minutes. Following incubation, cells were harvested from the chamber by incubation with 0.25% trypsin/EDTA at 37°C for 1-2 minutes. The trypsin reaction was stopped with 0.5mL DMEM containing 10% FBS. Cells were pelleted by centrifugation at 207g for 10 min, washed with 1 ml FACS buffer, and resuspended in 100ul FACS buffer for staining. Microglia were stained with PE-conjugated- anti-rat CD11c (Ox42) (BD-Biosciences, San Diego, CA) or mouse IgG1-PE isotype control (eBioscience, San Diego, CA) for 1hr on ice. After washing with FACS buffer, cell-associated fluorescence was measured using FACScaliber and analyzed by using FlowJo software (Tree Star, Ashland, OR).
LPS-stimulated Cytokine Assay
Microglia isolated from the glial culture as described were plated on HSA or C1q coated LabTek chambers at 1 × 106 cells/ml in HL-1 media supplemented with 1% L-alanyl L-glutamine and centrifuged at 70 g for 3 min. Following incubation for 30 min. at 37°C, microglia were treated with 0 or 150 ng/ml LPS. After 18 hour incubation, supernatants were collected and stored at -70°C until cytokine analysis. For cytokine analysis after the uptake of apoptotic cells, uptake assays were performed essentially as described above, with the following exceptions. Unlabeled live or apoptotic neurons or neuronal blebs were incubated without or with 300μg/ml C1q for 30minutes at 37°C in PBS/1% ovalbumin (OVA). Cells were washed twice to remove unbound C1q, prior to incubation with microglia. After 60 minute incubation, cells were washed twice with PBS and HL-1 medium supplemented with 1% L-alanyl L-glutamine was added to the cells. Where indicated, LPS (150 ng/ml) was added directly to the cells. Supernatants were collected as described above.
The levels of IL-1α, IL-1β, IL-6, IL-10, TNFα and MCP-1/CCL2 were measured in cell culture supernatants in a multiplex cytokine assay. Assays were carried out using rat cytokine multiplex kit (Linco/Millipore, St.Charles, MO) according to the manufacturer's protocol. Mean fluorescent intensities of each cytokine were read on a Luminex100 v.1.7 (Luminex, Austin, TX). Determination of cytokine concentrations was calculated from standard curves of each cytokine tested using Miraibio Master Plex QT software (Miraibio, Alameda, CA).
Results
C1q contributes to serum-mediated enhancement of microglial ingestion of apoptotic cells and neuronal blebs
Rapid clearance of dying neurons and/or neuronal blebs is critical to avoid detrimental inflammation and extracellular release of neurotoxic amounts of excitatory transmitters, such as glutamate, or other toxic intracellular molecules. To investigate the influence of complement proteins in the uptake of primary apoptotic neurons by microglia, primary rat cortical neurons were treated with varying concentrations of etoposide to induce apoptosis. The amount of annexin V and/or propidium iodide labeling, indicative of cell death was examined by FACS. While approximately 15% of non-treated neurons demonstrate Annexin V labeling, treatment with 1 μM etoposide for 18 hours greatly increases Annexin V binding to 91% +/-4% (n=3) with 70% +/-7% of these cells also being positive for propidium iodide (indicative of “late” apoptosis) (Figure S1). Higher concentrations of etoposide (5 and 10 μM) did not greatly increase Annexin V labeling (data not shown). Based on this data, 1 μM etoposide was chosen as the concentration with which to induce neuronal apoptosis for subsequent experiments.
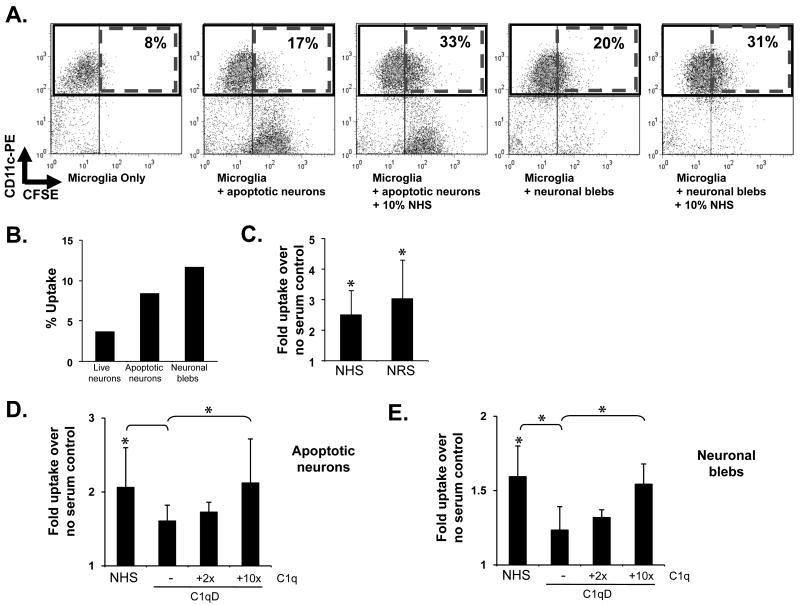
Live or apoptotic cells or neuronal blebs were added to microglial cells in phagocytosis buffer in the presence or absence of normal human serum and the percentage of ingested CSFE-labeled targets was assessed by flow cytometry (Figure 1A). Apoptotic neurons were preferentially ingested over live cells (Figure 1B), similar to previous reports (Magnus et al. 2002). In the presence of human serum, the uptake of apoptotic neurons by microglia is enhanced 2.2 +/- 0.5-fold and the uptake of neuronal blebs is enhanced 1.8 +/- 0.2-fold over uptake in the absence of serum (Figure 1D-E). Normal rat serum also enhances uptake of apoptotic cells equivalent to NHS (Figure 1C). Microglial uptake after incubation with human serum which is depleted of C1q (Figure 1D-E), heat-inactivated serum or serum from a C1q knock-out mouse (data not shown), was significantly reduced compared to whole serum, suggesting that C1q is one of the factors responsible for the serum-induced enhancement of apoptotic neuron and neuronal bleb uptake. Addition of C1q to C1qD enhanced the serum-mediated ingestion of both apoptotic neurons and neuronal blebs dose dependently, although full reconstitution of normal serum-levels of ingestion was seen only with the addition of excess C1q to C1qD (Figure 1D and E).
