Summary
The haematological and neurological consequences of cobalamin deficiency define the essential role of this vitamin in key metabolic reactions. The identification of cubilin-amnionless as the receptors for intestinal absorption of intrinsic factor-bound cobalamin and the plasma membrane receptor for cellular uptake of transcobalamin bound cobalamin have provided a clearer understanding of the absorption and cellular uptake of this vitamin. As the genes involved in the intracellular processing of cobalamins and genetic defects of these pathways are identified, the metabolic disposition of cobalamins and the proteins involved are being recognized. The synthesis of methylcobalamin and 5’deoxyadenosylcobalamin, their utilization in conjunction with methionine synthase and methylmalonylCoA mutase, respectively, and the metabolic consequences of defects in these pathways could provide insights into the clinical presentation of cobalamin deficiency.
Keywords: Vitamin B12, Transcobalamin-receptor/CD320, Intrinsic factor, haptocorrin
1. Introduction
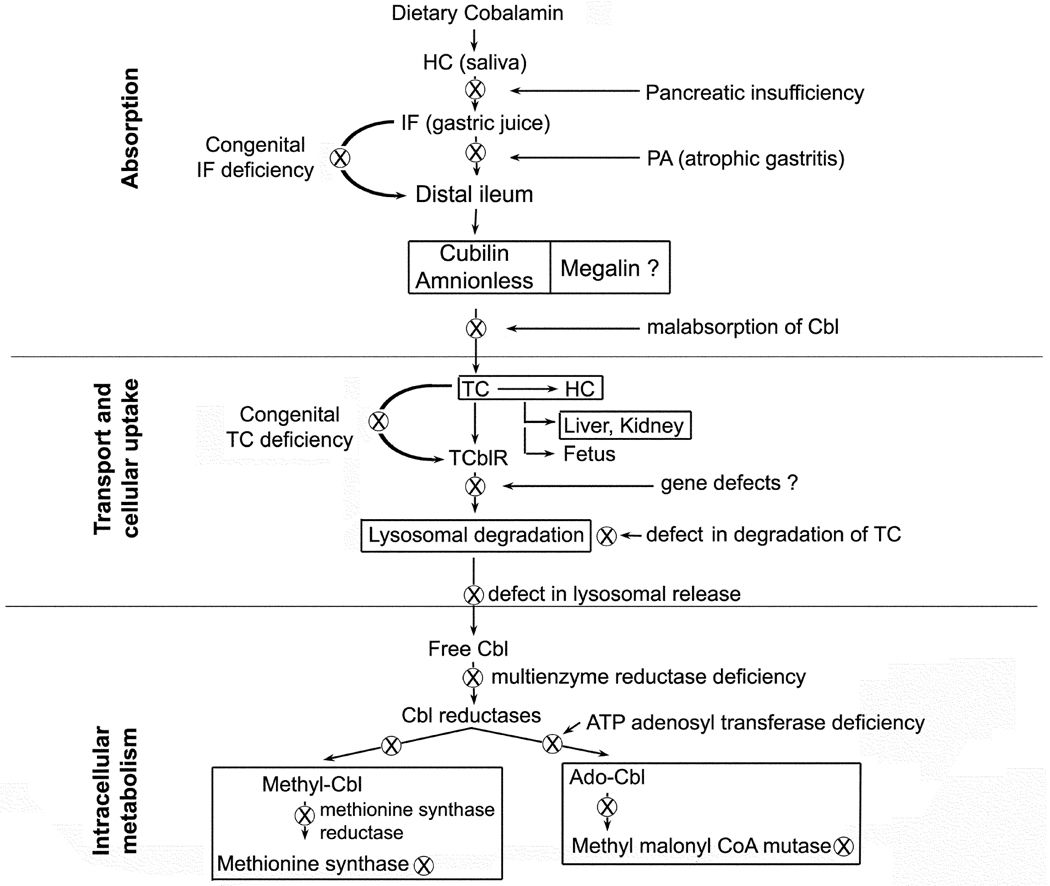
During the eighty plus years since the discovery of vitamin B12 (cobalamin, Cbl), much has been written to define the clinical, haematological and neuropathological manifestations of the disorders that result from Cbl deficiency (Watkins et al, 2009). Even though the haematological presentation of Cbl deficiency cannot be distinguished from that of folate deficiency (Wickramasinghe, 2006), the clinical disorder has, to a large extent, been defined as presenting with neuropathological changes that are unique to Cbl deficiency (Healton et al, 1991). However, this assumption may be changing due to the association of folate deficiency with gross structural alterations of neural development during embryonic and fetal life (Finnell et al, 2008) and functional abnormalities observed in children with cerebral folate deficiency syndrome (Ramaekers & Blau, 2004). The neurological presentation of dementia and diminishing cognitive function in the older population may well result from the combined deficiency of Cbl and folate in the central nervous system (Reynolds, 2006) however, definitive proof of this hypothesis is lacking. Recent advances in the purification of proteins and molecular genetics have provided new and definitive information on the Cbl binding proteins, their receptors, and enzymes involved in the intracellular processing of Cbl. This fundamental information has also enabled us to identify gene defects involving pathways of absorption, cellular uptake and intracellular metabolism of Cbl (Figure 1).
Figure 1.
Pathways and proteins involved in the assimilation of cobalamin and inborn or acquired defects (⊗) in these pathways.
2. Cobalamin homeostasis
In recent years, there has been much debate on Cbl status and subclinical Cbl deficiency. Approximately 6% of the western population over the age of 60 years has low plasma Cbl and as much as 20% may have marginal Cbl status (Allen, 2009). This subtle deficiency may play a role in decreased cognitive function and dementia in the ageing population (Smith & Refsum, 2009). Decreased dietary intake and poor absorption may contribute to low Cbl status in this population (Lindenbaum et al, 1994). The daily intake of Cbl in a western diet is probably greater than 5µg. This is likely to be considerably less in eastern and vegetarian diets due to lower meat intake. Based on daily loss of 1 – 2 µg of Cbl, a similar amount will have to be absorbed to maintain normal Cbl status. In Cbl deficiency, body stores would probably deplete in 6 – 12 months based on a half-life of about 12 months and loss of about 2 –4 µg/day because of impaired reabsorption (Chanarin, 1979). The folate supplementation of diet to reduce the incidence of neural tube defect pregnancy and to lower homocysteine (HCY) to decrease the risk of cardio-vascular disease (Selhub & Rosenberg, 2008) has raised issues about the need to improve the Cbl level along with the folate (Thompson et al, 2009; Green, 2009). Therefore, the current perception of normal plasma Cbl in the 200 – 800 pg / ml range, may need re-evaluation and a higher daily intake of Cbl may have to be considered. This may be particularly relevant in the elderly population with decreased absorption of dietary Cbl (Carmel, 1997). Given that approximately 80% of plasma Cbl is bound to haptocorrin (HC) and because it is the fraction bound to transcobalamin (TC) that is available for cellular uptake, a daily higher intake would provide more Cbl for tissue cells. Even though including Cbl in food fortification has been debated, universal agreement on this issue has not been reached. One potential objection has been the issue of stability of Cbl and the likelihood of introducing analogs or breakdown products that may be harmful antimetabolites. A resolution to this dilemma may be to fortify a food source, such as milk, with Cbl. With a short shelf life and storage under refrigerated conditions, and because some quantity is consumed in various forms by most of the population, milk could provide a vehicle to increase the daily intake to raise the Cbl status in the population. The percentage of Cbl absorbed decreases with increasing dose and less than 2µg of a 50µg dose is absorbed. This may be considered as the limit of Cbl absorption via the intrinsic factor (IF) receptor pathway since the amount of IF produced is far in excess of the amount of Cbl absorbed. At doses of 1–2mg, approximately 10µg of the vitamin may be absorbed via nonspecific internalization in both normal subjects and in patients with malabsorption (Carmel, 2008). Such high doses of oral Cbl are considered as effective as periodic intramuscular injections in restoring Cbl status (Butler et al, 2006).
