Abstract
Pulmonary arterial hypertension (PAH) is a chronic and progressive disease characterized by a persistent elevation of pulmonary artery pressure accompanied by right ventricular hypertrophy (RVH). The current treatment for pulmonary hypertension is limited and only provides symptomatic relief due to unknown etiology and pathogenesis of the disease. Both vasoconstriction and structural remodeling (enhanced proliferation of VSMC) of the pulmonary arteries contribute to the progressive course of PAH, irrespective of different underlying causes. The exact molecular mechanism of PAH, however, is not fully understood. The purpose of this review is to provide recent advances in the mechanistic investigation of PAH. Specifically, this review focuses on nitric oxide (NO), oxidative stress and inflammation and how these factors contribute to the development and progression of PAH. This review also discusses recent and potential therapeutic advancements for the treatment of PAH.
Keywords: pulmonary arterial hypertension, pulmonary arterial remodeling, nitric oxide, cGMP, oxidative stress, NADPH oxidase, inflammation, cytokines, chemokines
1. Introduction
Pulmonary arterial hypertension (PAH) is a severe and life-threatening disease with still largely unknown pathogenesis. The development of PH is multi-factorial, with genetic background and environmental stress as two critical components. PAH is defined as a persistent elevation in pulmonary artery (PA) blood pressure (>25 mmHg at rest or >30 mmHg with exertion) (1). PAH is characterized by symptoms of dyspnea, chest pain, and syncope. PAH results in right ventricular hypertrophy (RVH), progressive right heart failure, low cardiac output, and ultimately death if left untreated (2, 3). A patient diagnosed with PAH must meet specific hemodynamic criteria including a mean pulmonary artery pressure >25mm Hg at rest, a pulmonary capillary wedge or left ventricular end diastolic pressure <15mm Hg and a pulmonary vascular resistance value of 3 Wood units or greater (4). Mean diagnosis of a patient with PAH is relatively high with reports ranging between 36-41 years of age (4). This is most likely due to the vague symptoms reported during physical examinations and the relatively slow progression of symptoms. Further complicating this issue are the relatively nonspecific symptoms that are associated with PAH such as angina, dyspnea, syncope and dizziness (5).
The current classification system of PAH was last revised by the World Health Organization in 2003 and it includes 5 categories of PAH (5). The first group includes idiopathic (sporadic), familial, associated PH and persistent PH of the newborn. Idiopathic PAH or IPAH is diagnosed when PAH develops spontaneously, for an unknown cause. Familial PAH (FPAH) is diagnosed when PAH occurs in 2 or more members of the same family. Patients with certain conditions or diseases are at an increased risk to develop PAH. In particular, patients with connective tissue diseases, portal hypertension or human immunodeficiency virus (HIV) are at risk to develop PAH. Group 2 includes patients who develop PAH as a result of left heart disease. Group 3 comprises patients who develop PAH as a result of a lung disease or hypoxemia. Patients with PAH that suffer from chronic thrombotic and/or embolic disease comprise Group 4. And the last classification group of PAH includes patients who develop PAH from miscellaneous conditions (some examples are sarcoidosis and lymphangiomyomatosis).
PAH by definition is a rare disease, with only 2-5 persons per million diagnosed per year (5). The incidence of PAH associated with other diseases or conditions however, increases the overall incidence of PAH. For example, in connective tissue disorders such as scleroderma, estimates vary but as many as 35% of patients have been reported to develop PAH (6). In systemic sclerosis, PAH has an estimated prevalence of 25% (7). HIV associated PAH is estimated between 0.1 and 0.5% with the Sub-Saharan Africa, South Asia and Southeast Asia representing more than 80% of these cases (4, 6). Rates for patients suffering from portal hypertension to develop PAH are between 1 and 6% (4). A comprehensive evaluation is necessary to accurately diagnosis PAH and tests include pulmonary function exams, echocardiography, connective tissue disease serology and cardiac catheterization (4). Currently the standard in diagnosing PAH is right heart catheterization (5) but is usually performed only after preliminary tests indicate PAH and exclude other possible conditions such as thromboembolic disease. Initial estimates of survival after diagnosis of PAH are as low as 2.8 years (7) but this number was estimated prior to recent disease-specific targeted therapeutics and is now likely to be significantly higher.
Our current understanding of the pathogenesis of PAH has increased significantly in recent years which has resulted in an increase in available treatments and an improved prognosis but unfortunately there is no cure for the disease. Current approved therapeutics target the endothelin-1, nitric oxide and prostacyclin pathways (8). Recently combination therapy has attracted much attention and while early results have been promising, the transition into practical clinical settings has been limited. Although there are many factors involved in the pathogenesis of PAH, this review will focus on three specific areas; nitric oxide (NO), oxidative stress and inflammation and how these factors contribute to the development and progression of PAH. This review will also discuss current treatment of PAH, the recent therapeutic advancements made, the effects they are having on treatment of PAH and the potential for development of novel therapeutics in these areas.
2. Current Strategies for the Treatment of PAH
First line treatment for PAH is general and supportive healthcare including oxygen supplementation, calcium channel blockers, diuretics and avoiding physical exertion (9). In addition to primary care, the introduction of specific treatments directed at the pathogenesis of PAH has resulted in an improved quality of life for patients. Therapeutics currently approved for the treatment of PAH include; endothelin receptor antagonists, phosphodiesterase type-5 inhibitors and prostacyclin analogues.
2.1. Endothelin Receptor Antagonists
Currently there are three endothelin-1 receptor antagonists approved by the Food and Drug Administration (FDA) for use in various WHO classified PAH patients; bosentan, ambrisentan and sitaxsentan. These drugs act to reduce the activation of endothelin-1 receptors located on pulmonary vascular smooth muscle cells (ETA receptors) and pulmonary vascular endothelial cells (ETA and ETB) (10). A decrease in activation of endothelin receptors ultimately results in the reduction of intracellular calcium release thereby reducing vasoconstrictive effects. In addition to potent vasoconstrictive effects, endothelin-1 also acts as a powerful mitogen, inducing cell proliferation in a number of cell types (10). Endothelin receptor antagonists therefore also reduce the pro-mitogenic effects of endothelins on pulmonary arterial smooth muscle cell proliferation.
2.2. Phosphodiesterase Inhibitors
Phosphodiesterase type-5 (PDE-5) is an enzyme that is primarily expressed in the arterial walls of the lungs and penis. PDE-5 acts to decompose the second messenger cGMP that induces vasodilation. The first PDE-5 inhibitor, sildenafil citrate, was originally approved for the treatment of erectile dysfunction but in 2005 the FDA approved it for the treatment of PAH. It is marketed under the name of Revatio. Most PDE inhibitors have anti-inflammatory properties (68)
2.3. Inhibitors of cell proliferation
An important strategy for the treatment of PAH is to inhibit pulmonary artery smooth muscle cell (SMC) proliferation. Several growth factors including platelet-derived growth factor (PDGF) has been shown to be involved in the pathogenesis of PAH (69,70,88). Inhibition of PDGF suppresses inflammation, decreases pulmonary arterial SMC proliferation, and reverses PAH (88). Prostacyclin, a potent pulmonary vasodilator, also acts to inhibit SMC proliferation and platelet aggregation (11). Currently, prostacyclin synthetics are a vital form of treatment for PAH. There are several synthetic prostacyclins available for treatment of PAH, Epoprostenol (Flolan), Treprostinil (Remodulin) and Iloprost (Ventavis) (9).