Figure 1. Microglial uptake of apoptotic neurons and neuronal blebs.
CFSE-labeled apoptotic neurons (A–D) and neuronal blebs (A,B,E) were added to rat microglia at a ratio of 3:1 (neurons) or 10:1 (neuronal blebs) without serum (B.) or with normal human serum (NHS), normal rat serum (NRS) (C), C1q-depleted human serum (C1qD) or C1qD in which C1q had been reconstituted to 2× or 10× serum concentration (D,E), as indicated, at a final volume of 10%. After incubation for 1 hour at 37°C, microglia were stained with anti CD11b and ingestion of CSFE targets was assessed by flow cytometry with representative dot blots from a single experiment shown in (A.). % uptake was assessed by calculation of the number of CD11b+CFSE+ cells as a % of total CD11b+ cells, with data from a single representative experiment in the absence of serum given in (B.). Average fold enhancement of uptake by sera are shown in (C. – E.) Data are expressed as the average fold enhancement of uptake over no serum control +/- SD. * p<0.05, ANOVA. n>5 (neurons), n=5 (blebs)
C1q binds apoptotic cells and neuronal blebs and enhances their ingestion by microglia
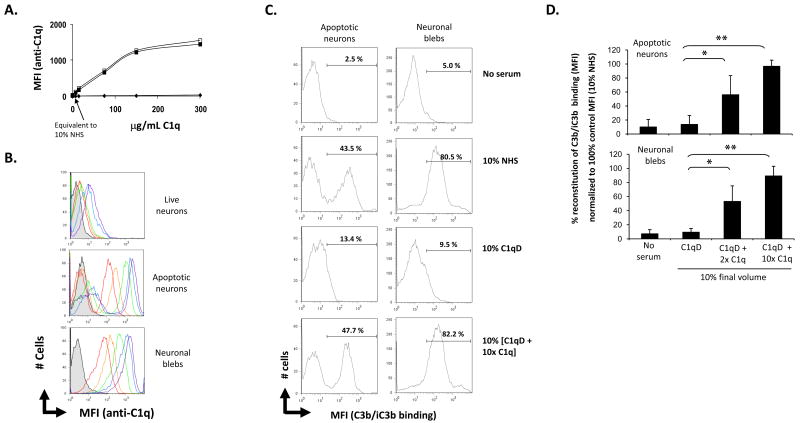
The observed C1q-dependent enhancement of apoptotic cell uptake could be due to C1q binding alone to the apoptotic cells or to the C1q-dependent activation of the classical complement cascade resulting in the deposition of C3b/iC3b on the apoptotic cell and facilitation of ingestion via CR1 and/or CR3 on the phagocyte. To examine the first possible mechanism live and apoptotic neurons or neuronal blebs were incubated with 0 to 300μg/mL C1q. Purified C1q bound to the apoptotic cells in a dose dependent manner, but not to live cells (Figure 2A & 2B). C1q binding to apoptotic neurons was 1.6-fold greater at 37°C than at 4°C (data not shown), and thus all subsequent studies used 37°C incubation with C1q. Similar to apoptotic neurons, neuronal blebs bound C1q in a dose dependent fashion. All blebs bound C1q essentially to a similar degree whereas there was always a residual population of apoptotic neurons (perhaps early apoptotic cells) that bound substantially (10-100 fold) less C1q than the majority of the apoptotic cells present. It should be noted that the MFI of the population of apoptotic neurons that bound C1q was 1.5-fold (+/- 0.05, n=3) higher than binding of C1q by neuronal blebs. Purified mouse C1q (75μg/ml) also bound to apoptotic cells but not to live cells (data not shown). Western blot analysis of washed live and apoptotic cells verified the association of C1q exclusively with apoptotic cells (data not shown). When apoptotic cells were incubated with 10% NHS for 30 min., the level of C1q binding detected was similar to that seen with the equivalent concentration of purified C1q (7 μg/ml) (data not shown).
Figure 2. C1q and C3b binding to apoptotic neurons and neuronal blebs.
Live neurons, apoptotic neurons and neuronal blebs were incubated with C1q or with normal human serum (NHS), C1q-depleted serum (C1qD) or C1qD in which C1q had been added to 2× or 10× serum concentration as described in Materials and Methods. A. C1q binding to live neurons (◆), apoptotic neurons (■) and neuronal blebs (□) incubated with amounts of human C1q from 7 to 300ug/mL, assessed by flow cytometry. MFI is calculated on the total population of cells B. Histogram depicting 0 (grey), 7 (red), 15 (orange), 75 (green), 150 (blue) or 300 (purple) μg/ml purified C1q binding to live neurons, apoptotic neurons or neuronal blebs assessed by flow cytometry. C. A typical histogram depicting the MFI of detection of anti- C3b/iC3b on apoptotic neurons (left) or neuronal blebs (right). Data in (A.), (B.) and (C.) are from a single experiment, representative of at least 3. D. Average C3b/iC3b binding (measured by MFI of antibody detection of C3d) expressed as % of the total average MFI of C3b/iC3b binding measured in samples incubated with 10% NHS. n=3-4 +/-SD. *p<0.05, **p<0.005, ANOVA.