3. Absorption of Cobalamin
The process of dietary Cbl absorption has evolved to provide selective absorption of Cbl and exclude structurally similar compounds of bacterial origin that may come from gut microflora and dietary sources. This process is mediated by a number of proteins, haptocorrin (HC) in the saliva, intrinsic factor (IF) in gastric juice and the receptor for IF-Cbl in the distal ileum.
Intrinsic Factor
This protein is produced by the acid secreting pareital cells of the stomach and binds ~50–60 ng Cbl per ml of gastric juice. Based on an average gastric juice production of 1–3 litres / 24 h, approximately 1–5mg of IF is produced to bind about 30–150 ug of Cbl (Allen, 1975). Thus, available IF is far in excess of the daily amount of Cbl absorbed. The 45 kDa protein is glycosylated and sialydated, accounting for the 58–60 kDa size by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) (Gueant et al, 1985). The gene (GIF) encodes a 417 amino acid protein with 9 exons, spans a region of 16.2 kb and is located at11q13 (Hewitt et al, 1991). The recombinant IF produced in plant has been well characterized. Based on these data, the protein shows two distinct domains with each domain capable of binding Cbl. However, as a single protein, both domains appear to cooperatively participate in binding Cbl with higher affinity than each domain separately (Fedosov, et al, 2005a). The corrin ring sits parallel to the central axis of the alpha barrel and forms multiple hydrogen bonds with both domains. Among the various Cbl binding proteins, HC has the broadest affinity for Cbl analogs, whereas IF specifically binds Cbl and reacts poorly with Cbl analogs, such as cobinamide, which lacks the nucleotide portion. The specificity for Cbl and Cbl analogs appears to reside in the beta 3 and beta 4 regions of the beta domain. The difference in amino acids in these regions among the three Cbl binding proteins accounts for the difference in affinity (Wuerges et al, 2007; Mathews et al, 2007). The amino acids in this region of IF appear to be critical for the specificity of Cbl binding and may have evolved to ensure selective absorption of Cbl in an environment where corrin-like compounds of bacterial origin or from break down of food Cbl may compete for the limited absorptive capacity of the IF receptor. The specificity for Cbl, coupled with the fact that the complete 50 kDa protein is required for receptor recognition, implies that the tertiary structure of the 50 kDa IF with the Cbl in position is necessary to generate the appropriate conformation for its interaction with the receptor for IF-Cbl.
The existence of a receptor in the distal ileum for absorption of Cbl was demonstrated in early studies by Ca++ dependent binding of IF-Cbl. The definitive identification of the receptor protein proved to be difficult because of the multiple protein bands in the purified product. The difficulty in obtaining a homogeneous preparation of the receptor protein for conclusive identification may be attributed to the fact that the protein now identified as the receptor for IF-Cbl is a receptor for multiple ligands and interacts with at least two other membrane proteins (Moestrup & Verroust, 2001). Decreased absorption of Cbl can result from many causes, the most common of which is pernicious anemia (PA) due to autoimmune destruction of parietal cells (Chanarin, 1979). Although of adult onset, this disorder can present in children and young adults as juvenile PA. Inborn errors of IF are rare and range from a total lack of IF to a non-functional protein. The latter could present as decreased binding of Cbl or to the receptor or as an unstable protein (Reviewed in Rosenblatt & Fenton, 1999). The genetic defects contributing to these abnormalities were not identified. The first GIF defect identified was a four base deletion in exon 2 (c183 - 186 delCAAT) leading to a frame shift and premature termination of translation. This was described in a female child of African descent (Yassin et al, 2004). Three additional cases have been identified and the identical deletion in this population has been attributed to a founder effect (Tanner et al, 2005; Ament et al, 2009). This homozygous mutation appears to be due to autosomal recessive inheritance of the GIF defect. A number of other single mutations leading to loss of function and polymorphisms have been identified (Tanner et al, 2005). Polymorphism in exon 1 (Q5R) was reported in four patients with low IF (Gordon et al, 2004). However, a larger study has identified this polymorphism at a frequency of 3 –11% and may not be associated with decreased IF production (Ament et al, 2009) or low Cbl (Remacha et al, 2008).
Intrinsic Factor Receptor and the absorption of Cbl
The absorption of physiological concentrations of Cbl occurs via IF and the receptor for IF-Cbl, cubilin (CUB), a peripheral membrane-associated protein expressed in the distal ileum. This 3600 aa, 460 kDa protein contains 8 epidermal growth factor ( EGF)-like repeats followed by 27 repeats of 110 aa of CUB (complement components C1r/C1s, the sea urchin protein Uegf, and bone morphogenic protein-1) domains found in many developmentally regulated proteins and involved in ligand binding (Kozyraki & Gofflot 2007). The binding of IF-Cbl has been localized to CUB domains 5–8 (Kristiansen et al, 1999). The human gene (CUBN) is located at10p12.31 and spans a region of 306kb with 39 exons (Kozyraki et al, 1998). The internalization of CUB with IF-Cbl is facilitated by amnionless (AMN), a transmembrane protein originally identified as a visceral endoderm protein essential for embryonic development. Congenital malabsorption of Cbl can result from mutations in either protein because AMN appears to be involved in the apical targeting as well as internalization of CUB (Fyfe et al, 2004). Even though megalin (MAG) and receptor-associated protein (RAP) can interact with CUB, the precise role if any, of these proteins in CUB-mediated absorption of IF-Cbl has not been determined.
3. Transport of Cobalamin
The IF-Cbl absorbed in the distal ileum appears in the circulation bound to TC. The events following translocation of IF-Cbl to the ileal enterocyte, release from IF and binding to TC are not fully defined. However, based on in vivo and in vitro studies, it is evident that apo TC is available in the ileum to bind the free Cbl. Evidence in support of this conclusion is based on: 1. In human studies, orally administered radiolabeled Cbl appeared bound to TC (Chanarin et al, 1978); 2. IF-Cbl absorption studies in the guinea pig showed the appearance of TC-Cbl from the isolated ileum (Rothenberg et al, 1978). The synthesis of TC in the intestinal villous and its transport to the serosal side has been confirmed by in situ hybridization studies and in the isolated ileum (Quadros et al, 1999). The distribution of the mRNA and the appearance of apoTC in the villous would support the origin of TC in the abundant microvascular endothelium. The release of Cbl from IF and its exit from the ileal enterocyte is not well defined. The Cbl may exit the cell by simple or facilitated diffusion against a concentration gradient or a yet unidentified active transport system may operate to release the Cbl into the basolateral side. A transport system for free Cbl was proposed for the exit of Cbl from L-1210 cells cultured in vitro (Quadros and Jacobsen, 1995) and may operate in all cell types for disposition and recycling of Cbl.
Cbl concentration in plasma has been used as a measure of nutrient status for decades and provided a reasonable parameter to distinguish normal from severe deficiency. However, the availability of assays for sensitive indicators of intracellular metabolites, such as methylmalonic acid (MMA), homocysteine (HCY) and 5-methyl citrate (Savage et al, 1994) have questioned the validity of total Cbl estimation, which does not always correlate with the clinical presentation of Cbl deficiency. Given that ~80% of the Cbl in serum is bound to HC and is not readily available for cellular uptake, the determination of Cbl specifically bound to TC (holotranscobalamin; holo-TC) was suggested as an alternative measure of Cbl status (Herzlich & Herbert, 1988). This would provide a measure of daily Cbl intake and Cbl readily available for cellular uptake because newly absorbed Cbl would be bound to TC. The availability of sensitive and direct assays for holo-TC (Brady et al, 2008) have overcome many of the technical limitations and recent studies provided convincing proof of the merits of assaying holo-TC (Morkbak et al, 2005). Even though this assay could replace total Cbl determination for mass screening, ultimately, intracellular metabolites, especially MMA, in conjunction with serum holo-TC may provide the most information on Cbl status and utilization.