Notably, the three strategies above have strong implications for reducing oxidative stress levels and increasing NO bioavailability. Activation of the endothelin system has been demonstrated to contribute to oxidative stress and endothelin receptor antagonists were shown to decrease levels of oxidative stress (12,13). PDE-5 inhibitors have been shown to decrease oxidative stress levels by virtue of inhibiting superoxide production through decreasing levels of NADPH oxidase (14-16). Inhibition of PDEs increases intracellular cGMP levels, which is similar to activation of the NO signaling pathway. Prostacyclins and their analogues have also been demonstrated to reduce oxidative stress levels and improve cardiac functions in addition to providing beneficial effects in vascular endothelium (17-19). Recent studies indicate that inflammation may play an important role in the pathogenesis of hypertension. This review will discuss the role of nitric oxide, oxidative stress and inflammation and their relationship in the pathogenesis of PAH.
3. Oxidative Stress and PAH
Oxidative stress is characterized by an increase in oxidants (e.g. hydrogen peroxide and superoxide) with or without a decrease in antioxidants or antioxidant enzymes (20). Oxidants cause direct damage in tissue by the oxidation of certain biological molecules; known mechanisms include lipid peroxidation (breakdown of biological membranes) and DNA damage. Oxidant damage can also occur in the form of alteration of transcription factors such as nuclear factor-κB (NFκB) or hypoxia-inducible factor-1(HIF-1) (20). In cardiovascular tissue, oxidative stress has been implicated in the pathogenesis of conditions such as heart failure, ventricular hypertrophy and both systemic and pulmonary hypertension (21).
3.1. NADPH Oxidase
NADPH oxidase, or nicotinamide adenine dinucleotide phosphate, is a molecular complex known to generate reactive oxygen species such as superoxides within vascular endothelial and smooth muscle cells by transferring their terminal electron to available oxygen molecules. NADPH oxidases play vital roles in many biological processes including host defense, cell signaling, post-translational modification and hormone synthesis (22, 23). Other superoxide producing complexes in cardiovascular tissue include xanthine oxidase, the mitochondrial transport chain and the uncoupled nitric oxide synthase (NOS) (21). Known activators of NADPH oxidases include hormones, growth factors, cytokines and shear stress (21). Gp91phox (Nox2), was the first superoxide generating NADPH oxidase to be discovered and initially was only considered to function in phagocytes for the purpose of host defense (23). Upon further investigation, superoxide production by Nox2 was also found in non-phagocytic cells, as well as additional Nox family members with homology to Nox2. Currently five mammalian oxidases (Nox1-5) have been identified (23).
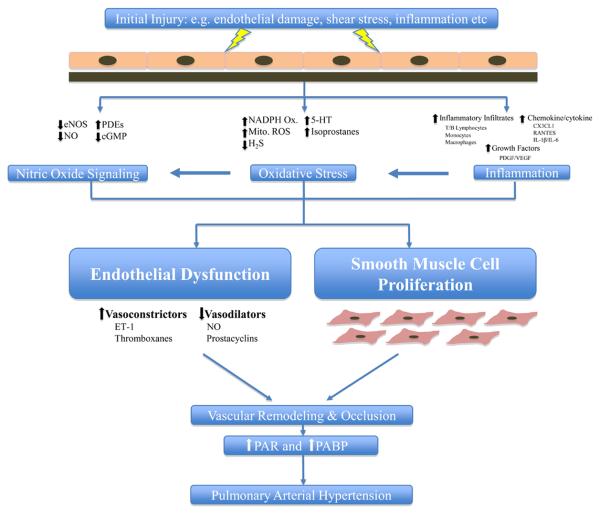
The production of superoxides has implication in the cardiovascular system because the generation of ROS has been demonstrated to be important regulators of vascular tone and function (23). Additional studies have shown that ROS generating systems stimulate both pulmonary artery smooth muscle cell (PASMC) and systemic arterial smooth muscle cell proliferation (23). Both vasoconstriction and structural remodeling (enhanced proliferation of vascular smooth muscle cells or VSMCs) of the pulmonary arteries contribute to the progressive course of PAH, irrespective of different underlying causes (24, 25). Administration of antioxidants, such as superoxide dismutase, has been demonstrated to significantly attenuate pulmonary vasoconstriction caused by hypoxia (26). In animal models where PAH is induced by means of chronic hypoxia, ROS production has been directly linked to vascular remodeling (27, 28). These data implicate a role for the generation of ROS species in vascular remodeling and the pathogenesis of PAH (Fig. 1).
Figure 1.
Nitric Oxide, Oxidative Stress and Inflammation in Pulmonary Hypertension. Despite advancements in PAH research in the last 20 years, the initiating factors in most patients remains unclear. Whatever the initiating insult, endothelial cell activation occurs leading to an increase in expression of cell adhesion molecules and production of chemokines and cytokines. Infiltration of inflammatory mediators (lymphocytes, monocytes and macrophages) occurs as well as production of growth factors. Disturbance of the endothelial cells also results in the alteration of nitric oxide signaling leading to a decrease in eNOS expression and NO production. Shear stress on vascular wall and inflammatory infiltrates can lead to the activation of oxidative stress mechanisms such as superoxide generating NADPH oxidases, mitochondrial reactive oxygen species (ROS) production, decreases in hydrogen sulfide (H2S) levels and increases in serotonin (5-HT) levels. These mechanisms act cumulatively causing a decrease in vasodilators (NO, prostacyclins) and a increase in vasoconstrictors (ET-1, thromboxanes). This imbalance of vasoactive substance leads to endothelial cell dysfunction and smooth muscle cell proliferation and hyperplasia eventually causing vascular remodeling and narrowing of the pulmonary arteries. Occlusion of the pulmonary arteries increases pulmonary vascular resistance (PVR) and pulmonary artery blood pressure (PABP) leading to pulmonary arterial hypertension.
3.2. Mitochondrial-derived ROS
Mitochondria are an additional major source for the production of ROS and recent data suggest that ROS produced by NADPH oxidase may contribute to the alteration in mitochondrial function (29). ROS generated by NADPH oxidase activity can interact with NO to form peroxynitrite that can damage respiratory complexes leading to mitochondrial dysfunction and release of additional ROS (29). This ROS can then activate more NADPH oxidases to release additional ROS creating a positive feedback system(29). An increase in ROS can lead to a decreased NO bioavailability that could attribute to vascular endothelial dysfunction and vascular remodeling. The data supporting mitochondrial dysfunction contributing to additional ROS production and endothelial dysfunction suggest that antioxidant therapeutics aimed specifically at mitochondria may be beneficial in the study of the pathogenesis of PAH.
3.3. HIF-1
Hypoxia-inducible factor-1 (HIF-1) is a transcription factor responsible for mediating physiological responses due to hypoxia and is found in a wide variety of organisms. The HIF-1α domain is thought to be an oxygen sensing domain and in the presence of oxygen allows for the eventual degradation of HIF-1 by the ubiquitin-proteosome pathway (30). The absence of oxygen results in a decrease in degradation, allowing HIF-1 to accumulate in the cell which activates transcription of genes. It is thought that HIF-1 in addition to being oxygen sensitive is oxidant and redox sensitive as well (31) and therefore it is thought that oxidants generated by oxygen sensors, such as NADPH oxidase, eventually lead to the activation of HIF-1. HIF-1 causes an increase in transcription of certain genes referred to as “early-response genes” which have been demonstrated to modulate cardiovascular function and vascular remodeling in the lung (30, 32, 33).