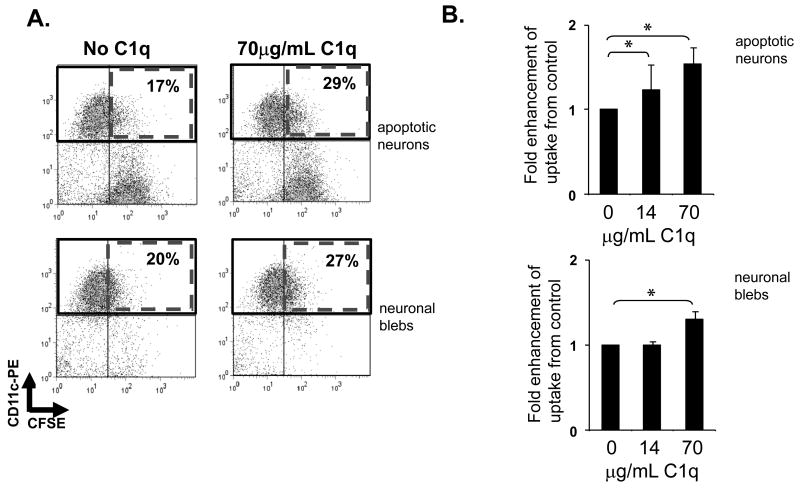
Preincubation of apoptotic neurons or neuronal blebs with purified C1q or addition of C1q during the ingestion period at concentrations resulting in approximately half maximal binding, enhanced the % of microglia ingesting apoptotic neurons and neuronal blebs (Figure 3) by microglia. That is, a concentration of C1q equivalent to the concentration in serum (70μg/mL) enhanced uptake by 1.5+/-0.2 fold while at lower concentrations of C1q which resulted in much less stable C1q binding to the cells, only minimal enhancement of ingestion occurred. These data suggest that C1q binding alone is sufficient to enhance uptake by microglia. However, since serum enhanced uptake to a greater extent than C1q alone (Figure 1C and 1D vs. 3B), additional factors appear to be involved in serum mediated enhancement of ingestion of apoptotic cells.
Figure 3. Microglial uptake of apoptotic neurons and neuronal blebs.
CFSE-labeled apoptotic neurons and neuronal blebs were added to rat microglia in the presence or absence of C1q as described in Materials and Methods. Uptake of CSFE-labeled targets by anti CD11b-labeled microglia was assessed by flow cytometry, with a single representative experiment depicted in (A) and expressed as average fold enhancement of uptake over control levels in the absence of C1q (B). +/- SD. * p<0.05 ANOVA, n=5 (apoptotic neurons), n=4 (neuronal blebs)
C3 binds apoptotic cells and enhances microglial uptake of apoptotic cells
To examine the possibility that C1q contributes to the enhancement of apoptotic cell uptake by serum via facilitation of C3b deposition, apoptotic neurons and neuronal blebs were incubated with 10% serum and C3b/iC3b binding assessed. Anti C3 bound to neuronal blebs, and to a subset of apoptotic neurons incubated with NHS, as measured by mean fluorescence intensity (MFI, Figure 2C). Very low/no C3b deposition was measured on live neurons (data not shown, n= 3). No enhancement of C3b/iC3b binding above background was detected on apoptotic neurons or neuronal blebs on cells incubated with C1q-deficient serum (C1qD) (Figure 2C, D). However, when apoptotic neurons or neuronal blebs were incubated with C1qD to which purified C1q had been added back at 2×, 4×, 6×, 8× or 10× normal serum concentration, C3b deposition was detected (Figure 2D and data not shown). The C3b binding (assessed by MFI) increased dose responsively with increased C1q concentration reaching 97% +/-9% of the level of deposition seen in NHS on a subset of the apoptotic neurons, while C3 was uniformly deposited on neuronal blebs in the presence of C1q (Figure 2C). This suggests that activation of complement by apoptotic cells and neuronal blebs as assessed by C3b/iC3b deposition can be classical pathway mediated. The C3b/iC3b binding in the C1q reconstituted C1qD correlated with the ingestion data supporting a role for the classical pathway deposited C3b in the serum-enhancement of uptake (Figure 1D,1E). Similar to the C1qD, C3-depleted serum (C3dpl), displayed a slight, but not significant, enhancement of apoptotic cell uptake over no serum control levels. Reconstitution of C3dpl serum with purified C3 to 1mg/ml (normal serum C3 concentration) restored C3 binding to apoptotic neurons and enhanced ingestion of apoptotic neurons up to 1.5-fold by microglia (n=3, data not shown). Interestingly with the primary microglia used here, the % of microglia ingesting apoptotic cells never exceeded 45% under any conditions, suggesting a mixed differentiation state of these primary microglia as prepared.
