Abstract
Overexposure to manganese is known to cause damage to basal ganglial neurons and the development of movement abnormalities. Activation of microglia and astrocytes has increasingly been associated with the pathogenesis of a variety of neurological disorders. We have recently shown that microglial activation facilitates manganese chloride (MnCl2, 10–300 μM)-induced preferential degeneration of dopamine neurons. In this study, we report that combinations of MnCl2 (1–30 μM) and endotoxin lipopolysaccharide (LPS, 0.5–2 ng/ml), at minimally effective concentrations when used alone, induced synergistic and preferential damage to dopamine neurons in rat primary neuron-glia cultures. Mechanistically, MnCl2 significantly potentiated LPS-induced release of tumor necrosis factor-alpha and interleukin-1 beta in microglia but not in astroglia. MnCl2 and LPS were more effective in inducing the formation of reactive oxygen species and nitric oxide in microglia than in astroglia. Furthermore, MnCl2 and LPS-induced free radical generation, cytokine release and dopamine neurotoxicity was significantly attenuated by pretreatment with potential anti-inflammatory agents minocycline and naloxone. These results demonstrate that the combination of manganese overexposure and neuroinflammation is preferentially deleterious to dopamine neurons. Moreover, these findings not only shed light on the understanding of manganese neurotoxicity but may also bear relevance to the potentially multifactorial etiology of Parkinson’s disease.
Keywords: neuroinflammation, neurotoxicity, dopamine, microglia, astroglia, free radical
Introduction
Excessive exposure to manganese (Mn), an essential trace metal important in mediating a variety of biological processes, in miners and smelters can result in significant neuronal damage to the basal ganglia and the development of movement disorders called manganism (Aschner et al. 2007, Cersosimo & Koller 2006). Clinically, classic cases of manganism overlap with that of idiopathic Parkinson’s disease (IPD) in the presentation of rigidity and bradykinesia but differ in the intensity of tremor and response to levodopa therapy (Calne et al. 1994, Cersosimo & Koller 2006, Pal et al. 1999). Pathologically, neuronal damage in manganism is most prominent in globus pallidus and less severe in striatum and other structures of the basal ganglia (Aschner et al. 2007, Perl & Olanow 2007).
A number of studies have associated occupational overexposure to Mn with increased incidence and/or decreased onset age of developing Parkinsonism (Gorell et al. 1997, Gorell et al. 1999, Mergler et al. 1994, Racette et al. 2001, Racette et al. 2005) although increased PD incidence has not been consistently associated with welding (Fored et al. 2006, Marsh & Gula 2006). Furthermore, several studies strongly suggest that environmental exposure to elevated levels of Mn through airborne, dietary and other routes contributes to IPD development (Finkelstein & Jerrett 2007, Lucchini et al. 2007, Powers et al. 2003, Tan et al. 2003).
IPD represents the vast majority of PD cases and cardinal symptoms (i.e., rigidity, bradykinesia, rest tremor and posture instability) do not start to appear before the 5–6th decade of life and a massive degeneration of the nigrostriatal dopaminergic (DA) pathway. Development of IPD most likely is a result of complex interactions among multiple factors that include the innate vulnerability of the nigrostriatal DA pathway to oxidative damage, exposure to environmental toxicants and potential genetic predisposition superimposed over decades of the aging process (Di Monte 2003, Klein & Schlossmacher 2007, Logroscino 2005, Manning-Bog & Langston 2007, Sulzer 2007). Neuroinflammation has increasingly been recognized to be a key contributor to DA neurodegeneration based on observations that include the presence of inflammatory markers in the SN of postmortem PD brains, reduced PD incidence in users of certain nonsteroidal anti-inflammatory drugs, neuroprotection achieved with anti-inflammatory agents in animal PD models and recapitulation of pathological PD features in animals with immuno-stimulators such as endotoxin lipopolysaccharide (LPS) (Chen et al. 2005, Liu 2006, McGeer & McGeer 2008, Przedborski 2007, Dutta et al. 2008). Activation of microglia and astroglia, main players of neuroinflammation, can lead to overproduction and accumulation of various proinflammatory and neurotoxic factors that include cytokines tumor necrosis factor-alpha (TNFα) and interleukin-1 beta (IL-1β), reactive oxygen species (ROS), reactive nitrogen species (RNS) such as nitric oxide (NO), and lipid mediators that impact DA neurons to induce and/or exacerbate DA neurodegeneration (Liu 2006, Liu et al. 2003).
Activation of microglia significantly facilitates the Mn-induced preferential degeneration of DA neurons (Zhang et al. 2009) that are vulnerable to Mn neurotoxicity (Stanwood et al. 2009). Mn-stimulated ROS and RNS generation primarily in microglia and astroglia respectively are key mediators of the DA neurotoxicity (Zhang et al. 2007, Zhang et al. 2009). Several groups have also reported that Mn potentiates LPS and/or cytokine-induced glial iNOS upregulation and microglial release of TNFα and IL-6 (Bae et al. 2006, Barhoumi et al. 2004, Chang & Liu 1999, Filipov et al. 2005, Moreno et al. 2008, Spranger et al. 1998). However, the impact of the Mn and inflammation-enhanced glial activation on neurons and the relative contribution of microglia and astroglia to neurotoxicity remain undefined.
In this study, using various primary glial and neuronal cultures, we determined the combined neurotoxicity of Mn and LPS. Combinations of Mn and LPS were used at concentrations that, when used alone, were ineffective or minimally effective in causing neuronal damage (Gao et al. 2002b, Zhang et al. 2009). Mechanistically, the capacity of microglia and astroglia to produce ROS, RNS, and proinflammatory cytokines in response to treatment with Mn and LPS was determined. The contribution of Mn and LPS-induced glial activation to neurodegeneration was determined using minocycline and naloxone, agents that are known to modulate glial activation (Kim & Suh 2009, Liu & Hong 2003c).
Materials and methods
Primary microglia and astroglia cultures
Primary microglia and astroglia were prepared from mixed glia cultures which were prepared from brains of 1-day-old Fisher F344 rats as described (Liu & Hong 2003b, Liu et al. 2001, Zhang et al. 2007). Microglia and astroglia were seeded at 5 × 104 cells/well in 96-well in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and grown overnight (microglia) or to near confluence (astroglia) before treatment.