3.4. ROS Overproduction
Vasoconstriction, through means of nitric oxide (NO) reduction, has also been observed in systems that overproduce ROS. This reduction in available NO is achieved when enough ROS, such as superoxide, are produced to start reacting readily with NO to form the intermediate peroxynitrite (34). Peroxynitrite can react with protein tyrosines to form 3-nitrotyrosine that can cause lung epithelial damage. In support of this, it was discovered that an increase in iNOS expression, causing an increase in NO production, is associated with an increase in 3-nitrotyrosine concentrations in the lungs (35). The uncoupling of eNOS has also been demonstrated to cause a reduction of available NO through its interaction with the protein cav-1 (See 4.6). Wunderlich et al propose that in cav-1 knockout animals, the interrupted interaction between cav-1 and eNOS leads to the uncoupling of eNOS that results in the production of superoxides instead of NO (36).
3.5. Hydrogen Sulfide
The term gasotransmitter is a relatively new term that is used to describe gaseous molecules that are synthesized within an organism and act as a neuromodulator and signal transmitter (37). Hydrogen sulfide (H2S), NO and carbon monoxide are a few examples of gasotransmitters. Recent evidence suggests that H2S acts as an antioxidant and may play a role in the regulation of oxidative stress in chronic hypoxia induced hypertension (38). H2S in the cardiovascular system is thought to act through the scavenging of oxygen-free radicals and attenuation of damage from lipid peroxidation (39). In support of this hypothesis, Zhang et al demonstrated a reduction of endogenous H2S in chronic hypoxia exposed rats and that delivery of exogenous H2S successfully attenuated pulmonary hypertension compared to controls (40).
3.6. Serotonin
Through its ability to induce pulmonary vascular vasoconstriction and PASMC proliferation, serotonin (5-HT) has been recognized as a major contributing factor to the development of PAH (41). In PAH animal models, high levels of 5-HT were observed compared to controls but more importantly in human patients with PAH, higher 5-HT levels were observed as well (42). Also, 5-HT was shown to promote generation of ROS in PASM cells (41). In the right heart, 5-HT was demonstrated to promote protein carbonlylation (an oxidation process) which would support the idea of protein oxidation occurring in the right heart leading to oxidative stress. The exact mechanism of how this might be occurring unfortunately remains unclear at this time. Initially it was proposed that the generation of ROS by 5-HT was occurring through a decrease of the mediator MAO-A (a protein that degrades 5-HT and promotes production of the superoxide and hydrogen peroxide) in the right heart (41). The application of clorgyline, an MAO-A inhibitor, did not attenuate right ventricular protein carbonlylation however and suggests MAO-A may not be the mediator of 5-HT protein oxidation (41). Although the mechanism is still unclear, 5-HT remains as an attractive therapeutic target for the treatment of PAH.
3.7. Isoprostanes
Recently, a new group of compounds called isoprostanes have gained attention in lung vascular pathology. Isoprostanes, isomers of prostanoids, are formed when ROS products (particularly peroxynitrite) react with unsaturated bonds of membrane lipids such as arachodonic acid (43). In many vascular diseases, isoprostanes were found to significantly accumulate during oxidative stress and have since been used as indicators of severity of disease state. New evidence has been produced however exploring a causal role for isoprostanes in pulmonary vascular diseases instead of a simple disease marker. While the mechanisms remain unclear, isoprostanes have been shown to have powerful vasoconstrictor effects upon the pulmonary artery and can induce pulmonary endothelium to release the potent vasoconstrictor endothelin (44). Isoprostanes have also been suggested to cause lung inflammation through increases in production of pro-inflammatory cytokines in smooth muscle and endothelial cells that can lead to vascular remodeling (43). Importantly, isoprostane levels have been found to be elevated in patients with PAH as well as animals with hypoxia induced PAH (43). Due to their biological diversity, it is possible that isoprostanes could be contributing to many vascular diseases, including PAH. Currently, many questions remain unanswered such as the mix of isoprostanes produced during disease states and potential sub-types that could be contributing to disease that have not been tested pharmacologically.
4. Nitric Oxide Pathway and PAH
It is well accepted that nitric oxide (NO) is the primary pulmonary vasodilator which is both produced and released by the endothelium. The primary function of NO is the regulation of vascular tone, inhibition of VSMC proliferation and platelet aggregation (45). Also it has long been believed that one factor in the progression of PAH is an imbalance between the vasodilation and vasoconstriction of the pulmonary circulation (46). This is most likely due to a decrease in the amount of available NO and prostacyclin, accompanied by an increase in production of vasoconstrictors such as endothelin and thromboxanes (46). Indeed lower levels of NO and higher levels of thromboxanes (A2 specifically) have been reported in PAH patients compared to controls (6). The imbalance of vasoactive mediators (e.g. NO) can lead to endothelial cell dysfunction and when accompanied by VSMC proliferation, results in the chronic obstruction of small pulmonary arteries, resulting in PAH (47) (Fig.1).
4.1. NO Production
NO is produced by a family of enzymes called nitric oxide synthases (NOS). Currently three isoforms of NOS have been identified; endothelial (eNOS), inducible (iNOS) and neuronal (nNOS) (48). Studies have indicated that eNOS is the primary mediator of vasodilation in the pulmonary circulation (48). The NOS enzymes convert the amino acid L-arginine into two products; NO and L-citrulline (45). Upon its release by endothelium, NO diffuses into VSMCs where it acts to stimulate production of the second messenger cyclic guanosine monophosphate (cGMP) from guanylyl cyclase ultimately leading to dilation of blood vessels via dephosphorylation of myosin light chain. Along with its dilatory actions, NO also has the ability to inhibit VSMC proliferation (6).
4.2. eNOS Knockout
Interestingly, eNOS knockout (ko) animals have yielded surprising results. Studies have shown that eNOS ko animals result in only slight pulmonary pressure increases (45). But under pathophysiological conditions, such as hypoxia where vasoconstriction occurs, pulmonary pressure was significantly higher in eNOS ko animals compared to controls (45). These results provided key insight into PAH, demonstrating that NO is not only involved in maintaining low pulmonary vascular tone but plays an important role in pulmonary vascular responses to environmental stress as well (45).
4.3. Phosphodiesterases
Several other factors in the NO pathway could be contributing to the decreased NO effect. An up-regulation of phosphodiesterases (PDE), key enzymes that regulate the intracellular concentration of cGMP, has been suggested to play a role in patients with PAH (48). PDE enzymes play an important role in regulating intracellular signals by degrading the second messengers such as cGMP and cAMP. PDE 5 and 1 have especially been implicated due to their tissue distribution (48). PDE 5 is expressed throughout human tissues but is especially abundant in the lungs and pulmonary vasculature (48). Although PDE 1 is expressed in relatively low amounts in pulmonary tissues under normal conditions, a significant increase in expression is observed in proliferating pulmonary vasculature (48). Together, findings have implicated that the up-regulation of PDEs play a role in pulmonary vasculature remodeling and as a result several drugs have been developed aimed at inhibiting endogenous PDE activity. Sildenafil, tadalafil and vardenafil are three examples of such drugs and all have been approved for use in humans.