C1q modulates cytokine expression in response to LPS
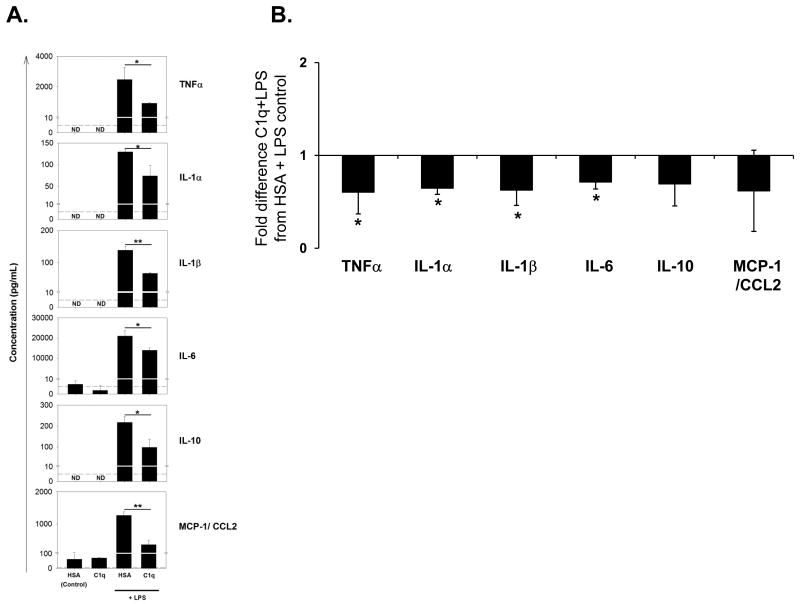
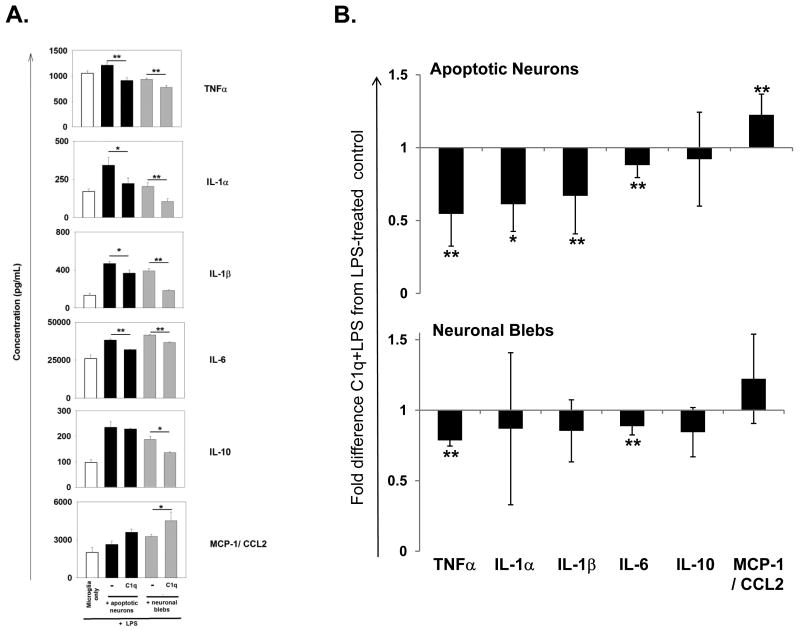
C1q has been shown to modulate LPS induced cytokine transcription and secretion in human monocytes under conditions in which phagocytosis is enhanced (Fraser et al. 2006). To determine if C1q similarly regulates microglial cytokine production, microglia were treated with 150 ng/ml LPS for 18 h with or without C1q and levels of cytokines/chemokines were measured in supernatants by Luminex multiplex assay. As expected, proinflammatory cytokines IL-1α, IL-1β, IL-6, TNF-α, and IL-10 and chemokine MCP-1/CCL-2 are upregulated by LPS (Figure 4A). However, when microglia were incubated on C1q coated slides, LPS-induction of the proinflammatory cytokines was significantly inhibited relative to microglia incubated on HSA protein control (Figure 4A and 5B). It has previously been reported that LPS-activated microglia cultured with apoptotic neuron-like cell lines alter their proinflammatory cytokine profile, down-regulating production of TNFα and nitric oxide (De Simone et al. 2003). Therefore to investigate if cytokine production by microglia may be influenced by the particle being ingested and/or opsonic molecules bound to those targets, cytokine production following stimulation with LPS was compared in microglia which have taken up apoptotic cells or neuronal blebs opsonized with C1q or in the absence of C1q. Contrary to data suggesting that apoptotic cell uptake induces a non-inflammatory state, proinflammatory cytokines IL-1α, IL-1β, IL-6 and TNFα were increased in LPS-stimulated microglia that have ingested apoptotic cells or neuronal blebs when compared to microglia that have not ingested apoptotic cells (Figure 5A, and data not shown). However, when C1q was pre-bound to the apoptotic neurons, levels of these proinflammatory cytokines were significantly reduced compared to levels in the absence of C1q (Figure 5B). In contrast to immobilized C1q, the presence of C1q on apoptotic neurons enhanced levels of LPS-induced chemokine MCP-1/CCL2 secreted by microglia. Although there was a degree of variability between experiments, on average, C1q did not significantly modify levels of anti-inflammatory cytokine IL-10, released by microglia during the uptake of apoptotic neurons (Figure 5B). The presence of C1q on neuronal blebs also significantly down-modulated levels of TNFα and IL-6 which were released by LPS-stimulated microglia, but levels of IL-1α, IL-1β, IL-10 and MCP-1 while not significantly altered showed similar trends toward modulation by the presence of C1q as was seen for apoptotic neurons (Figure 5B). These data suggest that the extent of C1q modulation of cytokine responses in microglia is dependent on the ingested particle, and may also be linked to the differential expression and/or density of co-receptor ligands on apoptotic neurons and neuronal blebs.