Transcobalamin (TC)
This nonglycosylated protein in plasma carries between 10–30 % of the total Cbl and is mostly present in its apo form (apotranscobalamin, apo-TC). Cohn fraction III of human plasma was the source of apo-TC for obtaining highly purified TC (Quadros, et al, 1986). TC serves the essential function of binding and transporting the newly absorbed Cbl in the distal ileum to tissue cells throughout the body where it is internalized by receptor-mediated cellular uptake (Rothenberg &Quadros, 1995). Even though all cells may produce some TC, the synthesis of large amounts of this protein by human umbilical vein endothelial cells suggests the endothelial cell as the likely source of this protein in circulation (Quadros et al, 1989). The abundant vascular endothelium is an ideal source of this protein with a relatively short half-life of 1–2 h for holo-TC and rapid uptake by tissue cells. This major source of TC also facilitated the cloning of the cDNA from an endothelial cDNA library (Platica et al, 1991). The gene encoding TC (TCN2) is located at 22 q11-13.1, spans a region of 18kb and has 9 exons. The similarity in the organization of the genes and amino acid sequences encoding Cbl binding proteins suggests their origin from a common ancestral gene (Regec et al 1995). The 1.9 kb cDNA encodes a peptide of 409 aa, 45.5 kDa secreted protein. Single nucleotide substitutions were identified at codon 198, 219, 259 and 376 (Li et al, 1993) however, the two isoforms of TC observed are due to the difference in two amino acids in the N terminus of the secreted protein (Quadros et al, 1993). TC is more discriminating than HC in binding Cbl analogues. Within the corrin ring, the amide side chains appear to be critical for binding, with modification of the amide group at the “e” position having the least effect (Pathare et al, 1996). Modifications or addition of ligands to the central cobalt or to the ribose moiety of the nucleotide base does not affect binding to TC. The crystallographic analysis of bovine holo-TC has provided the structure of TC and Cbl binding (Wuerges et al, 2006). The amino terminal major domain and the carboxy terminal minor domain appear to form a pocket for the Cbl molecule. It is the folded conformation of TC with the Cbl embedded that provides the epitope(s) for interaction with the receptor (TCblR). Based on the deduced structure and epitope mapping of the TC protein with monoclonal antibodies (Quadros et al, 1996), residues 103–159 and the positively charged heparin-binding region in residues 207 – 227 are likely to be involved in receptor binding (Fedosov et al, 2005b).
Genetic abnormalities of Transcobalamin
Congenital TC deficiency is not embryonically lethal and infants with this defect are born normal. However, these infants develop Cbl deficiency soon after birth and therefore prompt diagnosis and treatment with pharmacological doses of Cbl are critical to preventing hematological and neurological complications. Congenital abnormalities of TC include complete absence of TC, immunoreactive TC that does not bind to the receptor or that does not bind Cbl (reviewed in Rothenberg & Quadros, 1995). Mutations in TCN2 leading to TC deficiency have been identified and these can be deletions, nonsense mutations (Li et al, 1994), errors in RNA editing of the primary transcript (Qian et al, 2002) and a point mutation in the intron 3 splice site, generating a cryptic splice site in exon 3 and an in-frame deletion of 81 nucleotides (Namour et al, 2003).
Haptocorrins (HC)
The proteins referred to in older literature as R-binder, TC1 or TCIII are collectively named haptocorrins and differ only in glycosylation (Alpers & Russell-Jones, 1999). They are the product of a single gene (TCN1) encoded by 9 exons on chromosome11 and are present in many body fluids and secreted by many cell types including glandular cells (Johnston et al, 1989). The 410 aa core polypeptide accounts for a molecular weight of 45.5 kDa and the 30–40% glycosylation accounts for the ~66 kDa size (Burger et al, 1975). The desialylated protein can be taken up and degraded by the asialoglycoprotein receptor in the liver (Alpers & Russell-Jones 1999). No specific function has been assigned to this protein other than binding a large fraction of Cbl in circulation. This may prevent loss of free Cbl and its lack of specificity for Cbl may aid in clearing corrin–like compounds from entering the cell via the TC-receptor pathway.
4. Cellular uptake of Cbl
Early studies had recognized that free Cbl is not internalized in cells and a serum component is required for cellular uptake of physiological concentrations of Cbl. The serum component was subsequently identified as TC. Studies to define the pathway for cellular uptake of Cbl provided clear evidence of enhanced uptake of TC bound Cbl. This uptake involved binding of TC-Cbl to the cell surface and internalization of the TC-Cbl complex. The process required the divalent cation Ca++ and metabolic energy as indicated by enhanced uptake at 37°C (DiGirolamo & Huennekens, 1975). Even though the existence of a receptor on the plasma membrane for cellular uptake of TC-Cbl could be readily inferred from binding and uptake studies in live cells, the purification of the receptor protein proved to be a formidable challenge to investigators. However, functional receptor could be demonstrated by binding of TC-Cbl to a detergent soluble fraction of placental membranes analyzed by gel filtration chromatographic separation of the receptor/TC-Cbl complex (Nexo & Hollenberg, 1980).
The Receptor for Transcobalamin-Cbl
The very first concerted attempt to purify the soluble receptor used human placental membranes as the source of the receptor and affinity chromatography on a TC-Cbl matrix as the main purification step (Seligman & Allen 1978). The final product was, at best, partially pure and therefore, the data obtained on the size of the protein and amino acid/carbohydrate composition cannot be considered as accurate. Our initial attempts to purify the receptor from human placenta proved difficult. However, we were able to obtain information on the binding kinetics of the membrane bound and soluble receptor and on the size of the protein by cross-linking experiments and identified a ~58 kDa protein that was extensively glycosylated (Quadros et al, 1994). Following our publication, purification of the receptor was reported by another group that identified a 72 kDa monomer / 144 kDa dimer as the receptor on the plasma membrane for TC-Cbl (Bose et al, 1995). The reported abundance of this protein in placenta and the lack of any confirmation of specific binding to TC-Cbl, convinced us that the protein described with an apparent FC-like domain (Vanamala et al, 2003) may not be the receptor for TC-Cbl. We experimentally proved that the protein solubilized from human placental membrane that specifically binds TC-Cbl with high affinity does not have a FC-like domain (Quadros et al, 2005). This was followed by about two years of studies using refinements in conventional protein purification, coupled with multiple affinity purification steps that ultimately provided what appeared to be a homogeneous single protein by SDS-PAGE. The 58 kDa size of the protein and its retention through multiple affinity purification steps in association with TC-Cbl convinced us that the protein was the receptor for TC-Cbl. The receptor protein (TCblR) was identified by liquid chromatography / mass spectroscopy analysis of the trypsin-digested peptide fragments and the gene (CD320) encoding this receptor was identified from the human genome data bank (Quadros et al, 2009), Structurally this 282 aa protein belongs to the LDL receptor family with two LDLR type A domains separated by a 55 aa cysteine rich CUB-like domain, a 21 aa transmembrane region and a 32 aa cytoplasmic domain. There are three N glycosylation sites in the 198 aa extracellular region with potential O glycosylation sites both in the extracellular as well as cytoplasmic domains. The aberrant migration in SDS-PAGE as a 58 kDa protein is probably due to extensive glycosylation of the protein. The two LDLR type A domains and the intervening cysteine-rich CUB-like domain show remarkable similarity to other receptors of this family of proteins. However, the small size, the presence of only two LDLR-A domains and the sequences unique to these two domains probably provide the specificity for TC-Cbl binding because LDL and RAP, the two proteins known to affect binding of ligands to this class of receptors, do not affect TC-Cbl binding.
The expression of TCblR on the cell surface is fairly low with maximum expression in actively dividing cells and down-regulation of functional receptors in quiescent cells (Lindemans et al, 1989; Amagasaki et al, 1990). This cell cycle association of receptor expression and the efflux of free Cbl out of the cell may be physiologically relevant in that receptors are expressed to meet increased Cbl requirement during DNA synthesis and once this requirement has been met, the excess Cbl is effectively transported out of the cell and is captured by circulating apo-TC for mobilization to where it is needed most. Studies using in vitro culture of cells have supported the notion that the receptor is cell cycle associated, the TC-Cbl is internalized by endocytosis via clathrin-coated pits, the TC-Cbl is degraded in the lysosome and the receptor is recycled to the plasma membrane in a manner similar to the transferrin receptor (Takahashi et al, 1980). These conclusions need confirmation and with the identification of the receptor protein and available tools, such as epitope specific antibodies and receptor tagged with fluorescent proteins, it is now possible to study in detail the process of TC-Cbl binding, internalization and fate of the receptor.