Primary neuron-glia, neuron-enriched, neuron-microglia and neuron-astroglia cultures
Primary neuronal and neuron-glia cultures were prepared as described (Gao et al. 2002a, Liu et al. 2000, Qin et al. 2005, Zhang et al. 2009). Briefly, mixed neuron-glia cultures were prepared by seeding, in poly-D-lysine coated 24-well plates, cells (5 × 105/well) isolated from gestation day 14 Fisher F344 rat mesencephalon and maintaining cultures at 37°C and 5% CO2 in MEM containing 10% FBS and 10% horse serum (HS). Seven-day old cultures were used for treatment. Neuron-enriched cultures were prepared by adding cytosine β-D-arabinocide (10 μM) 2 days after seeding the neuron-glia cultures to suppress glial proliferation. Cultures were used for treatment 5 days later. Neuron-microglia cultures were prepared by adding microglia (105 cells/well) to the neuron-enriched cultures 1 day before treatment. Neuron-astroglia cultures were prepared by adding L-leucine methyl ester (0.5 mM) to neuron-glia cultures to eliminate microglia 3 days before treatment.
Treatment
Stock solutions of LPS (E. coli 0111:B4) and MnCl2 were prepared in de-ionized and distilled water (Gao et al. 2002b, Zhang et al. 2009). For treatment, equal volumes of treatment media (MEM-2% FBS and 2% HS) containing vehicle (0.2% water), MnCl2 or LPS were added to the cultures (0.1 and 1 ml/well final volume for 96 and 24-well plates respectively). For the conditioned media experiment, microglia cultures (1.2 × 105/well in 48-well plates) were treated for 24 hr with vehicle control or 30 μM MnCl2 and 2 ng/ml LPS in DMEM-2% FBS (300 μl/well). Supernatants (250 μl/well) were removed and added (250 μl/well) to neuron-enriched cultures containing 750 μl/well MEM-2% FBS and 2% HS.
Immunocytochemistry (ICC)
ICC analysis was performed as described (Gao et al. 2002b, Liu et al. 2000, Zhang et al. 2009). DA neurons, neurons in general, microglia and astroglia were recognized with monoclonal antibodies against tyrosine hydroxylase (TH), neuron-specific nuclear protein (Neu-N), complement type-3 receptor antibody (OX-42) and glial fibrillary acidic protein (GFAP) respectively. ICC images were recorded with a Micropublisher microscope CCD camera using the QCapture Suite software.
Assessment of neurotoxicity
Neurotoxicity was assessed using a combination of structural and functional parameters as described (Gao et al. 2002a, Liu et al. 2000, Zhang et al. 2009). For DA neurons, the number of TH-immunoreactive (TH-ir) neurons was counted and neurite length of individual TH-ir neurons was measured. DA uptake was determined using [3H]DA and DA uptake inhibitor mazindol. Degeneration of neurons in general was determined by counting the number of Neu-N-ir neurons (Gao et al. 2002b, Zhang et al. 2009). Uptake of gamma-aminobutyric acid (GABA) was determined using [3H]GABA and GABA uptake blocker, NO-711.
Measurement of NO, ROS, TNFα and IL-1β
The amounts of nitrite and cytokines (TNFα and IL-1β) in the supernatants were determined using the Griess reagent and enzyme-linked immunosorbent assays respectively (Liu et al. 2000, Zhang et al. 2009). ROS production was determined using 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) as described (Zhang et al. 2009).
Statistical Analysis
Data were analyzed for statistical significance with ANOVA followed by Bonferroni/Dunn post hoc analysis using the StatView program (SAS Institute, Cary, NC). A p value of < 0.05 was considered statistically significant.
Results
Mn and LPS induce synergistic and preferential degeneration of DA neurons
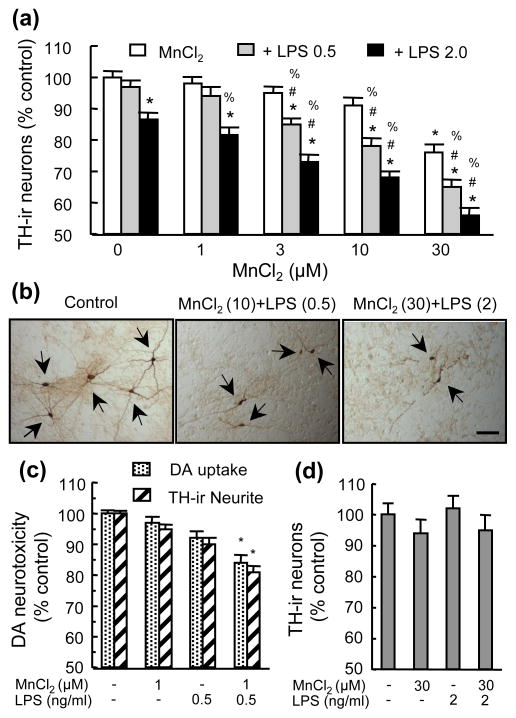
When neuron-glia cultures were treated for 7 days with combinations of MnCl2 and LPS at low concentrations (Gao et al. 2002b, Zhang et al. 2009), the combinations were more effective than individual agents in damaging DA neurons (Fig. 1). While 1–10 μM MnCl2 or 0.5 ng/ml LPS were ineffective when used alone, combinations of 3–10 μM MnCl2 with 0.5 ng/ml LPS caused a significant loss of TH-ir neuron (Fig. 1a). Significant potentiation of DA neurotoxicity was also observed for combinations of 3–30 μM MnCl2 with 2 ng/ml LPS and 30 μM MnCl2 with 0.5 ng/ml LPS (Figs. 1a & 1b). The combination of 1 μM MnCl2 with 0.5 ng/ml LPS, while incapable of causing significant loss of TH-ir neurons (Fig. 1a), caused significant degeneration in TH-ir neurites and reduction in [3H]DA uptake capacity (Fig. 1c). In contrast, in neuron-enriched cultures, treatment with the combination of 30 μM MnCl2 with 2 ng/ml LPS did not result in any cooperative DA neurotoxicity (Fig. 1d).
Fig. 1.