Figure 4. Modulation of LPS-induced proinflammatory cytokines released by rat microglia by C1q.
Cytokine levels were measured by Luminex multiplex analysis of the supernatant of unstimulated microglia plated on LabTek chamber slides coated with 8 μg/mL C1q or HSA control, or identically treated cells stimulated with 150ng/mL LPS for 18 hours. (A.) Data shown are average cytokine levels from an individual experiment, measured in triplicate +/- SD. *p<0.05, **p<0.01, students t-test. Dashed line represents the lower limit threshold of detection. ND – not detected. (B.) Results are expressed as fold difference in expression in supernatants of microglia on C1q-coated slides and stimulated with LPS compared to control levels from cells incubated in wells coated with HSA and stimulated with LPS. Data are the average values of 3 separate experiments, each performed in triplicate +/- SD. * p<0.05, ANOVA.
Figure 5. C1q modulation of cytokines released from microglia during the uptake of apoptotic neurons and neuronal blebs.
(A.) Cytokine levels were measured by Luminex multiplex analysis of the supernatant of microglia stimulated with 150 ng/ml LPS (white bar), or fed apoptotic neurons (black bars) or neuronal blebs (grey bars) in the absence or presence of 300 μg/mL C1q and stimulated with 150ng/mL LPS for 18 hours. Data are average cytokine levels from an individual experiment, measured in duplicate +/- SD. *p<0.05, **p<0.01, students t-test. (B.) Cytokine levels in the supernatant of LPS-stimulated microglia fed apoptotic neurons or neuronal blebs as measured in (A.), with results expressed as average fold difference in expression when C1q is present compared to control levels from identically treated microglia, in the absence of C1q, from n=5-7 individual experiments performed in duplicate with apoptotic neurons, or n=3-5 experiments with neuronal blebs) +/- SD. * p<0.05 **p<0.005, ANOVA.
Discussion
C1q expression is upregulated following neuronal injury and is synthesized early in neurodegenerative disorders such as Alzheimer's disease (reviewed in (Tenner and Pisalyaput 2008)). A multifunctional protein, C1q recognizes apoptotic cells and plays a vital role in their clearance as evidenced by the association of C1q deficiency with an accumulation of apoptotic cells and lupus-like disease in mice (Botto et al. 1998). Immobilized plate-bound C1q enhances phagocytosis of suboptimally opsonized targets by microglia, similar to other phagocytes (Webster et al. 2000). In addition, studies in our lab and others have shown that C1q can modulate phagocytosis of apoptotic cells by monocytes, macrophages and dendritic cells (Nauta et al. 2004b;Fraser et al. 2009), and contribute to non-inflammatory synaptic pruning during development (Stevens et al. 2007). Here we present data showing that C1q binds to neuronal blebs as well as to apoptotic neurons and significantly enhances their phagocytosis by microglia, the primary phagocytic cell of the CNS. In addition, while it is known that many serum factors contribute to uptake of apoptotic cells, here we show that activation of the classical complement pathway leads to C3b deposition and enhanced microglial uptake of both apoptotic neurons and neuronal blebs. However, the responses of microglia to C1q engagement are not identical, in terms of modulating cytokine gene expression (i.e. IL-10), to that of peripheral myeloid cells (Fraser et al. 2009).
When apoptotic cells were incubated with 10 % normal serum, C3b/iC3b was deposited on the cells, via classical pathway activation, and ingestion by microglia was enhanced. Reconstitution of C1qD with purified C1q provided a dose-dependent restoration of C3 deposition and uptake by microglia. Reductions in the level of uptake of apoptotic neurons by microglia compared to NHS were also observed using C3-depleted serum. These data are consistent with a sequence of events in which apoptotic neurons and neuronal blebs activate complement, and C3b is deposited on the surface of apoptotic neurons and blebs contributing to a more rapid uptake by microglia. It should be noted that the conclusions derived from the use of depleted serum are limited if the matching undepleted sera is not available for comparison and if other factors are diminished as a result of the depletion method. (For example, factor P depleted sera, intended for use in assessing alternative pathway activity, is also C1q depleted, and thus amplification of the classical pathway by factor P cannot be assessed unless both factor P and C1q are added back.) Even in these studies, levels of C3 binding were restored to just 55%, and target ingestion was returned to 80%, of the NHS levels with addition of near serum levels of C1q, with activity fully restored only by addition of much higher concentration of purified C1q to the matched C1q-depleted serum. Nevertheless, the results suggest that, while all serum proteins may not be present especially at early stages of neurodegenerative disorders, the upregulated synthesis of C1q following neuronal injury or early in neurodegenerative diseases such as Alzheimer's disease, may locally promote ingestion via synergistic effects of C1q with C3b as has been seen in other systems of phagocytic uptake (Bobak et al. 1988).