Genetic abnormalities of CD320 have not been reported. However, the recent identification of the gene provides the opportunity to investigate mutations of this essential receptor contributing to decreased cellular uptake of Cbl. Newborns identified with elevated MMA and HCY that cannot be attributed to known causes of defects in Cbl metabolism should be investigated for potential defects in CD320 contributing to decreased cellular uptake of Cbl.
Embryonic uptake of Cbl
The observation of higher holo-HC and TC levels in cord blood than in maternal blood suggests increased demand and turnover of Cbl in the fetus (Obeid et al, 2006). In this case, maternal Cbl status is an important determinant of transplacental transport of Cbl to meet the fetal Cbl requirement. In congenital TC deficiency, the fetus is not Cbl-deficient and develops normally to full term. In this case, maternal TC must provide the required Cbl. Our recent work on the receptor for TC-Cbl has indicated that the receptor gene knockout is not lethal to the embryo (unpublished data). Therefore, a surrogate TC receptor must function in the developing embryo to provide the necessary Cbl. IF and HC are present in amniotic fluid (Aimone-Gastin et al, 1999) and both CUB and MAG are expressed very early, during 8–16 cell stage of the embryo (Assemat et al, 2005), and therefore could serve as ligands and receptors for Cbl uptake. Gene knockout or antibodies to CUB and MAG are lethal and show multiple malformations. These abnormalities cannot be attributed to specific nutrients because of the diverse role of these receptors in internalizing numerous ligands (Kozyraki & Gofflot 2007). Increases in Cbl binders and decreases in total Cbl observed in mothers during pregnancy and at term, may be related to hormonal changes during pregnancy (Fernandes-Costa & Metz 1982). Follow up studies of women from preconception to delivery indicates progressive decrease in total and holo-TC and an increase in MMA in the cord blood at the time of delivery (Murphy et al, 2007). Increased Cbl requirement and perhaps up regulation of propionate metabolism may account for this. During early infancy, the Cbl requirement is met by breast milk, the only source of nutrition during this period, because a substantial portion of the Cbl intake of the mother is transferred to breast milk (Linnell, 1975).
The liver and kidney in cobalamin homeostasis
The primary storage sites for Cbl are the liver and kidney. The liver contains ~10 µg Cbl/g protein or 1 µg/g wet weight and can carry between 1–1.5 mg of the vitamin. The stored amount increases with age from newborn to adult (Rappazzo et al 1970). As the bulk of the liver mass is due to hepatocytes, HC-bound Cbl taken up via the asialoglycoprotein receptor could account for most of the stored Cbl (Alpers & Russel-Jones, 1999). Similarly, in the kidney, it would be due to renal reabsorption of TC-Cbl by megalin (Moestrup et al, 1996). The precise mechanism by which this storage occurs is not clear. As very little Cbl is distributed as free Cbl in the tissue, the assumption is that it is mostly bound to the two enzymes, methionine synthase (MS) and methylmalonylCoA Mutase (MMU). However, both enzymes are only partially saturated with Cbl even under Cbl replete conditions (Kolhouse et al, 1980) and no direct correlation between the activity of these enzymes and Cbl-replete status exists. The mechanism of Cbl release from its storage site under Cbl deficient conditions is also not known. Typically, the fall in serum Cbl precedes the fall in liver stores (Booth & Spray 1960). Based on the in vitro cell culture model (Quadros & Jacobsen, 1995) and tracer studies in animal models (Linnell 1975), it appears that the uptake, utilization and release of Cbl is a fairly dynamic process, with the Cbl and the various proteins associated with its utilization in constant flux. The accumulation of Cbl in some tissues like liver and kidney appears to be the exception that cannot be explained by the dynamic flux and raises a number of questions. For example, what factors regulate the accumulation of Cbl in liver and kidney? How does the Cbl-replete or Cbl-deficient status affect this process and the two enzymes? Does the stored Cbl compartmentalize to a location other than the two enzymes? Does the Cbl status affect synthesis, turnover and saturation of Cbl-dependent enzymes?
5. Metabolic utilization of Cbl and Inborn errors
Our current understanding of intracellular Cbl interconcersion indicates that once Cbl is released from TC, it undergoes processing to coenzyme forms and is directed to the two Cbl-dependent enzymes, MS and MMU. The intracellular distribution of various forms of Cbl has been well documented and in all tissues 5’ deoxyadenosyl Cbl (AdoCbl) is the predominant form with lesser amounts of hydroxo-Cbl. Methyl Cbl (MeCbl) is a minor component of intracellular Cbl. However, MeCbl is the major form of Cbl in the plasma and it is this form that is disproportionately reduced in Cbl deficiency. Higher MeCbl levels were observed in fetal tissues in association with higher MS enzyme activity (Linnell, 1975). A similar association was observed with cells cultured in medium containing methyl folate and HCY that resulted in a 3-fold increase in the levels of both holo- and apo-MS and a 9-fold increase in MeCbl, suggesting that the synthesis of MeCbl was tied to the activity and turnover of MS enzyme (Quadros & Jacobsen, 1995). The identification of methionine synthase reductase (MSR) enzyme (Leclerc et al, 1999) and the current understanding of the action of MSR in conjunction of MS enzyme activity (Wolthers & Scrutton 2009), provides compelling proof that MeCbl synthesis is linked to the catalytic activity of MS and the level of MeCbl in the plasma is a direct reflection of effective functioning of this pathway. The initial steps in the synthesis of Cbl coenzymes appear to be in the efficient removal of the upper axial ligand attached to the central cobalt, irrespective of the form of Cbl transported into the cell as shown by efficient and rapid interconversion of labeled cyano-Cbl, AdoCbl and MeCbl (Quadros et al, 1979). This function was ascribed to Cbl reductases (Watanabe & Nakano 1997) involved in the stepwise reduction of trivalent cobalt to the divalent and then to the monovalent state and in the process, removing the upper axial ligand for the chaperoning of the Cbl to cytosolic MS or mitochondrial MMU where it would be converted to the respective coenzyme form (Banerjee, 2006). A recent report has identified a gene product of MMACHC (CblC) as the protein specifically involved in decyanation as well as dealkylation of Cbl (Hannibal et al, 2009). Therefore, MMACHC protein probably has broad specificity for removing all upper axial ligands in the neosynthesis of intracellular AdoCbl and MeCbl. A system for efficient reduction of cobalt in Cbl and interconversion of all forms of Cbl probably involves multiple enzymes and cofactors. The ATP-dependent reductive alkylation of the cobalt in Cbl has been specifically assigned to the mitochondria, however, occurrence of this in the cytoplasm can not be ruled out.
The genetic defects of Cbl metabolism specifically involve the intracellular processing and utilization of Cbl and include lysosomal release of free Cbl and enzymes involved in the synthesis and utilization of Cbl cofactors (Table 1). Disorders involving the synthesis of Cbl cofactors are identified as cblA to cbl G based primarily on the order in which they were discovered. Both cblA and cblB disorders involve defects in mitochondrial synthesis of AdoCbl. The two disorders differ in that cell extracts from cblA mutants can convert cyano Cbl to AdoCbl whereas cblB mutants lack this ability (Whitehead, 2006). In vivo, a step common to these processes, such as transport of Cbl into mitochondria and / or initial steps in reductive alkylation, may be defective. The defect in cblA− has been ascribed to the MMAA gene (Dobson et al, 2002a) whose function is yet to be confirmed and that of cblB−, to the MMAB gene, which encodes the enzyme for transfer of the adenosyl moiety from ATP to form AdoCbl. (Dobson et al, 2002b). The cblC, cblD and cblF phenotypes affect the synthesis of both MeCbl and AdoCbl and present with homocysteineuria and methylmalonic aciduria (Morel et al, 2005). These disorders are not fully characterized, however, the defect in cblF phenotype appears to be in the lysosomal exit of Cbl due to a defect in LMBRD1 (Rutsch et al, 2009). Mutations in MMACHC result in the cblC phenotype (Lerner-Ellis et al, 2006). MMADHC carrying the cblD genotype results in elevated MMA and HCY. Mutations in exons 3 – 4 have been associated with MMA and with HCY in exons 6–8. In the case of combined MMA and HCY, additional mutation in exon 5 has been identified. Based on the phenotypes of the disorder, two functional domains have been suggested for this protein (Coehlo et al, 2008). The cblE and cblG defects are due to decreased activity of MS. The cblG disorder is due to mutations in the gene encoding MS (MTR) while the cblE disorder is due to mutations in MTRR, which encodes MSR associated with MS and is involved in the activation of MS and conversion of enzyme bound Cbl to MeCbl (Leclerc et al, 1999). One of the earliest genetic abnormalities of Cbl identified was in infants with MMA due to defects in the MMU enzyme. The gene defect can present as partial (MUT−) or complete loss (MUTo) of enzyme activity (Ledley & Rosenblatt, 1997).