4.4. L-arginine
Dietary supplement of L-arginine has been shown to attenuate pulmonary arterial pressures in patients with PAH (45). This is most likely due to the fact that L-arginine is used by NOS to produce NO. Increased production of NO in these patients can be demonstrated by the increase in circulating levels of L-citrulline, the alternate product produced by the enzymatic reaction of NOS (45). Infusion of L-arginine or oral administration of the amino acid have resulted in a decrease in pulmonary vascular resistance, a decrease in mean pulmonary arterial pressure and an improvement in physical exercise capacity in PAH patients (45).
4.5. Inhaled NO
Before the development of specific therapeutics, inhaled NO was one of the few treatments available to patients with PAH. Inhaled NO was demonstrated to produce vasodilation in the pulmonary system without any adverse effects on the systemic circulation. This is attributed to the fact that inhaled NO is delivered primarily to the lungs causing an increase in perfusion in areas that can participate in gas exchange (46). Despite this benefit, there are still concerns with the administration of inhaled NO to PAH patients. Examples include the cost of treatment, short effects, methemoglobinemia concerns, alteration in the immune system and the potential for “rebound PH” (46). Currently, inhaled NO therapy is only approved for use in infants who have respiratory distress syndrome (46).
4.6. Caveolin-1
Caveolin-1 (cav-1) is the major resident protein that provides structural integrity to caveolae. Caveolae are flask-shaped invaginations of the surface plasma membrane enriched in lipids and cholesterol that are found in many cells including endothelial and smooth muscle cells (49). Caveolae facilitate the endocytosis of particles involved in vesicular trafficking. In addition to providing structural support to caveolae, cav-1 has been demonstrated to be involved in regulating intracellular signaling pathways. Cav-1 can associate with many signaling associated proteins that reside in the caveloae resulting in the modulation of enzymatic processes, usually in an inhibitory manner (49). Cav-1 in normal functioning animals also plays a role in inhibiting growth factor activation and subsequent downstream signaling pathways as well as the negative regulation of smooth muscle cell proliferation (49). eNOS was demonstrated to be one such protein that associates with caveolae (50). Cav-1 ko mice have exhibited significant cardiac defects including increased right ventricular volume and hypertrophy, systemic hypotension and cardiac fibrosis (49). Despite systemic hypotension, cav-1 ko animals develop significant pulmonary hypertension and RVH (51). Endothelial cell specific reconstitution of cav-1 in ko animals restored pulmonary and vascular defects evidenced by the reversal of pulmonary hypertension and RVH (51).
Cav-1 ko animals display an increase in expression of eNOS in cardiac tissue as well as an increase in NO production and release from vascular smooth muscle cells (49). Just as a decrease of NO can have significant effects on the cardiovascular system, too much NO can be harmful as well. When NO is present at high rates, it can readily react with available reactive oxygen species resulting in the formation of peroxynitrite. This highly reactive molecule can cause cellular damage but it also reacts with tyrosine residues to form nitrotyrisine that can impair lung epithelial cells and further promote the development of PAH (45). Along with these discoveries, a decrease in cav-1 expression has been observed in lungs of patients with PAH compared to controls. These results indicate that the loss of cav-1 in PAH patients results in smooth muscle cell proliferation and vascular damage induced by an overproduction of NO.
4.7. Recent NO Research
Most recent research involving NO and its use in treating PAH patients has been aimed at combination therapies. The goal of combination therapy is that when two drugs are combined, they produce a synergistic effect that achieves better results than when the drugs are administered alone. Some of the recent therapies being studied include the combination of inhaled NO, PDE inhibitors and prostacyclin analogues. Voswinckel et el showed that the combination of sildenafil (PDE-5 inhibitor) with inhaled treprostinil (prostacyclin analogue) resulted in a reduction of pulmonary vascular resistance and mean arterial blood pressure accompanied by an increase in cardiac output (52). Currently phase III trials are underway. Similar improvements in pulmonary hemodynamics have been reported when inhaled nitric oxide was combined with milirinone, a PDE-3 inhibitor (53). And finally there are several drugs currently undergoing clinical trials that are targeted at stimulating NO-independent soluable guanylyl cyclases. These drugs are aimed at a critical subunit in NO-mediated signal transduction pathway in the pulmonary but not systemic circulation that ultimately leads to an increase in cGMP release initiating vasodilation (54).
5. Inflammation and PAH
Within the last decade, an increasing amount of evidence has been produced supporting the role of inflammation in the progression of PAH. In patients who suffer from systemic inflammatory diseases such as systemic lupus erythematosus or scleroderma, PAH is a common associated symptom (47). In fact, histological examinations of lung tissue in systemic inflammation patients are similar to those of PAH patients (47). The most prominent observations are pulmonary arterial medial hypertrophy, intimal lesions and plexiform lesions (47). In 1994, Tuder et al were the first to identify inflammatory infiltrates within the plexiform lesions of PAH patients (55). Later, Cool et al reported the presence of mononuclear inflammatory cells surrounding vascular sites of plexiform growth but not in extra-vascular lung structures (56). In parallel with these data, other studies have reported an up-regulation of inflammatory mediators such as intracellular adhesion molecule-1 (ICAM-1) and endothelial leukocyte adhesion molecule-1 (ELAM-1) in patients with PAH (57). Autoimmunity has also been implicated in PAH patients. Isern et al showed detectable levels of circulating antinuclear antibodies as well as elevated levels of pro-inflammatory cytokines in PAH patients (58). These findings indicate that inflammation may play a role in the pathogenesis or progression of PAH (Fig. 1).
5.1. Chemokines
Currently, the role of chemokines has been implicated in almost every step of the inflammatory process. In particular, the chemokine fraktalkine (FKN/CX3CL1) and its receptor (CX3CR1) have been suggested to play critical roles in monocyte/T-cell recruitment to the vessel wall (59, 60). Recent studies by Dorfmuller et al (61) and Balabanian et al (62) have shown that CX3CR1 was up-regulated in circulating T-cells from PAH patients compared to controls, elevated soluble FKN plasma concentrations were found in PAH patients compared to controls, an increase in FKN mRNA expression was detected in PAH patients compared to controls and that pulmonary artery endothelial cells from PAH patients expressed FKN.
RANTES (Regulated upon Activation, Normal T-cell Expressed and Secreted) another chemoattractant for monocytes and T-cells, has been studied for its role in PAH by causing an induction of vasoconstrictors such as endothelin-1 and mitogenic activators such as endothelin-converting enzyme-1 (63). Studies have shown a detectable expression of RANTES mRNA in control patients as well as PAH patients but noticeably that the number of RANTES mRNA copies was significantly elevated in PAH patients (47). The authors also confirmed that the major source of RANTES in PAH patients was endothelial cells from lung tissue (47). Since elevated levels of RANTES were found in lung tissues of PAH patients, it would be logical to hypothesize an increase in endothelin-1, a potent vasoconstrictor, as well as endothelin-converting enzyme, a mitogenic activator that could be contributing to vascular proliferation and to the progression of PAH.