It is important to note that depletion of C1q lowers, but does not completely abrogate the ability of serum to enhance uptake over levels seen in the absence of serum, suggesting that other complement factors or serum factors may also play a role in the recognition and clearance of apoptotic cells. Interestingly, we also saw a reduction in the level of uptake of apoptotic neurons by microglia in the presence of factor P-depleted serum (data not shown) and a 1.4-fold enhancement of microglial uptake of apoptotic neurons which had pre-bound factor P (data not shown), similar to levels seen with 70 μg/mL C1q. Factor P/properdin was recently identified as a serum factor capable of binding to apoptotic T-cells and mediating an enhancement of clearance both directly and via complement activation (Kemper et al. 2008) and thus may also play a role in microglial clearance of apoptotic neurons. Indeed, levels of properdin, a highly positively charged serum protein, and Factor D were determined to be much lower in our C1qD than in the matched NHS (7% and 1%, respectively) (P.Giclas, National Jewish Health, personal communication). Since Factor P is an initiator of alternative pathway activation by apoptotic cells and Factor D also contributes to alternative pathway amplification, their depletion from C1qD may thus be the basis for the lack of complete reconstitution of C3b deposition (Figure 2D) and apoptotic cell uptake with normal serum levels of C1q (Figure 1D,E), as Factor P and Factor D can also participate in alternative pathway amplification of C1q-mediated classical pathway of complement activation. Clearly redundancy of the processes could be advantageous to the host.
Regardless of the amplification mechanisms in the presence of serum factors, the data thus far suggest a role for C1q in the uptake of apoptotic neurons and neuronal blebs by microglia. Purified C1q binds to apoptotic neurons and neuronal blebs (Figure 2), and enhanced uptake, similar to C1q binding to other apoptotic cells and enhanced ingestion by peripheral phagocytes (Nauta et al. 2004b;Fraser et al. 2009). C1q binding to apoptotic neurons displays two distinct populations (Figure 2B), which may reflect C1q binding preferentially to late apoptotic cells (AV+PI+) than early apoptotic (AV+PI-) as we and others have previously shown (Nauta et al. 2004a;Fraser et al. 2009). In our studies, C1q binding was unaffected by the mechanism of induction of neuronal apoptosis, as binding of C1q to UV-irradiated apoptotic neurons was identical to the binding seen in etoposide-treated apoptotic neurons (data not shown), suggesting that the motif(s) recognized by C1q are common to both pathways of apoptotic induction. However, the ligands/apoptotic cell-associated molecular patterns (ACAMPs) recognized by C1q on the surface of apoptotic neurons or neuronal blebs (in the absence of serum) are unknown and may not be restricted to a single molecular species, as C1q has previously been reported to bind both the membrane and intracellular contents of apoptotic endothelial cells and blebs (Navratil et al. 2001). Candidate molecules include phosphatidylserine and/or nucleic acids as these have been shown to be potential ligands on other apoptotic cell types (Paidassi et al. 2008;Elward et al. 2005). Differences in the response of microglia to apoptotic neurons and neuronal blebs with bound C1q suggest that either the C1q density differs or other signals on these targets are contributing to the signals received by the phagocyte.
C1q modulated the LPS-induced cytokines released by microglia when C1q was either immobilized on a plate or bound to apoptotic neurons or neuronal blebs, similar to studies in which C1q modulates LPS-induced proinflammatory cytokine production by human monocytes (Fraser et al. 2006). As with other myeloid cell populations (Fraser et al. 2009), the quantity and direction of modulation varied substantially depending on the particle ingested. Microglia added to C1q coated wells or fed apoptotic cells coated with C1q downregulated the LPS-induced production of IL-1α, IL-1β, IL-6, and TNF-α (Figures 4 and 5). TNFα and IL-6 were also downregulated by microglia which had ingested neuronal blebs coated with C1q, while an observed trend for the downregulation of IL-1α and IL-1β by C1q did not reach statistical significance. Microglial activation by C1q alone, which was immobilized on a plate, did not modulate the very low/undetectable levels of cytokines secreted by unstimulated microglial cells (Figure 4A). This is different from a recent report in which addition of C1q to microglial cultures triggered TNFα and IL-6 release at similar levels as LPS stimulation (Farber et al. 2009). These differences may be due to differences in interaction of soluble C1q with phagocytic cells versus a multivalent interaction as presented by C1q immobilized on a plate or bound to an apoptotic cell surface or to the length of time exposed to C1q. Indeed, phagocytic cells by nature are sensing their environments, and the sum of the signals they receive direct the subsequent immune response (such as ingestion, gene expression, and instruction of the adaptive response). Previous studies have shown that levels and type of costimulation can have a profound effect on the cytokine responses of microglia. For example, C1q has been shown to downregulate Aβ-stimulated levels of IL-1α, IL-1β, IL-6 and TNFα released by microglia in a models system of Alzheimer's disease. However, in the same study, when SAP was also included, C1q enhanced the Aβ stimulation of these cytokines (Veerhuis et al. 2003). Therefore, although the putative ACAMPs on apoptotic neurons and neuronal blebs recognized by C1q may be common, it is possible that while C1q is suppressing inflammatory responses during the uptake of apoptotic neurons, the density of ACAMP signals on the surface of neuronal blebs is different, and therefore triggers differential responses.
The inhibition of IL-10 and MCP-1/CCL2 production by C1q-coated surfaces, while not statistically significant was somewhat unexpected as similar experiments with human monocytes showed an enhanced secretion of those two mediators by C1q. Rather, microglia resembled immature dendritic cells (Fraser et al. 2009) in the response to surface immobilized C1q. However, regulation of IL-10 production is complex (Chung et al. 2007;Mosser and Zhang 2008), and thus more molecular delineation of these signaling pathways in the CNS is clearly needed.
It can be noted that apoptotic neurons themselves induced a significant increase of IL-1α, IL-1β and TNFα by microglia in response to LPS (figure 5A) in contrast to a report by DeSimone et al in which apoptotic PC12 cells (a neuronal cell line) decreased levels of TNFα secreted by microglia. However, those studies were performed in the presence of 10% FBS, and therefore a suppressive role for serum molecules present in the assay system cannot be ruled out (De Simone et al. 2003). Nevertheless, C1q bound to the apoptotic cells suppressed the increase in proinflammatory cytokines (Figure 4 and 5).