Table 1.
Inborn Errors of Intracellular Cobalamin Metabolism
| Disorder |
*Gene involved |
Chromosomal location Size/Exons |
Protein involved |
Disease phenotype | Biochemical abnormality |
|---|---|---|---|---|---|
| cblA | MMAA | 4q31.21 39kb/6ex |
46.5 kDa 418 aa |
AdoCbl deficiency in cells/AdoCbl formation in cell free extracts |
Methylmalonic aciduria |
| cblB | MMAB | 12q24.11 19.8kb/9ex |
27.4 kDa 250 aa |
No AdoCbl formation in cells or in cell extracts |
Methylmalonic aciduria |
| cblC | MMACHC | 1p34.1 10.8kb/5ex |
31.7 kDa 282 aa |
Lack of AdoCbl and MeCbl synthesis |
Homocysteinuria Methylmalonic aciduria |
| cblD cblD1 |
MMADHC | 2q23.2 18kb/7ex |
32.8 kDa 296 aa |
Lack of AdoCbl and MeCbl synthesis 1No MeCbl, normal AdoCbl |
Homocysteinuria Methylmalonic aciduria |
| cblE | MTRR | 5q15.2 32kb/14ex |
77.7 kDa 698 aa |
Methionine synthase reductase deficiency |
Low MeCbl Low MS activity Elevated HCY |
| cblF | LMBRD1 | 6q13 121kb/31ex |
61.4 kDa 540 aa |
Defective lysosomal exit |
Lack of MeCbl and AdoCbl |
| cblG | MTR | 1q42.3 1400.3kb/31ex |
140 kDa 1265 aa |
Decreased methionine synthase |
Homocysteinuria Lack of MeCbl |
| MUT o MUT − |
MUT | 6p12.3 30kb/13ex |
83 kDa 750 aa |
oLack MUT protein −Partial MUT activity |
Methylmalonic aciduria |
MMAA: methylmalonic aciduria cblA type
MMAB: methylmalonic aciduria cblB type
MMACHC: methylmalonic aciduria cblC type with homocysteinuria
MMADHC: methylmalonic aciduria cblD type with homocysteinuria
MTRR: 5-methyltetrahydrofolate-homocysteine methyltransferase reductase/methionine synthase reductase.
LMBRD1: Initially labeled as limb regeneration domain 1(Identified as a lysosomal exporter)
MTR: 5-methyltetrahydrofolate-homocysteine methyltransferase/ methionine synthase
MUT: methylmalonyl Coenzyme A mutase
Targeting the cobalamin pathways in cancers
The successful use of inhibitors of dihydrofolate reductase and thymidylate synthatase proved the utility of inhibiting folate pathways in cancer therapy. Since the identification of the role of Cbl-dependent MS enzyme in the recycling of folate, the use of Cbl antimetabolites as inhibitors of MS has been suggested as a potential strategy for cancer therapy (Huennekens et al, 1976). Even though numerous compounds were produced and tested none proved effective inhibitors of MS- or Cbl-dependent pathways. Newer approaches have explored the use of fluorescent Cbl compounds to visualize tumours, Cbl-drug conjugates to deliver these compounds preferentially to tumor cells (Hogenkamp et al, 1999), and Cbl depletion as a strategy to inhibit the proliferation of cancer cells (McLean et al, 1997). Using epitope-specific monoclonal antibodies to TC and TCblR, it is possible to block Cbl uptake or deliver Cbl drug conjugates via this pathway (Quadros et al, 1996). The increased Cbl requirement in highly proliferative cancer cells and the over expression of TCblR to meet the higher demand for Cbl, renders the TC-TCblR pathway an ideal target for this approach. Elevated haptocorrin has been reported in hepatocellular carcinomas and myelomas. This protein provides a marker for these malignancies and could serve as a vehicle for targeted therapy of tumors that express high levels of HC and receptors for this protein (Waibel et al 2008)
Epilogue
Over a century of research into the understanding of the pathology and metabolism of Cbl has resulted in our current understanding of the physiology and biochemistry of this essential vitamin and the transport proteins, receptors and genes involved in this process. The metabolic utilization of Cbl in the cell and the proteins involved is being unravelled; aided primarily by our understanding of inborn errors in these pathways. As a more complete picture of Cbl homeostasis emerges, strategies for effective prevention and treatment of Cbl deficiency disorders will be implemented.
Acknowledgments
The current research in the author’s laboratory related to the work reported in this publication is supported by grant R01 DK064732 from the National Institutes of Health, USA
References
- Aimone-Gastin I, Gueant JL, Plenat F, Muhale F, Maury F, Djalali M, Gerard P, Duprez A. Assimilation of [57Co]-labeled cobalamin in human fetal gastrointestinal xenografts into nude mice. Pediatr Res. 1999;45:860–866. doi: 10.1203/00006450-199906000-00014. [DOI] [PubMed] [Google Scholar]
- Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr. 2009;89:693S–696S. doi: 10.3945/ajcn.2008.26947A. [DOI] [PubMed] [Google Scholar]
- Allen RH. Human vitamin B12 transport proteins. Prog Hematol. 1975;9:57–84. [PubMed] [Google Scholar]
- Alpers DH, Russell-Jones GJ. In: Intrinsic factor, haptocorrin and their receptors. Banerjee R, editor. Wiley-Interscience; 1999. pp. 411–440. [Google Scholar]
- Amagasaki T, Green R, Jacobsen DW. Expression of transcobalamin II receptors by human leukemia K562 and HL-60 cells. Blood. 1990;76:1380–1386. [PubMed] [Google Scholar]
- Ament AE, Li Z, Sturm AC, Perko JD, Lawson S, Masterson M, Quadros EV, Tanner SM. Juvenile cobalamin deficiency in individuals of African ancestry is caused by a founder mutation in the intrinsic factor gene GIF. Br J Haematol. 2009;144:622–624. doi: 10.1111/j.1365-2141.2008.07496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assemat E, Vinot S, Gofflot F, Linsel-Nitschke P, Illien F, Chatelet F, Verroust P, Louvet-Vallee S, Rinninger F, Kozyraki R. Expression and role of cubilin in the internalization of nutrients during the peri-implantation development of the rodent embryo. Biol Reprod. 2005;72:1079–1086. doi: 10.1095/biolreprod.104.036913. [DOI] [PubMed] [Google Scholar]
- Banerjee R. B12 trafficking in mammals: A for coenzyme escort service. ACS Chem Biol. 2006;1:149–159. doi: 10.1021/cb6001174. [DOI] [PubMed] [Google Scholar]
- Booth MA, Spray GH. Vitamin B12 activity in serum and liver of rats after total gastrectomy. Br. J. Haematol. 1960;6:288–295. [Google Scholar]
- Bose S, Seetharam S, Seetharam B. Membrane expression and interactions of human transcobalamin II receptor. J Biol Chem. 1995;270:8152–8157. doi: 10.1074/jbc.270.14.8152. [DOI] [PubMed] [Google Scholar]
- Brady J, Wilson L, McGregor L, Valente E, Orning L. Active B12: a rapid, automated assay for holotranscobalamin on the Abbott AxSYM analyzer. Clin Chem. 2008;54:567–573. doi: 10.1373/clinchem.2007.096784. [DOI] [PubMed] [Google Scholar]
- Burger RL, Mehlman CS, Allen RH. Human plasma R-type vitamin B12-binding proteins. I. Isolation and characterization of transcobalamin I. transcobalamin III. and the normal granulocyte vitamin B12-binding protein. J Biol Chem. 1975;250:7700–7706. [PubMed] [Google Scholar]
- Butler CC, Vidal-Alaball J, Cannings-John R, McCaddon A, Hood K, Papaioannou A, McDowell I, Goringe A. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Fam Pract. 2006;23:279–285. doi: 10.1093/fampra/cml008. [DOI] [PubMed] [Google Scholar]