DA neurotoxicity induced by combined treatment with MnCl2 and LPS. Rat primary mesencephalic neuron-glia cultures (a–c) or neuron-enriched cultures (d) were treated for 7 days with vehicle control (0.2% water), indicated concentrations of MnCl2 or LPS alone, and combinations of MnCl2 and LPS. Following treatment, the cultures were either immunostained for TH-ir neurons (a–d) or subject to measurement of [3H]DA uptake capacity (c). The number of TH-ir neurons (a & d) and neurite length of TH-ir neurons (c) were determined. Results are expressed as a percentage of the vehicle-treated control cultures and are mean ± SEM of three (d) or five (a & c) independent experiments performed in triplicate. *p < 0.05 compared to vehicle-treated control cultures, #p < 0.05 compared to corresponding LPS-treated cultures, %p < 0.05 compared to corresponding MnCl2-treated cultures. In (b), the numbers in parentheses of indicate 10 or 30 μM and 0.5 or 2 ng/ml respectively. ICC images (b) are from a representative experiment. Arrowheads: TH-ir neurons. Scale bar: 100 μm.
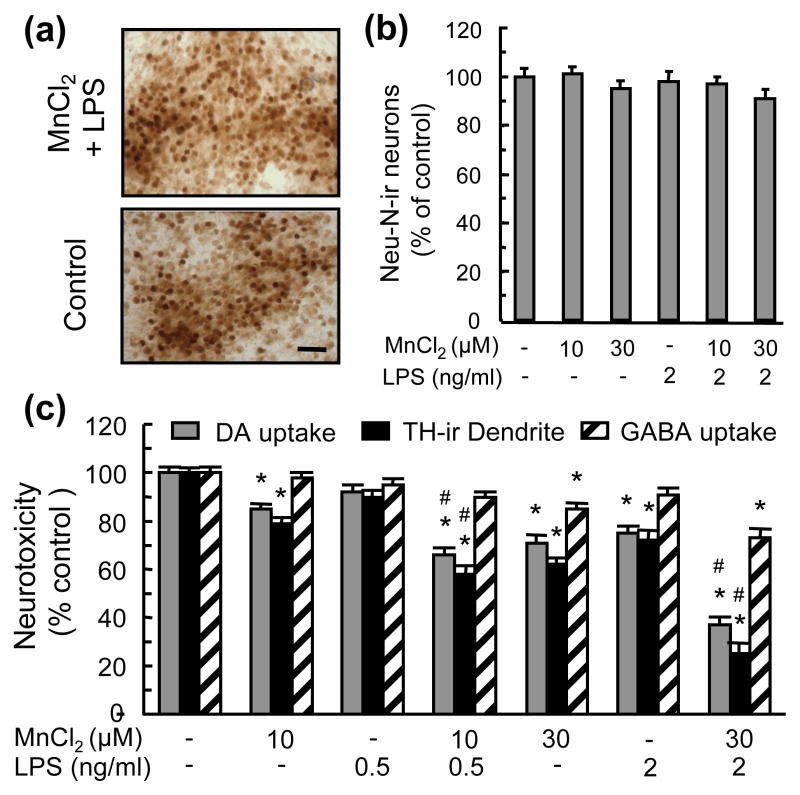
The combination of 30 μM MnCl2 and 2 ng/ml LPS was not equally effective in causing the loss of TH-ir neurons and that of neurons in general (i.e, Neu-N-ir neurons, Fig. 2a). Compared to a 44% loss of TH-ir neurons (Fig. 1a), the same treatment resulted in a statistically insignificant 9% reduction in Neu-N-ir neurons (Fig. 2b). In addition, combinations of MnCl2 and LPS were significantly more effective in damaging TH-ir neurites and reducing DA uptake capacity of DA neurons than reducing GABA uptake capacity of non-DA neurons (Fig. 2c). For example, 30 μM MnCl2 and 2 ng/ml LPS caused a 63% and 75% the reduction in DA uptake capacity and TH-ir neurite length respectively but only a 27% reduction in GABA uptake capacity (Fig. 2c).
Fig. 2.
Preferential DA neurotoxicity induced by MnCl2 and LPS. Neuron-glia cultures were treated for 7 days with vehicle, MnCl2 or LPS alone, and combinations of MnCl2 and LPS. Cultures were then immunostained for counting Neu-N-ir neurons (b) and measurement of TH-ir neurites (c), or assayed for [3H]DA and [3H]GABA uptake (c). For ICC analysis (a), cultures were treated with vehicle (Control) or 30 μM MnCl2 and 2 ng/ml LPS (MnCl2 + LPS), scale bar: 50 μm. Results are expressed as a percentage of the vehicle-treated control cultures and are mean ± SEM of four (b) or five (c) experiments performed in triplicate. *p< 0.05 compared to vehicle-treated control cultures, #p < 0.05 compared to corresponding MnCl2 or LPS-treated cultures.
Effect of Mn and LPS on the production of proinflammatory and neurotoxic factors in neuron-glia cultures
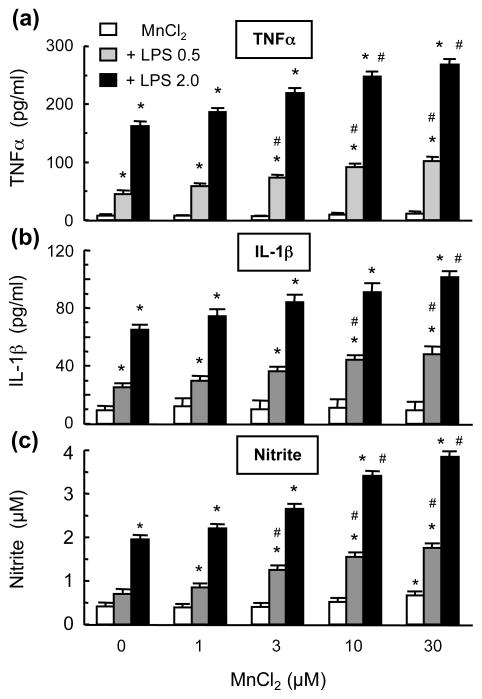
Glial activation and release of cytokines TNFα and IL-1β and free radicals are known to contribute to neurodegeneration (Liu 2006). In neuron-glia cultures treated for 7 days with 1–30 μM MnCl2, no significant release of TNFα or IL-1β was detected (Fig. 3). However, MnCl2, in a concentration-dependent manner, augmented the LPS-induced release of TNFα or IL-1β. In cultures treated with 0.5 ng/ml and 2 ng/ml LPS, significant augmentation of TNFα release was observed with ≥ 3 μM and ≥ 10 μM MnCl2 respectively (Fig. 3a). Significant enhancement of 0.5 ng/ml and 2 ng/ml LPS-stimulated IL-1β release was observed for ≥ 10 μM and 30 μM MnCl2 respectively (Fig. 3b). Different from a lack of effect on stimulating TNFα or IL-1β release, MnCl2 caused a modest increase in NO production with significant elevation observed with 30 μM MnCl2 (Fig. 3c). Moreover, significant enhancement of 0.5 ng/ml and 2 ng/ml LPS-induced nitrite production was observed for ≥ 3 μM and 10 μM MnCl2 respectively (Fig. 3c).