In summary, the observations reported here extend the involvement of complement in the enhancement of uptake of apoptotic cells by phagocytic cells to microglia, the primary phagocytic cell of the CNS, and define contributions of C1q and C3b/iC3b to microglial ingestion of apoptotic cells and at least one form of cellular debris, neuronal blebs. The induction of C1q synthesis following injury is therefore consistent with a protective response facilitating clearance of apoptotic cells or cell debris, and promoting the resolution of inflammation before further damage ensues. More rapid ingestion by microglia of apoptotic neurons or neuronal blebs which have bound C1q may prevent toxic intracellular contents (such as glutamate) from being released into the CNS environment which could accelerate damage to surrounding neurons. The rapid clearance of cell debris would be particularly important following injuries such as stroke and also in neurodegenerative diseases such as Alzheimer's disease, when a large number of apoptotic neurons/neuronal blebs may be generated. These studies suggest the potential benefit of development of therapeutic strategies for CNS injury, which allow for the protective effects of C1q in the removal of cell debris, while minimizing potential harmful downstream consequences of complement activation (such as release of potent proinflammatory mediator, C5a) (Fonseca et al. 2009). In addition, the recent reports of complement proteins involved in normal development and remodeling of synapses in the CNS as well as synapse loss during neurodegeneration (reviewed by (Perry and O'Connor 2008) suggests that further investigation of complement proteins in these processes may provide novel insights for targeted control of events in the CNS.
Acknowledgments
This work was supported by NIH grants AI 41090, NS35144, and AG 00538. The authors thank Ozkan Yazan, Rahasson Ager and Emily Volger for assistance with neuronal and microglial cell preparation.
Abbreviations used
- BSA
bovine serum albumin
- CFSE
Carboxyfluorescein succinimidyl ester
- FBS
fetal bovine serum
- HBSS++
Hank's buffered salt solution
- HSA
human serum albumin
- IL
interleukin
- LPS
lipopolysaccharide
- NHS
normal human serum
- OVA
ovalbumin
- PE
phycoerythrin
Reference List
- Bobak DA, Frank MM, Tenner AJ. C1q acts synergistically with phorbol dibutyrate to activate CR1-mediated phagocytosis by human mononuclear phagocytes. Eur J Immunol. 1988;18:2001–2007. doi: 10.1002/eji.1830181220. [DOI] [PubMed] [Google Scholar]
- Botto M, Dell'agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology. 2002;205:395–406. doi: 10.1078/0171-2985-00141. [DOI] [PubMed] [Google Scholar]
- Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. Interleukin-10 Expression in Macrophages during Phagocytosis of Apoptotic Cells Is Mediated by Homeodomain Proteins Pbx1 and Prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Tirassa P, Minghetti L. Apoptotic PC12 cells exposing phosphatidylserine promote the production of anti-inflammatory and neuroprotective molecules by microglial cells. J Neuropathol Exp Neurol. 2003;62:208–216. doi: 10.1093/jnen/62.2.208. [DOI] [PubMed] [Google Scholar]
- Elward K, Griffiths M, Mizuno M, Harris CL, Neal JW, Morgan BP, Gasque P. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. J Biol Chem. 2005;280:36342–36354. doi: 10.1074/jbc.M506579200. [DOI] [PubMed] [Google Scholar]
- Farber K, Cheung G, Mitchell D, Wallis R, Weihe E, Schwaeble W, Kettenmann H. C1q, the recognition subcomponent of the classical pathway of complement, drives microglial activation. J Neurosci Res. 2009;87:644–652. doi: 10.1002/jnr.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, Taylor SM, Woodruff TM, Tenner AJ. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease. J Immunol. 2009;183:1375–1383. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DA, Arora M, Bohlson SS, Lozano E, Tenner AJ. Generation of inhibitory NF{kappa}B complexes and phosphorylated cAMP response element-binding protein correlates with the activity of complement protein C1q in human monocytes. J Biol Chem. 2007;282:7360–7367. doi: 10.1074/jbc.M605741200. [DOI] [PubMed] [Google Scholar]
- Fraser DA, Bohlson SS, Jasinskiene N, Rawal N, Palmarini G, Ruiz S, Rochford R, Tenner AJ. C1q and MBL, components of the innate immune system, influence monocyte cytokine expression. J Leukoc Biol. 2006;80:107–116. doi: 10.1189/jlb.1105683. [DOI] [PubMed] [Google Scholar]
- Fraser DA, Laust AK, Nelson EL, Tenner AJ. C1q differentially modulates phagocytosis and cytokine responses during ingestion of apoptotic cells by human monocytes, macrophages, and dendritic cells. J Immunol. 2009;183:6175–6185. doi: 10.4049/jimmunol.0902232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DA, Tenner AJ. Directing an appropriate immune response: the role of defense collagens and other soluble pattern recognition molecules. Curr Drug Targets. 2008;9:113–122. doi: 10.2174/138945008783502476. [DOI] [PubMed] [Google Scholar]
- Goodwin JL, Uemura E, Cunnick JE. Microglial release of nitric oxide by the synergistic action of beta-amyloid and IFN-gamma. Brain Res. 1995;692:207–214. doi: 10.1016/0006-8993(95)00646-8. [DOI] [PubMed] [Google Scholar]