- Carmel R. Cobalamin, the stomach, and aging. Am J Clin Nutr. 1997;66:750–759. doi: 10.1093/ajcn/66.4.750. [DOI] [PubMed] [Google Scholar]
- Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood. 2008;112:2214–2221. doi: 10.1182/blood-2008-03-040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanarin I. Megaloblastic Anemias. UK: Blackwell Scientific Publications; 1979. [Google Scholar]
- Chanarin I, Muir M, Hughes A, Hoffbrand AV. Evidence for intestinal origin of transcobalamin II during vitamin B12 absorption. Br Med J. 1978;1:1453–1455. doi: 10.1136/bmj.1.6125.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho D, Suormala T, Stucki M, Lerner-Ellis JP, Rosenblatt DS, Newbold RF, Baumgartner MR, Fowler B. Gene identification for the cblD defect of vitamin B12 metabolism. N Engl J Med. 2008;358:1454–1464. doi: 10.1056/NEJMoa072200. [DOI] [PubMed] [Google Scholar]
- DiGirolamo PM, Huennekens FM. Transport of vitamin B12 into mouse leukemia cells. Arch Biochem Biophys. 1975;168:386–393. doi: 10.1016/0003-9861(75)90267-2. [DOI] [PubMed] [Google Scholar]
- Dobson CM, Wai T, Leclerc D, Wilson A, Wu X, Dore C, Hudson T, Rosenblatt DS, Gravel RA. Identification of the gene responsible for the cblA complementation group of vitamin B12-responsive methylmalonic acidemia based on analysis of prokaryotic gene arrangements. Proc Natl Acad Sci U S A. 2002a;99:15554–15559. doi: 10.1073/pnas.242614799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CM, Wai T, Leclerc D, Kadir H, Narang M, Lerner-Ellis JP, Hudson TJ, Rosenblatt DS, Gravel RA. Identification of the gene responsible for the cblB complementation group of vitamin B12-dependent methylmalonic aciduria. Hum Mol Genet. 2002b;11:3361–3369. doi: 10.1093/hmg/11.26.3361. [DOI] [PubMed] [Google Scholar]
- Fedosov SN, Fedosova NU, Berglund L, Moestrup SK, Nexo E, Petersen TE. Composite organization of the cobalamin binding and cubilin recognition sites of intrinsic factor. Biochemistry. 2005a;44:3604–3614. doi: 10.1021/bi047936v. [DOI] [PubMed] [Google Scholar]
- Fedosov SN, Orning L, Lovli T, Quadros EV, Thompson K, Berglund L, Petersen TE. Mapping the functional domains of human transcobalamin using monoclonal antibodies. Febs J. 2005b;272:3887–3898. doi: 10.1111/j.1742-4658.2005.04805.x. [DOI] [PubMed] [Google Scholar]
- Fernandes-Costa F, Metz J. Levels of transcobalamins I, II, and III during pregnancy and in cord blood. Am J Clin Nutr. 1982;35:87–94. doi: 10.1093/ajcn/35.1.87. [DOI] [PubMed] [Google Scholar]
- Finnell RH, Shaw GM, Lammer EJ, Rosenquist TH. Gene-nutrient interactions: importance of folic acid and vitamin B12 during early embryogenesis. Food Nutr Bull. 2008;29:S86–S98. doi: 10.1177/15648265080292S112. discussion S99-100. [DOI] [PubMed] [Google Scholar]
- Fyfe JC, Madsen M, Hojrup P, Christensen EI, Tanner SM, de la Chapelle A, He Q, Moestrup SK. The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood. 2004;103:1573–1579. doi: 10.1182/blood-2003-08-2852. [DOI] [PubMed] [Google Scholar]
- Gordon MM, Brada N, Remacha A, Badell I, del Rio E, Baiget M, Santer R, Quadros EV, Rothenberg SP, Alpers DH. A genetic polymorphism in the coding region of the gastric intrinsic factor gene (GIF) is associated with congenital intrinsic factor deficiency. Hum Mutat. 2004;23:85–91. doi: 10.1002/humu.10297. [DOI] [PubMed] [Google Scholar]
- Green R. "Is it time for vitamin B-12 fortification? What are the questions?". Am J Clin Nutr. 2009;89(2):712S–716S. doi: 10.3945/ajcn.2008.26947E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueant JL, Kouvonen I, Michalski JC, Masson C, Grasbeck R, Nicolas JP. Purification of human intrinsic factor using high-performance ion-exchange chromatography as the final step. FEBS Lett. 1985;184:14–19. doi: 10.1016/0014-5793(85)80643-8. [DOI] [PubMed] [Google Scholar]
- Hannibal L, Kim J, Brasch NE, Wang S, Rosenblatt DS, Banerjee R, Jacobsen DW. Processing of alkylcobalamins in mammalian cells: A role for the MMACHC (cblC) gene product. Mol Genet Metab. 2009;97:260–266. doi: 10.1016/j.ymgme.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healton EB, Savage DG, Brust JC, Garrett TJ, Lindenbaum J. Neurologic aspects of cobalamin deficiency. Medicine (Baltimore) 1991;70:229–245. doi: 10.1097/00005792-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Herzlich B, Herbert V. Depletion of serum holotranscobalamin II. An early sign of negative vitamin B12 balance. Lab Invest. 1988;58:332–337. [PubMed] [Google Scholar]
- Hewitt JE, Gordon MM, Taggart RT, Mohandas TK, Alpers DH. Human gastric intrinsic factor: characterization of cDNA and genomic clones and localization to human chromosome 11. Genomics. 1991;10:432–440. doi: 10.1016/0888-7543(91)90329-d. [DOI] [PubMed] [Google Scholar]
- Hogenkamp HPC, Collins DA, Grissom CB, West FG. Diagnostic and therapeutic analogues of cobalamin. In: Banerjee R, editor. Chemistry and Biochemistry of B12. Wiley-Interscience; 1999. pp. 385–410. [Google Scholar]
- Huennekens FM, DiGirolamo PM, Fujii K, Jacobsen DW, Vitols KS. B12 -- dependent methionine synthetase as a potential target for cancer chemotherapy. Adv Enzyme Regul. 1976;14:187–205. doi: 10.1016/0065-2571(76)90013-3. [DOI] [PubMed] [Google Scholar]
- Johnston J, Bollekens J, Allen RH, Berliner N. Structure of the cDNA encoding transcobalamin I, a neutrophil granule protein. J Biol Chem. 1989;264:15754–15757. [PubMed] [Google Scholar]
- Kolhouse JF, Utley C, Allen RH. Isolation and characterization of methylmalonyl-CoA mutase from human placenta. J Biol Chem. 1980;255:2708–2712. [PubMed] [Google Scholar]
- Kozyraki R, Gofflot F. Multiligand endocytosis and congenital defects: roles of cubilin, megalin and amnionless. Curr Pharm Des. 2007;13:3038–3046. doi: 10.2174/138161207782110507. [DOI] [PubMed] [Google Scholar]
- Kozyraki R, Kristiansen M, Silahtaroglu A, Hansen C, Jacobsen C, Tommerup N, Verroust PJ, Moestrup SK. The human intrinsic factor-vitamin B12 receptor, cubilin: molecular characterization and chromosomal mapping of the gene to 10p within the autosomal recessive megaloblastic anemia (MGA1) region. Blood. 1998;91:3593–3600. [PubMed] [Google Scholar]
- Kristiansen M, Kozyraki R, Jacobsen C, Nexo E, Verroust PJ, Moestrup SK. Molecular dissection of the intrinsic factor-vitamin B12 receptor, cubilin, discloses regions important for membrane association and ligand binding. J Biol Chem. 1999;274:20540–20544. doi: 10.1074/jbc.274.29.20540. [DOI] [PubMed] [Google Scholar]
- Leclerc D, Odievre M, Wu Q, Wilson A, Huizenga JJ, Rozen R, Scherer SW, Gravel RA. Molecular cloning, expression and physical mapping of the human methionine synthase reductase gene. Gene. 1999;240:75–88. doi: 10.1016/s0378-1119(99)00431-x. [DOI] [PubMed] [Google Scholar]