Fig. 3.
Effect of MnCl2 and LPS on the release of TNFα and IL-1β and production of NO in neuron-glia cultures. Supernatants from cultures treated for 7 days with vehicle, MnCl2 or LPS alone, and combinations of MnCl2 and LPS were collected and the levels of TNFα, IL-1β, and nitrite were determined. Results are mean ± SEM of five experiments performed in triplicate. *p< 0.05 compared to vehicle-treated control cultures, #p < 0.05 compared to corresponding MnCl2 or LPS-treated cultures.
Microglia and astroglia are differentially involved in the Mn and LPS-induced DA neurodegeneration
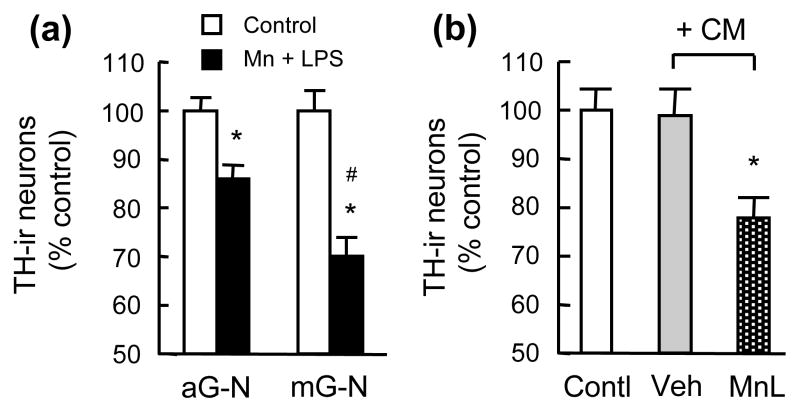
In analysis of the contribution of microglia and astroglia to the Mn and LPS-induced neurotoxicity, we treated astroglia-neuron and microglia-neuron cultures for 7 days with 30 μM MnCl2 and 2 ng/ml LPS. TH-ir neurons in the microglia-neuron cultures were found to be significantly more vulnerable than those in the astroglia-neuron cultures to the Mn and LPS-induced neurotoxicity (Fig. 4a). The magnitude of reduction in TH-ir neurons in the microglia-neuron cultures (Fig. 4a) was less than that observed in the mixed neuron-glia cultures (Fig. 1a). The elevated susceptibility of neurons in the microglia-neuron cultures to Mn and LPS toxicity prompted us to collect conditioned media from microglia treated for 1 day with 30 μM MnCl2 and 2 ng/ml LPS. When the conditioned media were used to treat neuron-enriched cultures (6 days), significant loss of TH-ir neurons was observed while vehicle-treated microglia-conditioned media had no effect (Fig. 4b). The degree of reduction in TH-ir neurons observed in neuron-enriched cultures treated with Mn and LPS-conditioned media (Fig. 4b) was less than that observed in Mn and LPS-treated neuron-microglia cultures (Fig. 4a).
Fig. 4.
Characterization of glial dependence for MnCl2 and LPS-induced DA neurotoxicity. (a). Astroglia-neuron (aG-N) or microglia-neuron (mG-N) cultures were treated for 7 days with vehicle and 30 μM MnCl2 and 2 ng/ml LPS (Mn+LPS). (b). Neuron-enriched cultures were treated for 6 days with treatment media alone (Contl) or treatment media containing conditioned media (CM) collected from microglia treated for 24 hr with vehicle (Veh) or 30 μM MnCl2 and 2 ng/ml LPS (MnL). Following treatment, cultures were immunostained and the number of TH-ir neurons determined. Results are mean ± SEM of four experiments performed in triplicate. *p< 0.05 compared to vehicle-treated control cultures, #p < 0.05 compared to MnCl2+LPS-treated astroglia-neuron cultures.