The hypothesis that chemokines potentially play a role in PAH pathogenesis was further supported by the work of Sanchez et al. Increased levels of monocyte chemotactic protein (MCP)-1, also known as CCL2 was demonstrated to be elevated in plasma and lung tissue of patients (64). Also, compared to controls, PA SMCs from patients demonstrated stronger migratory and proliferative responses to CCL2 (64).Taken together, these reports strongly support the role of chemokines in the pathogenesis of PAH.
5.2. Cytokines
Cytokines are proteins that are involved in inflammatory signaling. Both hematopoietic and non-hematopoietic cells can release cytokines. Cytokines can act in an autocrine (acts on itself), paracrine (acts on cells in the immediate vicinity) or endocrine (acts on cells throughout the body) manner. Cytokines regulate a variety of processes including immune cell proliferation and differentiation (both B and T-cells) and hematopoiesis. Therapeutically, anti-cytokine treatment presents a novel target in treatment of many inflammatory diseases and potentially PAH. Currently, cytokine antagonists have only been tested in the monocrotaline-induced PAH models in rats. Monocrotaline is a drug used to induce PAH and is thought to act by inducing inflammation of lung tissue, stimulating inflammatory cells such as neutrophils and the production of endothelin-1 and other vasoconstrictors (34). Studies have been conflicting, for example repeated injections of an antagonist for the pro-inflammatory cytokine IL-1 receptor reduced PAH and RVH in the monocrotaline-induced PAH accompanied by hypoxia rat model but not in the chronic hypoxia model alone (65). Nevertheless, considering the potential of anti-cytokine treatment and the numerous possible mechanisms and pathways involved in inflammation, further studies in anti-cytokine treatment is needed. Other examples of chemoattractants and cytokines that have been studied in PAH models include interleukin-1β, transforming growth factor-β1, bradykinin and leukotrienes (66).
5.3. Phosphodiesterases and Inflammation
It has been well studied that PDE inhibitors have anti-inflammatory properties by virtue of their ability to increase intracellular levels of cAMP or cGMP, depending on the PDE subtype (67). PDE subtype 4 has attracted the most attention in developing anti-inflammatory therapeutics, especially those targeted toward inflammatory pulmonary diseases such as asthma and COPD due to its identification as one of the major cAMP metabolizing enzymes in immune cells such as T and B lymphocytes, leukocytes and dendritic cells (68). The inhibition of PDE-4 has multiple biological consequences in immune cells such as the inhibition of cytokine release, inhibition of cell adhesion molecules (CAMs) and inhibition of TNF-α (68). Several drugs aimed at PDE-4 inhibition have been developed over the last two decades but all have been hampered by their dose limiting side effects including nausea and diarrhea. Several PDE-4 inhibitors have even been linked to complications such as heart failure and arrhythmias but in phase II and III clinical trials of two recent PDE-4 inhibitors, cilomilast and roflumilast, none of these events have materialized and offer promise for PDE-4 inhibitors (68).
5.4. Growth Factors and Inflammation
Growth factors such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) act as potent mitogens and chemoattractants for smooth muscle cells (SMCs), endothelial cells (ECs), fibroblasts and can cause resistance to apoptosis (69)As such, growth factors have strong implications in the proliferation and migration of pulmonary artery vascular cells.
PDGF can be synthesized in SMCs, ECs and macrophages and have been demonstrated to stimulate proliferation and migration of SMCs and fibroblasts (69). Increased levels of PDGF and its receptor, PDGFR were found to be elevated from lungs of IPAH patients supporting its role in the pathogenesis of PAH (70). Increased levels of VEGF were also found to be highly expressed in ECs of plexiform lesions in PAH patients (71). In the same study, decreased levels of C-Scr kinase, a VEGF mediator, were observed. Also, the EGF receptor inhibitor PKI166 reversed MCT-induced PH in rats (72). Combined, these studies provide evidence for the role of growth factors contributing to the pro-inflammatory condition observed in PAH.
Imatinib (Gleevac) was originally designed for anti-cancer therapeutics due to its tyrosine kinase inhibitor activity. However, imatinib is not strictly specific and was found to inhibit PDGFR as well. As such, clinical trails have been initiated examining the potential for use in PAH therapy. Sunitinib and sorafenib are two other drugs recognized for their ability to inhibit PDGF and VEGF signaling and are currently being evaluated for safety and tolerability for use in PAH patients (69).
5.5. Immunosuppression
With the implication of treating inflammation in PAH patients, the use of immunosuppressive therapy has been discussed but is generally considered only in PAH patients associated with connective tissue disorders (47). There has not been a large, placebo controlled study performed in humans to date so most data has been collected from case reports and observations in small groups (47). In the monocrotaline rat model however, two drugs have initially showed promise; rapamycin and triptolide. Rapamycin is a macrolide immunosuppressant currently used for chronic allograft rejection and triptolide is a traditional herb used in Chinese medicine to treat rheumatoid arthritis and other autoimmune diseases (47). According to the studies, both drugs showed significant results in lowering the mean pulmonary arterial blood pressure compared to control animals (73). In addition to lowering the arterial blood pressure, significant less RVH was observed (73). Immunosuppressant therapy provides a potential avenue for the development of novel therapeutics to treat PAH but considering the potential side effects and complications, extreme caution must be taken.
5.6. PBEF
A relatively new protein, pre-B-cell colony-enhancing factor (PBEF), has been implicated in a number of inflammatory processes due to its localization in lung epithelium, endothelium and leukocytes (74). Although most research involving PBEF and pulmonary inflammation has been studied in acute lung injuries, PBEF may have implications for PAH as well. Zhang and colleagues showed that PBEF plays a role in the generation of ROS in pulmonary artery and endothelial cells (75). Indeed, over-expression of PBEF increased ROS leading to oxidative stress that could be attenuated by the electron chain transport inhibitor rotenone (75). Also, PBEF has been shown to significantly increase pro-inflammatory cytokines such as IL-6 and IL-8 in the pulmonary vasculature (76, 77). Liu et al further demonstrated that knockdown of PBEF by siRNA attenuated the increase of cytokine production (76). PBEF was also shown to lead to NFκB activation which is a well known activator of inflammatory cascades (74). In a murine model, PBEF was also demonstrated to play a role in lung tissue damage by virtue of its neutrophil chemotactic property inducing leukocyte migration into the lungs and also by stimulating expression of other chemoattractants and macrophage inflammatory proteins (74, 76). Future studies, such as the over-expression or knockdown of PBEF combined with the available PAH animal models could be useful in investigation the potential role of PBEF in the development of PAH.