- Ledley FD, Rosenblatt DS. Mutations in mut methylmalonic acidemia: clinical and enzymatic correlations. Hum Mutat. 1997;9:1–6. doi: 10.1002/(SICI)1098-1004(1997)9:1<1::AID-HUMU1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Lerner-Ellis JP, Tirone JC, Pawelek PD, Dore C, Atkinson JL, Watkins D, Morel CF, Fujiwara TM, Moras E, Hosack AR, Dunbar GV, Antonicka H, Forgetta V, Dobson CM, Leclerc D, Gravel RA, Shoubridge EA, Coulton JW, Lepage P, Rommens JM, Morgan K, Rosenblatt DS. Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nat Genet. 2006;38:93–100. doi: 10.1038/ng1683. [DOI] [PubMed] [Google Scholar]
- Li N, Seetharam S, Lindemans J, Alpers DH, Arwert F, Seetharam B. Isolation and sequence analysis of variant forms of human transcobalamin II. Biochim Biophys Acta. 1993;1172:21–30. doi: 10.1016/0167-4781(93)90264-e. [DOI] [PubMed] [Google Scholar]
- Li N, Rosenblatt DS, Kamen BA, Seetharam S, Seetharam B. Identification of two mutant alleles of transcobalamin II in an affected family. Hum Mol Genet. 1994;3:1835–1840. doi: 10.1093/hmg/3.10.1835. [DOI] [PubMed] [Google Scholar]
- Lindemans J, Kroes AC, van Geel J, van Kapel J, Schoester M, Abels J. Uptake of transcobalamin II-bound cobalamin by HL-60 cells: effects of differentiation induction. Exp Cell Res. 1989;184:449–460. doi: 10.1016/0014-4827(89)90343-1. [DOI] [PubMed] [Google Scholar]
- Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994;60:2–11. doi: 10.1093/ajcn/60.1.2. [DOI] [PubMed] [Google Scholar]
- Linnell JC. In: The fate of cobalamins in vivo. Babior BM, editor. Wiley -Interscience; 1975. pp. 287–333. [Google Scholar]
- Mathews FS, Gordon MM, Chen Z, Rajashankar KR, Ealick SE, Alpers DH, Sukumar N. Crystal structure of human intrinsic factor: cobalamin complex at 2.6-A resolution. Proc Natl Acad Sci U S A. 2007;104:17311–17316. doi: 10.1073/pnas.0703228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean GR, Quadros EV, Rothenberg SP, Morgan AC, Schrader JW, Ziltener HJ. Antibodies to transcobalamin II block in vitro proliferation of leukemic cells. Blood. 1997;89:235–242. [PubMed] [Google Scholar]
- Moestrup SK, Verroust PJ. "Megalin- and cubilin-mediated endocytosis of protein-bound vitamins, lipids, and hormones in polarized epithelia.". Annu Rev Nutr. 2001;21:407–428. doi: 10.1146/annurev.nutr.21.1.407. [DOI] [PubMed] [Google Scholar]
- Moestrup SK, Birn H, Fischer PB, Petersen CM, Verroust PJ, Sim RB, Christensen EI, Nexo E. Megalin-mediated endocytosis of transcobalamin-vitamin-B12 complexes suggests a role of the receptor in vitamin-B12 homeostasis. Proc Natl Acad Sci U S A. 1996;93:8612–8617. doi: 10.1073/pnas.93.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel CF, Watkins D, Scott P, Rinaldo P, Rosenblatt DS. Prenatal diagnosis for methylmalonic acidemia and inborn errors of vitamin B12 metabolism and transport. Mol Genet Metab. 2005;86:160–171. doi: 10.1016/j.ymgme.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Morkbak AL, Heimdal RM, Emmens K, Molloy A, Hvas AM, Schneede J, Clarke R, Scott JM, Ueland PM, Nexo E. Evaluation of the technical performance of novel holotranscobalamin (holoTC) assays in a multicenter European demonstration project. Clin Chem Lab Med. 2005;43:1058–1064. doi: 10.1515/CCLM.2005.185. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Molloy AM, Ueland PM, Fernandez-Ballart JD, Schneede J, Arija V, Scott JM. Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. J Nutr. 2007;137:1863–1867. doi: 10.1093/jn/137.8.1863. [DOI] [PubMed] [Google Scholar]
- Namour F, Helfer AC, Quadros EV, Alberto JM, Bibi HM, Orning L, Rosenblatt DS, Jean-Louis G. Transcobalamin deficiency due to activation of an intra exonic cryptic splice site. Br J Haematol. 2003;123:915–920. doi: 10.1046/j.1365-2141.2003.04685.x. [DOI] [PubMed] [Google Scholar]
- Nexo E, Hollenberg MD. Characterization of the particulate and soluble acceptor for transcobalamin II from human placenta and rabbit liver. Biochim Biophys Acta. 1980;628:190–200. doi: 10.1016/0304-4165(80)90366-9. [DOI] [PubMed] [Google Scholar]
- Obeid R, Morkbak AL, Munz W, Nexo E, Herrmann W. The cobalamin-binding proteins transcobalamin and haptocorrin in maternal and cord blood sera at birth. Clin Chem. 2006;52:263–269. doi: 10.1373/clinchem.2005.057810. [DOI] [PubMed] [Google Scholar]
- Pathare PM, Wilbur DS, Heusser S, Quadros EV, McLoughlin P, Morgan AC. Synthesis of cobalamin-biotin conjugates that vary in the position of cobalamin coupling. Evaluation of cobalamin derivative binding to transcobalamin II. Bioconjug Chem. 1996;7:217–232. doi: 10.1021/bc9600022. [DOI] [PubMed] [Google Scholar]
- Platica O, Janeczko R, Quadros EV, Regec A, Romain R, Rothenberg SP. The cDNA sequence and the deduced amino acid sequence of human transcobalamin II show homology with rat intrinsic factor and human transcobalamin I. J Biol Chem. 1991;266:7860–7863. [PubMed] [Google Scholar]
- Qian L, Quadros EV, Regec A, Zittoun J, Rothenberg SP. Congenital transcobalamin II deficiency due to errors in RNA editing. Blood Cells Mol Dis. 2002;28:134–142. doi: 10.1006/bcmd.2002.0499. discussion 143-135. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Jacobsen DW. The dynamics of cobalamin utilization in L-1210 mouse leukemia cells: a model of cellular cobalamin metabolism. Biochim Biophys Acta. 1995;1244:395–403. doi: 10.1016/0304-4165(95)00037-c. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Jackson B, Hoffbrand AV, Linnell JC. Interconversion of cobalamins in human lymphocytes in vitro and the influence of nitrous oxide on the synthesis of cobalamin coenzymes. In: Zagalak B, Friedrich F, editors. Vitamin B12, Proceedings of the Third European Symposium on Vitamin B12 and Intrinsic Factor. Walter de Gruyter & Co.; 1979. pp. 1045–1054. [Google Scholar]
- Quadros EV, Rothenberg SP, Pan YC, Stein S. Purification and molecular characterization of human transcobalamin II. J Biol Chem. 1986;261:15455–15460. [PubMed] [Google Scholar]
- Quadros EV, Rothenberg SP, Jaffe EA. Endothelial cells from human umbilical vein secrete functional transcobalamin II. Am J Physiol. 1989;256:C296–C303. doi: 10.1152/ajpcell.1989.256.2.C296. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Sai P, Rothenberg SP. Functional human transcobalamin II isoproteins are secreted by insect cells using the baculovirus expression system. Blood. 1993;81:1239–1245. [PubMed] [Google Scholar]