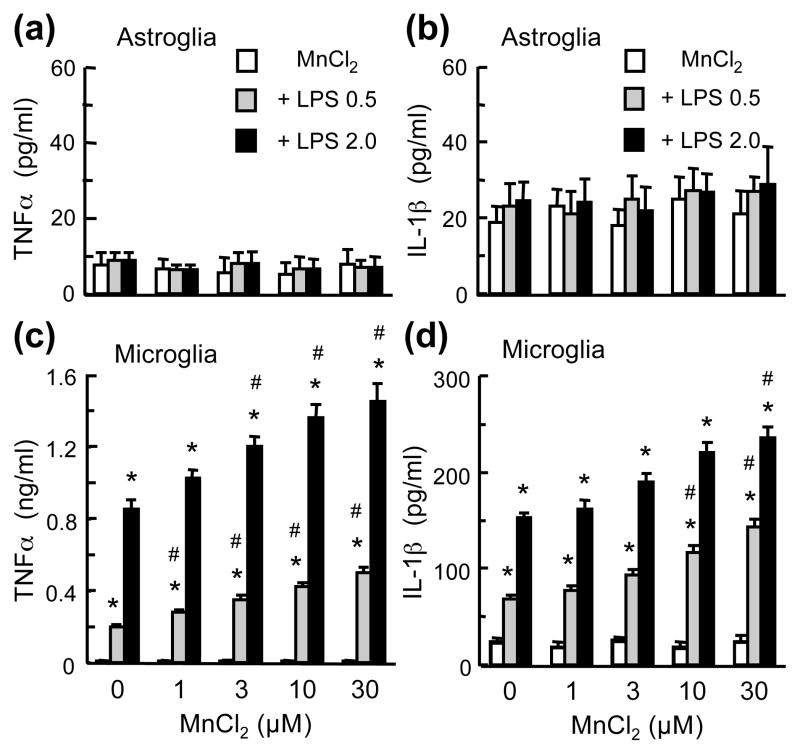
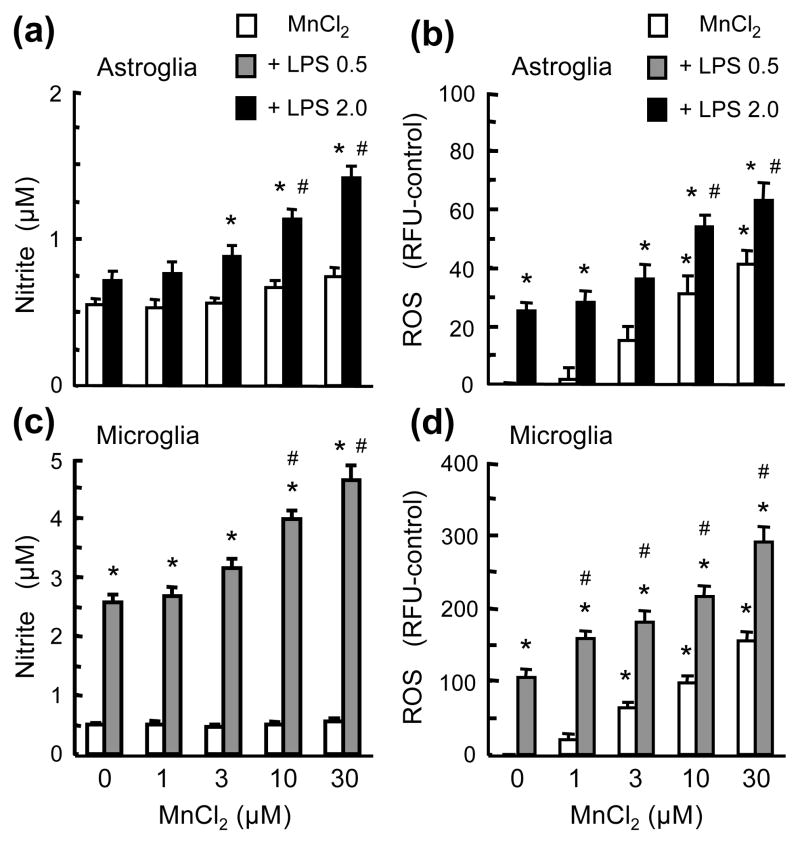
Next, we directly compared the effect of Mn and LPS on the production of cytokines TNFα and IL-1β, NO and ROS in microglia and astroglia cultures. First, in astroglia cultures, compared to the vehicle-treated control cultures, no significant release of TNFα and IL-1β was detected following treatment with MnCl2 (1–30 μM), LPS (0.5 and 2 ng/ml), or the combinations of MnCl2 and LPS for 24 (Figs. 5a & 5b), 48 or 72 hr (data not shown). In microglia cultures, although MnCl2 (1–30 μM; 24 hr) alone did not cause significant release of TNFα or IL-1β, MnCl2 markedly augmented the LPS-induced TNFα and IL-1β release (Figs. 5c & 5d). Significant augmentation of TNFα release was observed with the combinations of 0.5 ng/ml LPS and ≥1 μM MnCl2 and 2 ng/ml LPS and ≥3 μM MnCl2; and IL-1β release with 0.5 ng/ml LPS and 10–30 μM MnCl2 and 2 ng/ml LPS and 30 μM MnCl2 (Figs. 5c & 5d). Second, compared to the control cultures, no significant NO production was observed in astroglia or microglia cultures treated with 1–30 μM MnCl2 for up to 72 hr (Figs. 6a & 6c). However, the combinations of MnCl2 and LPS were more effective than either agent alone in causing NO production in both astroglia and microglia. At 10 and 30 μM, MnCl2 significantly augmented the LPS (2 ng/ml; 72 hr)-induced astroglial NO production from 130% to 205% and 256% of control and LPS (0.5 ng/ml, 24 hr)-induced microglial NO production from 526% to 723% and 843% of control respectively (Figs. 6a & 6c). Third, MnCl2 (1–30 μM; 24 hr) caused a more robust production of ROS in microglia than in astroglia (Figs. 6b & 6d). In astroglia, significant augmentation of LPS (2 ng/ml)-induced ROS production was observed for 10 and 30 μM MnCl2 and in microglia significant enhancement of LPS (0.5 ng/ml)-induced ROS production with 1–30 μM MnCl2 (Figs. 6b & 6d).
Fig. 5.
Comparison of the effect of MnCl2 and LPS on TNFα and IL-1β release in microglia and astroglia. Astroglia (a & b) microglia (c & d) cultures were treated for 24 hr with vehicle, MnCl2 or LPS alone, and the combinations of MnCl2 and LPS. The levels of TNFα (a & c) and IL-1β (b & d) in the culture supernatants were determined. Results are mean ± SEM of four to six experiments performed in triplicate. *p< 0.05 compared to vehicle-treated control cultures, #p < 0.05 compared to corresponding MnCl2 or LPS-treated cultures.
Fig. 6.
Effect of MnCl2 and LPS on NO and ROS production in microglia and astroglia. Astroglia (a & b) microglia (c & d) cultures were treated for 24 hr (b–d) or 72 hr (a) with vehicle, MnCl2 or LPS alone, and the combinations of MnCl2 and LPS and production of NO and ROS was determined. Results are mean ± SEM of four to six experiments performed in triplicate. *p< 0.05 compared to vehicle-treated control cultures, #p < 0.05 compared to corresponding MnCl2 or LPS-treated cultures.