Summary
While oxidative stress and its role in the pathogenesis of PAH is not a new discipline, there are several areas that are producing new potential therapeutic targets. The discovery of new antioxidants (e.g. H2S) and their potential as oxygen scavengers that could mediate the NO bioavailability in PAH patients, presents new therapeutic targets. Also the discovery of novel compounds that appear to be oxidant/redox sensors (e.g. HIF-1) and how they play a role in contributing to oxidative stress in PAH have opened up new possibilities. Also, the research of cav-1 interactions with nitric oxide synthases is relatively new field that could produce new targets for treatment in PAH. In the case of both serotonin and isoprostanes, more research is needed to determine their basic mechanisms and how they may contribute to the development of PAH but the preliminary results are promising for potential therapeutic targets. Lastly, sildenafil, the PDE inhibitor already approved for human use, has provided benefits to PAH patients when combined with other therapies, such as inhaled NO.The potent pulmonary vasodilator NO and its effect on the pulmonary circulation are well documented but NO also has effects as an inhibitor of platelet activation and smooth muscle cell proliferation. Studies indicate that endothelial dysfunction is the result of an imbalance of vasodilators such as NO and vasoconstrictors such as endothelin-1 and can lead to the progression of PAH. NO effects the pulmonary circulation primarily through the second messenger cGMP and its downstream signaling partners. Recent experiments with the amino acid arginine have demonstrated to effect the level of NO in the pulmonary circulation but more investigation is necessary before any clinical studies are performed. Also, a drug that stimulates guanylate cyclase independent of NO has been reported that results in an increase in cGMP levels (78) but the lack of specificity has limited its use in the clinical setting. As more research is directed at caveolin and eNOS, we will learn more about the exact interactions taking place. This could in turn lead to the potential for therapeutics targeted specifically to this interaction, possibly limiting the cell proliferation and endothelial dysfunction that is occurring in PAH patients.
The similarities observed in lung morphology between patients with PAH and systemic inflammation disorders indicate the potential role for the activation of inflammatory cascades in PAH patients. Inflammatory infiltrates in the lungs could stimulate the observed structural remodeling in the vasculature. Preliminary studies involving pro-inflammatory cytokines have yielded promising results as means of preventing inflammation in PAH patients. Anti-chemokine studies also show promising targets for novel therapies as indicated by the fracktalkine and RANTES studies. Immunosuppressive studies have shown promise but protocols for therapy vary wildly and mixed results have been observed. Currently there are clinical trials underway determining the safety and use of anti-PDGF therapy for PAH patients. Many hurdles remain in developing effective PDE inhibitors aimed at treating pulmonary conditions. For example, most PDE inhibitors are designed for oral administration but the inhaled route would be preferred to directly target cells in the lung and minimize complications occurring systemically (68). There is also evidence that PDE-4 inhibitors exhibit some pro-inflammatory activities in the lung so this could limit their potential to developing effective therapeutics (79). Further complicating the matter is that PDE-4's main target, cAMP, has such a profound role in many downstream pathways that it could be difficult to achieve specific therapeutic results in PAH patients without adverse side effects. Finally, the relatively novel protein PBEF could also provide novel information for the role of inflammation in PAH. PBEF has been linked to an increase in production of superoxides and as an activator of pro-inflammatory cascades, both processes that have strong implications in the progression of PAH.
Nitric oxide signaling, oxidative stress and inflammation represent three heavily studied disciplines of PAH research. While the roles of each have strongly been attributed to the pathophysiology of PAH, information explaining how these areas intimately interact and contribute to PAH development is however lacking. For example, after disruption of the endothelial lining (e.g. shear stress) the infiltration of inflammatory mediators (cytokines, leukocytes etc.) can activate not only inflammatory cascades but can also contribute to the development of oxidative stress. Pro-inflammatory mediators (such as interleukin-6) can activate oxidative stress mechanisms such as NADPH oxidases leading to generation of ROS (80). Excess ROS can lead to mitochondrial dysfunction further promoting oxidative stress. As stated earlier, an increase in ROS levels can significantly decrease the bio-availability of NO and ultimately lead to a reduction in NO-induced vasodilation of the pulmonary vasculature, promoting development of PAH. This is just one example of how an alteration in one process can lead to a disruption in another process promoting PAH development. (Fig. 1). Therefore, there remains a need to develop strategies to target not just one individual component of PAH but multiple components simultaneously. Recently the role of resveratrol, a polyphenic compound known to exert anti-oxidant and anti-inflammatory effects in the systemic circulation, was evaluated in a PAH animal model (81-83). It was shown to decrease pro-inflammatory cytokine expression, leukocyte infiltration and prevent PASMC proliferation while also increasing eNOS expression (82).
Perspectives on the Treatment for PAH
Recent advancements in the pathogenesis of PAH have resulted in new therapeutic strategies for treatment but the poor survival rate for patients diagnosed with PAH demonstrates the need for alternative therapeutics that are safer and more effective than those available today. As we continue to research and understand the pathogenesis of PAH, the more we are realizing that PAH represents a complex multi-factorial disease that appears to be initiated and sustained by a number of biological pathways. The complexity of PAH indicates that a number of drugs may be necessary for effective treatment. Currently approved drugs for treatment of PAH include the phosphodiesterase inhibitors, prostacyclin analogues and endothelin-1 antagonists. Future drugs for successful treatment of PAH will probably include drugs that not only target the vasodilation aspects of the disease but also the structural remodeling that occurs. Recently, intravenous injection of circulating endothelial progenitor cells (EPCs) was demonstrated to attenuate monocrotaline-induced PAH in rats and dogs (84-86). More importantly, it was demonstrated that injection of EPCs was not only a potential treatment but also effectively decreased certain hemodynamic criteria in human PAH patients (84). Certainly long term, randomized, placebo controlled studies need to be completed to ascertain the feasibility of treating PAH patients with EPCs. Angiotensin-converting enzyme 2 (ACE2) has also been suggested as a novel therapeutic target demonstrated by its ability to attenuate increases in right ventricular systolic pressure in a PAH rat model (87). Drugs that would be successful in attenuating the smooth muscle cell proliferation are of particular interest. Drugs that target vasodilation in the pulmonary but not systemic circulation, such as the NO-independent soluable guanylyl cyclase stimulators, also provide promise in future therapeutics for PAH. These drugs could offer the pulmonary specific treatments that many drugs in the past have failed to provide.
Acknowledgements
This work was supported by NIH R01 077490 and AHA GIA 0655257B.