- Quadros EV, Sai P, Rothenberg SP. Characterization of the human placental membrane receptor for transcobalamin II-cobalamin. Arch Biochem Biophys. 1994;308:192–199. doi: 10.1006/abbi.1994.1027. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Rothenberg SP, McLoughlin P. Characterization of monoclonal antibodies to epitopes of human transcobalamin II. Biochem Biophys Res Commun. 1996;222:149–154. doi: 10.1006/bbrc.1996.0713. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Regec AL, Khan KM, Quadros E, Rothenberg SP. Transcobalamin II synthesized in the intestinal villi facilitates transfer of cobalamin to the portal blood. Am J Physiol. 1999;277:G161–G166. doi: 10.1152/ajpgi.1999.277.1.G161. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Nakayama Y, Sequeira JM. The binding properties of the human receptor for the cellular uptake of vitamin B12. Biochem Biophys Res Commun. 2005;327:1006–1010. doi: 10.1016/j.bbrc.2004.12.103. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Nakayama Y, Sequeira JM. The protein and the gene encoding the receptor for the cellular uptake of transcobalamin-bound cobalamin. Blood. 2009;113:186–192. doi: 10.1182/blood-2008-05-158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers VT, Blau N. Cerebral folate deficiency. Dev Med Child Neurol. 2004;46:843–851. doi: 10.1017/s0012162204001471. [DOI] [PubMed] [Google Scholar]
- Rappazzo ME, Salmi HA, Hall CA. The content of vitamin B12 in adult and foetal tissue: a comparative study. Br J Haematol. 1970;18:425–433. doi: 10.1111/j.1365-2141.1970.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Regec A, Quadros EV, Platica O, Rothenberg SP. The cloning and characterization of the human transcobalamin II gene. Blood. 1995;85:2711–2719. [PubMed] [Google Scholar]
- Remacha AF, Del Rio E, Sarda MP, Canals C, Simo M, Baiget M. Role of (Glu --> Arg, Q5R) mutation of the intrinsic factor in pernicious anemia and other causes of low vitamin B12. Ann Hematol. 2008;87:599–600. doi: 10.1007/s00277-008-0465-0. [DOI] [PubMed] [Google Scholar]
- Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- Rosenblatt DS, Fenton WA. In: Inborn errors of cobalamin metabolisn. Banerjee R, editor. Wiley-Interscience; 1999. pp. 367–384. [Google Scholar]
- Rothenberg SP, Quadros EV. Transcobalamin II and the membrane receptor for the transcobalamin II-cobalamin complex. Baillieres Clin Haematol. 1995;8:499–514. doi: 10.1016/s0950-3536(05)80218-5. [DOI] [PubMed] [Google Scholar]
- Rothenberg SP, Weiss JP, Cotter R. Formation of transcobalamin II--vitamin B12 complex by guinea-pig ileal mucosa in organ culture after in vivo incubation with intrinsic factor--vitamin B12. Br J Haematol. 1978;40:401–414. doi: 10.1111/j.1365-2141.1978.tb05812.x. [DOI] [PubMed] [Google Scholar]
- Rutsch F, Gailus S, Miousse IR, Suormala T, Sagne C, Toliat MR, Nurnberg G, Wittkampf T, Buers I, Sharifi A, Stucki M, Becker C, Baumgartner M, Robenek H, Marquardt T, Hohne W, Gasnier B, Rosenblatt DS, Fowler B, Nurnberg P. Identification of a putative lysosomal cobalamin exporter altered in the cblF defect of vitamin B12 metabolism. Nat Genet. 2009;41:234–239. doi: 10.1038/ng.294. [DOI] [PubMed] [Google Scholar]
- Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96:239–246. doi: 10.1016/0002-9343(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Selhub J, Rosenberg IH. "Public health significance of supplementation or fortification of grain products with folic acid.". Food Nutr Bull. 2008;29(2 Suppl):S173–S176. doi: 10.1177/15648265080292S120. [DOI] [PubMed] [Google Scholar]
- Seligman PA, Allen RH. Characterization of the receptor for transcobalamin II isolated from human placenta. J Biol Chem. 1978;253:1766–1772. [PubMed] [Google Scholar]
- Smith AD, Refsum H. Vitamin B-12 and cognition in the elderly. Am J Clin Nutr. 2009;89:707S–711S. doi: 10.3945/ajcn.2008.26947D. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tavassoli M, Jacobsen DW. Receptor binding and internalization of immobilized transcobalamin II by mouse leukaemia cells. Nature. 1980;288:713–715. doi: 10.1038/288713a0. [DOI] [PubMed] [Google Scholar]
- Thompson MD, Cole DE, Ray JG. Vitamin B-12 and neural tube defects: the Canadian experience. Am J Clin Nutr. 2009;89:697S–701S. doi: 10.3945/ajcn.2008.26947B. [DOI] [PubMed] [Google Scholar]
- Tanner SM, Li Z, Perko JD, Oner C, Cetin M, Altay C, Yurtsever Z, David KL, Faivre L, Ismail EA, Grasbeck R, de la Chapelle A. Hereditary juvenile cobalamin deficiency caused by mutations in the intrinsic factor gene. Proc Natl Acad Sci U S A. 2005;102:4130–4133. doi: 10.1073/pnas.0500517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanamala SK, Seetharam S, Yammani RR, Seetharam B. Human transcobalamin II receptor binds to Staphylococcus aureus protein A: implications as to its structure and function. Arch Biochem Biophys. 2003;411:204–214. doi: 10.1016/s0003-9861(03)00005-5. [DOI] [PubMed] [Google Scholar]
- Watanabe F, Nakano Y. Purification and characterization of aquacobalamin reductases from mammals. Methods Enzymol. 1997;281:295–305. doi: 10.1016/s0076-6879(97)81036-1. [DOI] [PubMed] [Google Scholar]
- Watkins D, Whitehead VM, Rosenblatt DS. Megaloblastic anemia. In: Orkin SH, Ginsburg D, Nathan DA, Look AT, Fisher DE, editors. Nathan and Oski’s Hematology of Infancy and Childhood. 7th Edition. Philadelphia: Saunders Elsevier; 2009. pp. 467–520. [Google Scholar]
- Waibel R, Treichler H, Schaefer NG, van Staveren DR, Mundwiler S, Kunze S, Kuenzi M, Alberto R, Nuesch J, Knuth A, Moch H, Schibli R, Schubiger PA. New derivatives of vitamin B12 show preferential targeting of tumors. Cancer Res. 2008;68:2904–2911. doi: 10.1158/0008-5472.CAN-07-6771. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe SN. Diagnosis of megaloblastic anaemias. Blood Rev. 2006;20:299–318. doi: 10.1016/j.blre.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Whitehead VM. Acquired and inherited disorders of cobalamin and folate in children. Br J Haematol. 2006;134:125–136. doi: 10.1111/j.1365-2141.2006.06133.x. [DOI] [PubMed] [Google Scholar]
- Wolthers KR, Scrutton NS. Cobalamin uptake and reactivation occurs through specific protein interactions in the methionine synthase-methionine synthase reductase complex. Febs J. 2009;276:1942–1951. doi: 10.1111/j.1742-4658.2009.06919.x. [DOI] [PubMed] [Google Scholar]
- Wuerges J, Garau G, Geremia S, Fedosov SN, Petersen TE, Randaccio L. Structural basis for mammalian vitamin B12 transport by transcobalamin. Proc Natl Acad Sci U S A. 2006;103:4386–4391. doi: 10.1073/pnas.0509099103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuerges J, Geremia S, Fedosov SN, Randaccio L. Vitamin B12 transport proteins: crystallographic analysis of beta-axial ligand substitutions in cobalamin bound to transcobalamin. IUBMB Life. 2007;59:722–729. doi: 10.1080/15216540701673413. [DOI] [PubMed] [Google Scholar]
- Yassin F, Rothenberg SP, Rao S, Gordon MM, Alpers DH, Quadros EV. Identification of a 4-base deletion in the gene in inherited intrinsic factor deficiency. Blood. 2004;103:1515–1517. doi: 10.1182/blood-2003-07-2239. [DOI] [PubMed] [Google Scholar]