Blockade of proinflammatory and neurotoxic factor production protects DA neurons from Mn and LPS-induced degeneration
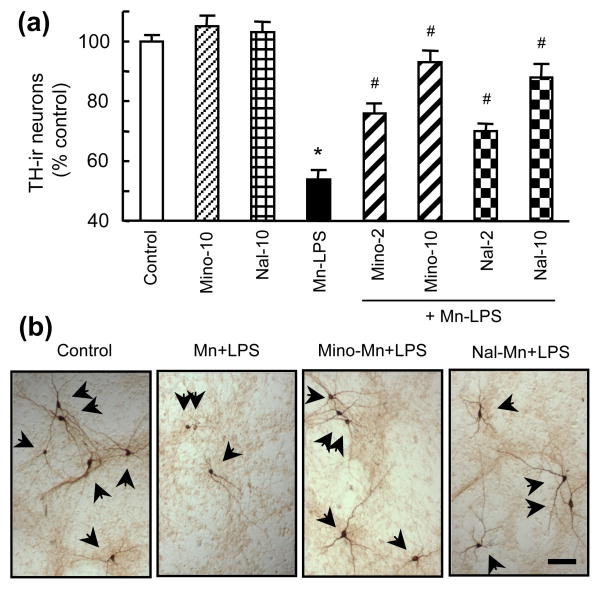
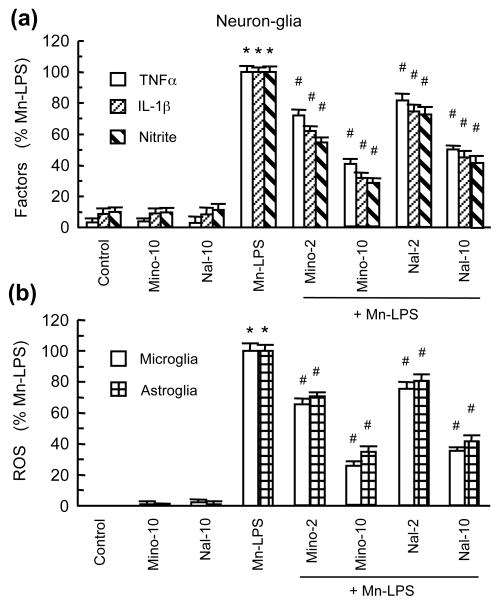
Minocycline and naloxone have been shown to inhibit glial activation and afford neuroprotection (Dutta et al. 2008, Liu 2006). In this study, neuron-glia cultures were pretreated with 2 and 10 μM minocycline or naloxone prior to treatment for 7 days with the combination of 30 μM MnCl2 and 2 ng/ml LPS. Minocycline and naloxone significantly reduced TH-ir neuron loss induced by MnCl2 and LPS (Fig. 7). At equal concentrations, minocycline appeared to be slightly more effective than naloxone in affording neuroprotection. The production of TNFα, IL-1β and NO was significantly reduced by minocycline and naloxone in neuron-glia cultures treated for 7 days with 30 μM MnCl2 and 2 ng/ml LPS (Fig. 8a). In addition, MnCl2 and LPS-induced microglial and astroglial ROS production was significantly reduced by minocycline or naloxone (Fig. 8b).
Fig. 7.
Effect of naloxone and minocycline on the MnCl2 and LPS-induced DA neurodegeneration. Neuron-glia cultures were treated for 7 days with vehicle, 10 μM minocycline (Mino-10) or naloxone (Nal-10) alone, the combination of 30 μM MnCl2 and 2 ng/ml LPS (Mn-LPS), or pretreated for 30 min with 2 or 10 μM minocycline (Mino-2, Mino-10) or naloxone (Nal-2, Nal-10) prior to treatment with 30 μM MnCl2 and 2 ng/ml LPS (+ Mn-LPS). Cultures were then immunostained for TH-ir neurons and the number of TH-ir neurons was determined. Results in (a) are mean ± SEM of five experiments performed in triplicate. *p< 0.05 compared to vehicle-treated control cultures, #p < 0.05 compared to MnCl2 and LPS-treated cultures. ICC images (b) are from cultures treated with vehicle (control), 30 μM MnCl2 and 2 ng/ml LPS (Mn+LPS), and pretreated with 10 μM minocycline or naloxone prior to treatment with MnCl2 and LPS (Mino-Mn+LPS or Nal-Mn+LPS). Bar: 100 μm.
Fig. 8.
Effect of minocycline and naloxone on the MnCl2 and LPS-induced production of neurotoxic factors. (a). The levels of TNFα, IL-1β, and nitrite in the supernatants from cultures treated as described for Fig. 7a were determined. (b). Microglia and astroglia cultures were treated for 24 hr with the same combinations of agents as described for Fig. 7a and ROS production was determined. Results in are mean ± SEM of five (a) or four (b) experiments performed in triplicate (a) or quadruplicate (b). *p< 0.05 compared to vehicle-treated control cultures, #p < 0.05 compared to MnCl2 and LPS-treated cultures.
Discussions
In this study, we have demonstrated, for the first time, that Mn (1–30 μM MnCl2) and LPS (0.5–2 ng/ml) work in concert to induce synergistic and preferential degeneration of DA neurons in mesencephalic neuron-glia cultures (Figs. 1 & 2). Microglia and astroglia are both involved in the Mn and LPS-induced neurotoxicity with microglia possibly playing a larger role than astroglia (Fig. 4). The more prominent role for microglia may be a result of their robust and synergistic production of proinflammatory cytokines including TNFα and IL-1β and free radicals including ROS and RNS in response to the combined stimulation of Mn and LPS (Figs. 5 & 6). While astroglia do not seem to respond to Mn, LPS or the combination of both to produce more TNFα and IL-1β than the basal levels, Mn and LPS induce astroglia to produce more ROS and RNS than that by either agent alone albeit at a subdued intensity compared to microglia (Figs. 5 & 6). The contribution of glial activation to the Mn and LPS-induced synergistic neurotoxicity is supported by the observation that minocycline or naloxone affords neuroprotection (Fig. 7) through blockade of glial production of proinflammatory and neurotoxic factors (Fig. 8).