- Kemper C, Mitchell LM, Zhang L, Hourcade DE. The complement protein properdin binds apoptotic T cells and promotes complement activation and phagocytosis. Proc Natl Acad Sci U S A. 2008;105:9023–9028. doi: 10.1073/pnas.0801015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- Li M, Pisalyaput K, Galvan M, Tenner AJ. Macrophage colony stimulatory factor and interferon-gamma trigger distinct mechanisms for augmentation of beta-amyloid-induced microglia-mediated neurotoxicity. J Neurochem. 2004;91:623–633. doi: 10.1111/j.1471-4159.2004.02765.x. [DOI] [PubMed] [Google Scholar]
- Lynch NJ, Willis CL, Nolan CC, Roscher S, Fowler MJ, Weihe E, Ray DE, Schwaeble WJ. Microglial activation and increased synthesis of complement component C1q precedes blood-brain barrier dysfunction in rats. Mol Immunol. 2004;40:709–716. doi: 10.1016/j.molimm.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Magnus T, Chan A, Savill J, Toyka KV, Gold R. Phagocytotic removal of apoptotic, inflammatory lymphocytes in the central nervous system by microglia and its functional implications. J Neuroimmunol. 2002;130:1–9. doi: 10.1016/s0165-5728(02)00212-6. [DOI] [PubMed] [Google Scholar]
- Magnus T, Korn T, Jung S. Chronically stimulated microglial cells do no longer alter their immune functions in response to the phagocytosis of apoptotic cells. J Neuroimmunol. 2004;155:64–72. doi: 10.1016/j.jneuroim.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, van Kooten C, Roos A. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol. 2004a;173:3044–3050. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, van KC, Roos A. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol. 2004b;173:3044–3050. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Trouw LA, Daha MR, Tijsma O, Nieuwland R, Schwaeble WJ, Gingras AR, Mantovani A, Hack EC, Roos A. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. 2002;32:1726–1736. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- Ogden C, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–796. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CA, Elkon KB. Role of complement and other innate immune mechanisms in the removal of apoptotic cells. Curr Dir Autoimmun. 2006;9:120–142. doi: 10.1159/000090776. [DOI] [PubMed] [Google Scholar]
- Paidassi H, Tacnet-Delorme P, Garlatti V, Darnault C, Ghebrehiwet B, Gaboriaud C, Arlaud GJ, Frachet P. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol. 2008;180:2329–2338. doi: 10.4049/jimmunol.180.4.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, O'Connor V. C1q: the perfect complement for a synaptic feast? Nat Rev Neurosci. 2008;9:807–811. doi: 10.1038/nrn2394. [DOI] [PubMed] [Google Scholar]
- Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol. 2005;35:252–260. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa F, Tsuji S, Nagasawa S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Lett. 1996;397:269–272. doi: 10.1016/s0014-5793(96)01197-0. [DOI] [PubMed] [Google Scholar]
- Tenner AJ, Fonseca MI. The double-edged flower: roles of complement protein C1q in neurodegenerative diseases. Adv Exp Med Biol. 2006;586:153–176. doi: 10.1007/0-387-34134-X_11. [DOI] [PubMed] [Google Scholar]
- Tenner AJ, Pisalyaput K. The Complement System in the CNS: Thinking again. In: Lane TE, Carson MJ, Bergmann C, Wyss-Coray T, editors. Central Nervous System Diseases and Inflammation. Springer; New York: 2008. pp. 153–174. [Google Scholar]
- Trouw LA, Bengtsson AA, Gelderman KA, Dahlback B, Sturfelt G, Blom AM. C4b-binding protein and factor H compensate for the loss of membrane-bound complement inhibitors to protect apoptotic cells against excessive complement attack. J Biol Chem. 2007;282:28540–28548. doi: 10.1074/jbc.M704354200. [DOI] [PubMed] [Google Scholar]
- Trouw LA, Blom AM, Gasque P. Role of complement and complement regulators in the removal of apoptotic cells. Mol Immunol. 2008a;45:1199–1207. doi: 10.1016/j.molimm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Trouw LA, Nielsen HM, Minthon L, Londos E, Landberg G, Veerhuis R, Janciauskiene S, Blom AM. C4b-binding protein in Alzheimer's disease: binding to Abeta1-42 and to dead cells. Mol Immunol. 2008b;45:3649–3660. doi: 10.1016/j.molimm.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Van Muiswinkel FL, Veerhuis R, Eikelenboom P. Amyloid β protein primes cultured rat microglial cells for an enhanced phorbol 12-myristate 13-acetate-induced respiratory burst activity. J Neurochem. 1996;66:2468–2476. doi: 10.1046/j.1471-4159.1996.66062468.x. [DOI] [PubMed] [Google Scholar]
- Veerhuis R, Van Breemen MJ, Hoozemans JM, Morbin M, Ouladhadj J, Tagliavini F, Eikelenboom P. Amyloid beta plaque-associated proteins C1q and SAP enhance the Abeta1-42 peptide-induced cytokine secretion by adult human microglia in vitro. Acta Neuropathol. 2003;105:135–144. doi: 10.1007/s00401-002-0624-7. [DOI] [PubMed] [Google Scholar]
- Webster SD, Yang AJ, Margol L, Garzon-Rodriguez W, Glabe CG, Tenner AJ. Complement component C1q modulates the phagocytosis of Abeta by microglia. Exp Neurol. 2000;161:127–138. doi: 10.1006/exnr.1999.7260. [DOI] [PubMed] [Google Scholar]