References
- 1.Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 3.LaRaia AV, Waxman AB. Pulmonary arterial hypertension: evaluation and management. South Med J. 2007;100:393–399. doi: 10.1097/SMJ.0b013e31802f2ff1. [DOI] [PubMed] [Google Scholar]
- 4.Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:1527–1538. doi: 10.1016/j.jacc.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Park MH. Advances in diagnosis and treatment in patients with pulmonary arterial hypertension. Catheter Cardiovasc Interv. 2008;71:205–213. doi: 10.1002/ccd.21389. [DOI] [PubMed] [Google Scholar]
- 6.Barst RJ. A review of pulmonary arterial hypertension: role of ambrisentan. Vasc Health Risk Manag. 2007;3:11–22. [PMC free article] [PubMed] [Google Scholar]
- 7.Highland KB. Pulmonary arterial hypertension. Am J Med Sci. 2008;335:40–45. doi: 10.1097/MAJ.0b013e31815d2647. [DOI] [PubMed] [Google Scholar]
- 8.Humbert M. Update in pulmonary hypertension 2008. Am J Respir Crit Care Med. 2009;179:650–656. doi: 10.1164/rccm.200901-0136UP. [DOI] [PubMed] [Google Scholar]
- 9.Hrometz SL, Shields KM. Role of ambrisentan in the management of pulmonary hypertension. Ann Pharmacother. 2008;42:1653–1659. doi: 10.1345/aph.1L014. [DOI] [PubMed] [Google Scholar]
- 10.Channick RN, Sitbon O, Barst RJ, Manes A, Rubin LJ. Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:62S–67S. doi: 10.1016/j.jacc.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 11.Yildiz P. Molecular mechanisms of pulmonary hypertension. Clin Chim Acta. 2009;403:9–16. doi: 10.1016/j.cca.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Dammanahalli KJ, Sun Z. Endothelins and NADPH oxidases in the cardiovascular system. Clin Exp Pharmacol Physiol. 2008;35:2–6. doi: 10.1111/j.1440-1681.2007.04830.x. [DOI] [PubMed] [Google Scholar]
- 13.Elgebaly MM, Portik-Dobos V, Sachidanandam K, Rychly D, Malcom D, Johnson MH, Ergul A. Differential effects of ET(A) and ET(B) receptor antagonism on oxidative stress in type 2 diabetes. Vascul Pharmacol. 2007;47:125–130. doi: 10.1016/j.vph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Shukla N, Rossoni G, Hotston M, Sparatore A, et al. Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. BJU Int. 2009;103:1522–1529. doi: 10.1111/j.1464-410X.2009.08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gur S, Sikka SC, Hellstrom WJ. Novel phosphodiesterase-5 (PDE5) inhibitors in the alleviation of erectile dysfunction due to diabetes and ageing-induced oxidative stress. Expert Opin Investig Drugs. 2008;17:855–864. doi: 10.1517/13543784.17.6.855. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch M, Kemp-Harper B, Weissmann N, Grimminger F, Schmidt HH. Sildenafil in hypoxic pulmonary hypertension potentiates a compensatory up-regulation of NO-cGMP signaling. Faseb J. 2008;22:30–40. doi: 10.1096/fj.06-7526com. [DOI] [PubMed] [Google Scholar]
- 17.Ohata S, Ishibashi Y, Shimada T, Takahashi N, et al. Effects of oral beraprost sodium, a prostaglandin I2 analogue, on endothelium dependent vasodilatation in the forearm of patients with coronary artery disease. Clin Exp Pharmacol Physiol. 2006;33:381–387. doi: 10.1111/j.1440-1681.2006.04379.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari R, Cargnoni A, Curello S, Boffa GM, Ceconi C. Effects of iloprost (ZK 36374) on glutathione status during ischaemia and reperfusion of rabbit isolated hearts. Br J Pharmacol. 1989;98:678–684. doi: 10.1111/j.1476-5381.1989.tb12643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinmura K, Tamaki K, Sato T, Ishida H, Bolli R. Prostacyclin attenuates oxidative damage of myocytes by opening mitochondrial ATP-sensitive K+ channels via the EP3 receptor. Am J Physiol Heart Circ Physiol. 2005;288:H2093–2101. doi: 10.1152/ajpheart.01003.2004. [DOI] [PubMed] [Google Scholar]
- 20.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 21.DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, et al. Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol. 2008;294:H2659–2668. doi: 10.1152/ajpheart.00953.2007. [DOI] [PubMed] [Google Scholar]
- 22.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. Febs J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 23.Sanders KA, Hoidal JR. The NOX on pulmonary hypertension. Circ Res. 2007;101:224–226. doi: 10.1161/CIRCRESAHA.107.158246. [DOI] [PubMed] [Google Scholar]
- 24.Humbert M, Morrell NW, Archer SL, Stenmark KR, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Simonneau G, Galie N, Rubin LJ, Langleben D, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 26.Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2003;285:L322–333. doi: 10.1152/ajplung.00337.2002. [DOI] [PubMed] [Google Scholar]
- 27.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 28.Hoshikawa Y, Ono S, Suzuki S, Tanita T, et al. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol. 2001;90:1299–1306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- 29.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 30.Gupte SA, Wolin MS. Oxidant and redox signaling in vascular oxygen sensing: implications for systemic and pulmonary hypertension. Antioxid Redox Signal. 2008;10:1137–1152. doi: 10.1089/ars.2007.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acker T, Fandrey J, Acker H. The good, the bad and the ugly in oxygen-sensing: ROS, cytochromes and prolyl-hydroxylases. Cardiovasc Res. 2006;71:195–207. doi: 10.1016/j.cardiores.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 33.Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest. 1995;95:1798–1807. doi: 10.1172/JCI117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, et al. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2008;177:219–226. doi: 10.1164/rccm.200702-264OC. [DOI] [PubMed] [Google Scholar]
- 35.Peng X, Abdulnour RE, Sammani S, Ma SF, et al. Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;172:470–479. doi: 10.1164/rccm.200411-1547OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wunderlich C, Schmeisser A, Heerwagen C, Ebner B, et al. Chronic NOS inhibition prevents adverse lung remodeling and pulmonary arterial hypertension in caveolin-1 knockout mice. Pulm Pharmacol Ther. 2008;21:507–515. doi: 10.1016/j.pupt.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Lowicka E, Beltowski J. Hydrogen sulfide (H2S) - the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 38.Wei HL, Zhang CY, Jin HF, Tang CS, Du JB. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol Sin. 2008;29:670–679. doi: 10.1111/j.1745-7254.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 39.Geng B, Chang L, Pan C, Qi Y, et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Du J, Bu D, Yan H, Tang X, Si Q, Tang C. The regulatory effect of endogenous hydrogen sulfide on hypoxic pulmonary hypertension. Beijing Da Xue Xue Bao. 2003;35:488–493. [PubMed] [Google Scholar]
- 41.Liu L, Marcocci L, Wong CM, Park AM, Suzuki YJ. Serotonin-mediated protein carbonylation in the right heart. Free Radic Biol Med. 2008;45:847–854. doi: 10.1016/j.freeradbiomed.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herve P, Launay JM, Scrobohaci ML, Brenot F, et al. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- 43.Janssen LJ. Isoprostanes and lung vascular pathology. Am J Respir Cell Mol Biol. 2008;39:383–389. doi: 10.1165/rcmb.2008-0109TR. [DOI] [PubMed] [Google Scholar]
- 44.Yi SL, Kantores C, Belcastro R, Cabacungan J, Tanswell AK, Jankov RP. 8-Isoprostane-induced endothelin-1 production by infant rat pulmonary artery smooth muscle cells is mediated by Rho-kinase. Free Radic Biol Med. 2006;41:942–949. doi: 10.1016/j.freeradbiomed.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 45.Dias-Junior CA, Cau SB, Tanus-Santos JE. Role of nitric oxide in the control of the pulmonary circulation: physiological, pathophysiological, and therapeutic implications. J Bras Pneumol. 2008;34:412–419. doi: 10.1590/s1806-37132008000600012. [DOI] [PubMed] [Google Scholar]
- 46.Granton J, Moric J. Pulmonary vasodilators--treating the right ventricle. Anesthesiol Clin. 2008;26:337–353. doi: 10.1016/j.anclin.2008.01.010. vii. [DOI] [PubMed] [Google Scholar]