Difference in primary site of neuronal damage and response to levodopa therapy indicates that excessive exposure to Mn-induced manganism is distinct from IPD (Aschner et al. 2007, Huang 2007, Perl & Olanow 2007). Excessive exposure to Mn by itself does not seem to result in PD development. However, the combination of Mn and other contributing factors may contribute to DA neurodegeneration in PD. In the present study, we show that Mn and inflammogen LPS work in concert to induce DA neurodegeneration in primary neuron-glia cultures. While the plasma Mn content in healthy individuals are at the low to mid nanomolar levels (Zheng et al. 1998), levels of Mn in the range of 4.4–334 μM have been reported for various brain regions, in particular the basal ganglia in brains of accidentally exposed human subjects and experimental animals (rodents and non-human primates) (Erikson et al. 2004). While low micromolar concentrations of Mn by itself exhibit little neurotoxicity [(Stanwood et al. 2009, Zhang et al. 2009) and this study], combinations of Mn (1–30 μM) with LPS (0.5–2 ng/ml) that by itself is very modestly effective (Gao et al. 2003a, Gao et al. 2002b) result in synergistic DA neurotoxicity (this study). While the potential relevance of environmental Mn exposure and episodic systemic infections and consequential neuroinflammation to PD development remains to be proven (Brydon et al. 2008, Teeling & Perry 2009), PD may represent a collection of highly similar pathological abnormalities caused by long-term interactions among intrinsic (the vulnerability of the SN DA pathway and aging), genetic and various combinations of environmental factors. Analogous to Mn and inflammation, cooperative DA neurotoxicity has been reported for combinations of herbicide paraquat and the Mn-containing fungicide maneb, pesticide dieldrin and neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), LPS and MPTP or 6-hydroxydopamine, and LPS and parkin gene deletion (Frank-Cannon et al. 2008, Gao et al. 2003a, Gao et al. 2003b, Ling et al. 2004a, Ling et al. 2004b, Richardson et al. 2006, Thiruchelvam et al. 2000).
DA neurotoxicity of Mn as well as LPS is dependent upon the concentrations of Mn or LPS. In the neuron-glia cultures, at low micromolar concentrations, Mn is more effective in damaging DA neurons than non-DA neurons and with increasing concentrations (high micromolar and low millimolar concentrations), non-DA neurons begin to be markedly affected (Milatovic et al. 2009, Stanwood et al. 2009, Zhang et al. 2009). For example, 1 mM MnCl2 induced >90% loss of TH-ir neurons compared to ~35–60% of Neu-N-ir neurons in cultures (Stanwood et al. 2009, Zhang et al. 2009). In animals, Mn-induced loss of TH-ir neurons is paralleled by a loss of Nissl-stained neuronal cell bodies in the SN (Stanwood et al. 2009), indicative of the destruction of TH-expressing neurons. Similarly, LPS-induced DA neurotoxicity involves the degeneration of TH-ir and Neu-N-ir neurons in cultures and in the SN with preferential DA neurotoxicity observed at lower LPS concentrations (Gao et al. 2002b, McCoy et al. 2006). The combination of low concentrations of Mn (≤30 μM) and LPS (≤2 ng/ml) seem to preferentially target DA neurons. While 30 μM MnCl2 and 2 ng/ml LPS resulted in a 63% reduction in DA uptake and 44% loss of TH-ir neurons, the same treatment only caused a 27% reduction in GABA uptake but not a statistically significant loss of Neu-N-ir neurons (Figs. 1 & 2). The loss of uptake capacity could also be due, at least in part, to glia-originated ROS and RNS since transporters of neurotransmitters are known targets of free radicals (Fleckenstein et al. 2007).
The preferential DA neurotoxicity observed in this study may be due to the innate vulnerability of DA neurons and the ability of Mn and LPS to stimulate glial production of deleterious factors. DA neurons are known to be particularly susceptible to oxidative and nitrosative stress (Jenner & Olanow 1996). In this study, the combination of low concentrations of Mn and LPS stimulate glial cells to produce ROS, RNS and cytokines TNFα and IL-1β, factors that are known to cause the demise of DA neurons (Liu 2006). The formation of more damaging intermediates such as peroxynitrite from ROS and RNS may render DA neurons more vulnerable to Mn and LPS-induced toxicity. It should be pointed out that the complete repertoire of factors that mediate the glial activation-enhanced Mn neurotoxicity will not only include ROS, NO, TNFα and IL-1β, but also IL-6 and prostaglandin E2, as well as additional and yet to be identified factors (Filipov et al. 2005, Milatovic et al. 2009).
While LPS depends on the activation of glial cells to induce neurotoxicity (Liu et al. 2002, Zhang et al. 2009), the involvement of glia in Mn neurotoxicity appears to be concentration-dependent. Direct comparison using cell culture models indicates that at 1–30 μM, MnCl2 is only effective in causing DA neurotoxicity in the presence of glia and at 1 mM, MnCl2 appears to be equally toxic regardless of glial presence (Zhang et al. 2009). Therefore, it is reasonable to speculate that Mn-induced neurodegeneration involves both glia-mediated and direct neurotoxicity and the degree of glial participation is dependent upon the concentrations of Mn. At low and perhaps more environmentally relevant doses, especially in combination with other agents such as glia-activating agents, Mn-induced neurotoxicity may involve a significant contribution from glial cells.
Microglia and astroglia are the main players of the neuroinflammatory process (Liu & Hong 2003c). Results from this study indicate that microglia and astroglia exhibit differential contribution to the Mn and LPS-induced generation of neurotoxic factors. Mechanistically, several groups have reported the involvement of members of the mitogen-activated protein kinases (MAPKs) and transcription factors in the Mn-potentiated production of neurotoxic factors in activated glia. For example, Barhoumi et al has shown that increased mitochondrial ROS production and activation of nuclear factor kappa B (NFκB) mediate Mn enhancement of LPS-induced iNOS upregulation in C6 astrocytic cells (Barhoumi et al. 2004). In cytokine-activated astroglia, Mn potentiation of iNOS expression is mediated through increased production of cGMP that leads to activation of MAPK and NFκB (Moreno et al. 2008). In N9 microglial cells, MAPK and NFkB activation is involved in Mn potentiation of LPS-induced TNFα and IL-6 release and iNOS upregulation (Crittenden & Filipov 2008, Filipov et al. 2005). Members of MAPKs are also involved, at least in part, in Mn-stimulated microglial release of hydrogen peroxide (Zhang et al. 2007). The precise mechanisms of action underlying the differential contribution of microglia and astrocytes to Mn and LPS-induced neurotoxicity remain to be further delineated. In addition, the potent effect of LPS at 0.5 ng/ml on glial production of free radicals and cytokines (Gao et al. 2002b) warrant future studies to determine the interaction of even lower and potentially subclinical doses of LPS with Mn.
The Mn and LPS-induced glial production of cytokines and free radicals and DA neurotoxicity is markedly reduced by minocycline and naloxone (Figs. 7 & 8). Minocycline, a tetracycline derivative and naloxone, a non-selective opioid receptor antagonist, have been shown to inhibit glial activation induced by LPS and neurotoxicants such as MPTP and 6-OHDA and afford neuroprotection (Dutta et al. 2008, Liu & Hong 2003c, Liu & Hong 2003a). In particular, minocycline and naloxone have been shown to be effective inhibitors of microglial ROS production (Liu et al. 2000, Wu et al. 2002). In addition, minocycline has been shown to possess anti-inflammatory and protective effects for a variety of disorders of the central nervous and peripheral systems (Kim & Suh 2009, Rempe et al. 2007).