- 47.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 48.Wilkins MR, Wharton J, Grimminger F, Ghofrani HA. Phosphodiesterase inhibitors for the treatment of pulmonary hypertension. Eur Respir J. 2008;32:198–209. doi: 10.1183/09031936.00124007. [DOI] [PubMed] [Google Scholar]
- 49.Ryter SW, Choi AM. Caveolin-1: a critical regulator of pulmonary vascular architecture and nitric oxide bioavailability in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294:L862–864. doi: 10.1152/ajplung.00074.2008. [DOI] [PubMed] [Google Scholar]
- 50.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 51.Maniatis NA, Shinin V, Schraufnagel DE, Okada S, Vogel SM, Malik AB, Minshall RD. Increased pulmonary vascular resistance and defective pulmonary artery filling in caveolin-1−/− mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L865–873. doi: 10.1152/ajplung.00079.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voswinckel R, Reichenberger F, Enke B, Kreckel A, et al. Acute effects of the combination of sildenafil and inhaled treprostinil on haemodynamics and gas exchange in pulmonary hypertension. Pulm Pharmacol Ther. 2008;21:824–832. doi: 10.1016/j.pupt.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Cai J, Su Z, Shi Z, Zhou Y, et al. Nitric oxide in conjunction with milrinone better stabilized pulmonary hemodynamics after fontan procedure. Artif Organs. 2008;32:864–869. doi: 10.1111/j.1525-1594.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- 54.Rhodes CJ, Davidson A, Gibbs JS, Wharton J, Wilkins MR. Therapeutic targets in pulmonary arterial hypertension. Pharmacol Ther. 2008 doi: 10.1016/j.pharmthera.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 56.Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol. 1997;28:434–442. doi: 10.1016/s0046-8177(97)90032-0. [DOI] [PubMed] [Google Scholar]
- 57.Okawa-Takatsuji M, Aotsuka S, Fujinami M, Uwatoko S, Kinoshita M, Sumiya M. Up-regulation of intercellular adhesion molecule-1 (ICAM-1), endothelial leucocyte adhesion molecule-1 (ELAM-1) and class II MHC molecules on pulmonary artery endothelial cells by antibodies against U1-ribonucleoprotein. Clin Exp Immunol. 1999;116:174–180. doi: 10.1046/j.1365-2249.1999.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isern RA, Yaneva M, Weiner E, Parke A, et al. Autoantibodies in patients with primary pulmonary hypertension: association with anti-Ku. Am J Med. 1992;93:307–312. doi: 10.1016/0002-9343(92)90238-7. [DOI] [PubMed] [Google Scholar]
- 59.Moatti D, Faure S, Fumeron F, Amara Mel W, et al. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- 60.McDermott DH, Halcox JP, Schenke WH, Waclawiw MA, et al. Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ Res. 2001;89:401–407. doi: 10.1161/hh1701.095642. [DOI] [PubMed] [Google Scholar]
- 61.Dorfmuller P, Zarka V, Durand-Gasselin I, Monti G, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:534–539. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- 62.Balabanian K, Foussat A, Dorfmuller P, Durand-Gasselin I, et al. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:1419–1425. doi: 10.1164/rccm.2106007. [DOI] [PubMed] [Google Scholar]
- 63.Molet S, Furukawa K, Maghazechi A, Hamid Q, Giaid A. Chemokine- and cytokine-induced expression of endothelin 1 and endothelin-converting enzyme 1 in endothelial cells. J Allergy Clin Immunol. 2000;105:333–338. doi: 10.1016/s0091-6749(00)90084-8. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez O, Marcos E, Perros F, Fadel E, et al. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2007;176:1041–1047. doi: 10.1164/rccm.200610-1559OC. [DOI] [PubMed] [Google Scholar]
- 65.Voelkel NF, Tuder RM, Bridges J, Arend WP. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am J Respir Cell Mol Biol. 1994;11:664–675. doi: 10.1165/ajrcmb.11.6.7946395. [DOI] [PubMed] [Google Scholar]
- 66.Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008;44:14–30. doi: 10.1016/j.yjmcc.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peter D, Jin SL, Conti M, Hatzelmann A, Zitt C. Differential expression and function of phosphodiesterase 4 (PDE4) subtypes in human primary CD4+ T cells: predominant role of PDE4D. J Immunol. 2007;178:4820–4831. doi: 10.4049/jimmunol.178.8.4820. [DOI] [PubMed] [Google Scholar]
- 68.Spina D. PDE4 inhibitors: current status. Br J Pharmacol. 2008;155:308–315. doi: 10.1038/bjp.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Perros F, Montani D, Dorfmuller P, Durand-Gasselin I, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:81–88. doi: 10.1164/rccm.200707-1037OC. [DOI] [PubMed] [Google Scholar]
- 71.Tuder RM, Chacon M, Alger L, Wang J, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 72.Merklinger SL, Jones PL, Martinez EC, Rabinovitch M. Epidermal growth factor receptor blockade mediates smooth muscle cell apoptosis and improves survival in rats with pulmonary hypertension. Circulation. 2005;112:423–431. doi: 10.1161/CIRCULATIONAHA.105.540542. [DOI] [PubMed] [Google Scholar]
- 73.Faul JL, Nishimura T, Berry GJ, Benson GV, Pearl RG, Kao PN. Triptolide attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med. 2000;162:2252–2258. doi: 10.1164/ajrccm.162.6.2002018. [DOI] [PubMed] [Google Scholar]
- 74.Hong SB, Huang Y, Moreno-Vinasco L, Sammani S, et al. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med. 2008;178:605–617. doi: 10.1164/rccm.200712-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang LQ, Adyshev DM, Singleton P, Li H, et al. Interactions between PBEF and oxidative stress proteins--a potential new mechanism underlying PBEF in the pathogenesis of acute lung injury. FEBS Letters. 2008;582:1802–1808. doi: 10.1016/j.febslet.2008.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu P, Li H, Cepeda J, Zhang LQ, Cui X, Garcia JG, Ye SQ. Critical role of PBEF expression in pulmonary cell inflammation and permeability. Cell Biol Int. 2008 doi: 10.1016/j.cellbi.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sommer G, Garten A, Petzold S, Beck-Sickinger AG, Bluher M, Stumvoll M, Fasshauer M. Visfatin/PBEF/Nampt: structure, regulation and potential function of a novel adipokine. Clin Sci (Lond) 2008;115:13–23. doi: 10.1042/CS20070226. [DOI] [PubMed] [Google Scholar]
- 78.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCluskie K, Klein U, Linnevers C, Ji YH, Yang A, Husfeld C, Thomas GR. Phosphodiesterase type 4 inhibitors cause proinflammatory effects in vivo. J Pharmacol Exp Ther. 2006;319:468–476. doi: 10.1124/jpet.106.105080. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H, Jiang Z, Chang J, Li X, et al. Role of NAD(P)H oxidase in transforming growth factor-beta1-induced monocyte chemoattractant protein-1 and interleukin-6 expression in rat renal tubular epithelial cells. Nephrology (Carlton) 2009;14:302–310. doi: 10.1111/j.1440-1797.2008.01072.x. [DOI] [PubMed] [Google Scholar]
- 81.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, et al. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2417–2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 82.Csiszar A, Labinskyy N, Olson S, Pinto JT, et al. Resveratrol Prevents Monocrotaline-Induced Pulmonary Hypertension in Rats. Hypertension. 2009 doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H1694–1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 84.Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH, Chen JZ. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant. 2008;12:650–655. doi: 10.1111/j.1399-3046.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 85.Wang XX, Zhang FR, Shang YP, Zhu JH, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 86.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 87.Yamazato Y, Ferreira AJ, Hong KH, Sriramula S, et al. Prevention of pulmonary hypertension by Angiotensin-converting enzyme 2 gene transfer. Hypertension. 2009;54:365–371. doi: 10.1161/HYPERTENSIONAHA.108.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]