In summary, this study demonstrates the synergistic DA neurotoxicity of Mn, an occupational and potential environmental neurotoxicant and LPS, an inflammatory stimulus. This study has identified differential involvement of microglia and astroglia in the combined Mn and LPS neurotoxicity with TNFα, IL-1β, ROS and RNS as key mediators. Continued delineation of the underlying molecular and cellular mechanisms of action will further advance our understanding of the contribution of combinations of multiple occupational and/or environmentally relevant agents to the development of disorders of the brain and peripheral systems.
Supplementary Material
Acknowledgments
This work was supported by a grant (ES013265) from the National Institute of Environmental Health Sciences of the National Institutes of Health.
Abbreviations used
- DA
dopamine
- DCF
dichlorodihydrofluorescein
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- HS
horse serum
- GABA
gamma-aminobutyric acid
- GFAP
glial fibrillary acidic protein
- ICC
immunocytochemistry
- IL-1β
interleukin-1beta
- LPS
lipopolysaccharide
- MEM
minimum essential medium
- MnCl2
manganese chloride
- Neu-N
neuron-specific nuclear protein
- NO
nitric oxide
- PD
Parkinson’s disease
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TH
tyrosine hydroxylase
- TNFα
tumor necrosis factor-alpha
References
- Aschner M, Guilarte TsR, Schneider JS, Zheng W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicology and applied pharmacology. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JH, Jang BC, Suh SI, Ha E, Baik HH, Kim SS, Lee MY, Shin DH. Manganese induces inducible nitric oxide synthase (iNOS) expression via activation of both MAP kinase and PI3K/Akt pathways in BV2 microglial cells. Neuroscience letters. 2006;398:151–154. doi: 10.1016/j.neulet.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Barhoumi R, Faske J, Liu X, Tjalkens RB. Manganese potentiates lipopolysaccharide-induced expression of NOS2 in C6 glioma cells through mitochondrial-dependent activation of nuclear factor kappaB. Brain Res Mol Brain Res. 2004;122:167–179. doi: 10.1016/j.molbrainres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. NeuroToxicology. 2006;27:340–346. doi: 10.1016/j.neuro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Chang JY, Liu LZ. Manganese potentiates nitric oxide production by microglia. Brain Res Mol Brain Res. 1999;68:22–28. doi: 10.1016/s0169-328x(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Annals of neurology. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- Crittenden PL, Filipov NM. Manganese-induced potentiation of in vitro proinflammatory cytokine production by activated microglial cells is associated with persistent activation of p38 MAPK. Toxicol In Vitro. 2008;22:18–27. doi: 10.1016/j.tiv.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte DA. The environment and Parkinson’s disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- Dutta G, Zhang P, Liu B. The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fundamental & clinical pharmacology. 2008;22:453–464. doi: 10.1111/j.1472-8206.2008.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Science of The Total Environment. 2004:334–335. 409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol Sci. 2005;84:139–148. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- Finkelstein MM, Jerrett M. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ Res. 2007;104:420–432. doi: 10.1016/j.envres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fored CM, Fryzek JP, Brandt L, Nise G, Sjogren B, McLaughlin JK, Blot WJ, Ekbom A. Parkinson’s disease and other basal ganglia or movement disorders in a large nationwide cohort of Swedish welders. Occup Environ Med. 2006;63:135–140. doi: 10.1136/oem.2005.022921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, et al. Parkin Deficiency Increases Vulnerability to Inflammation-Related Nigral Degeneration. Journal of Neuroscience. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002a;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. J Neurosci. 2003a;23:1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. Journal of neurochemistry. 2002b;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. Faseb J. 2003b;17:1957–1959. doi: 10.1096/fj.03-0203fje. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology. 1997;48:650–658. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology. 1999;20:239–247. [PubMed] [Google Scholar]
- Huang CC. Parkinsonism induced by chronic manganese intoxication--an experience in Taiwan. Chang Gung Med J. 2007;30:385–395. [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology. 1996;47:S161–170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Klein C, Schlossmacher MG. Parkinson disease, 10 years after its genetic revolution: multiple clues to a complex disorder. Neurology. 2007;69:2093–2104. doi: 10.1212/01.wnl.0000271880.27321.a7. [DOI] [PubMed] [Google Scholar]
- Ling Z, Chang QA, Tong CW, Leurgans SE, Lipton JW, Carvey PM. Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Exp Neurol. 2004a;190:373–383. doi: 10.1016/j.expneurol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Ling ZD, Chang Q, Lipton JW, Tong CW, Landers TM, Carvey PM. Combined toxicity of prenatal bacterial endotoxin exposure and postnatal 6-hydroxydopamine in the adult rat midbrain. Neuroscience. 2004b;124:619–628. doi: 10.1016/j.neuroscience.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Liu B. Modulation of microglial pro-inflammatory and neurotoxic activity for the treatment of Parkinson’s disease. Aaps J. 2006;8:E606–621. doi: 10.1208/aapsj080369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. The Journal of pharmacology and experimental therapeutics. 2000;293:607–617. [PubMed] [Google Scholar]
- Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environmental health perspectives. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Annals of the New York Academy of Sciences. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Neuroprotective effect of naloxone in inflammation-mediated dopaminergic neurodegeneration. Dissociation from the involvement of opioid receptors. Methods in molecular medicine. 2003a;79:43–54. doi: 10.1385/1-59259-358-5:43. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Primary rat mesencephalic neuron-glia, neuron-enriched, microglia-enriched, and astroglia-enriched cultures. Methods in molecular medicine. 2003b;79:387–395. doi: 10.1385/1-59259-358-5:387. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. The Journal of pharmacology and experimental therapeutics. 2003c;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang K, Gao HM, Mandavilli B, Wang JY, Hong JS. Molecular consequences of activated microglia in the brain: overactivation induces apoptosis. Journal of neurochemistry. 2001;77:182–189. doi: 10.1046/j.1471-4159.2001.t01-1-00216.x. [DOI] [PubMed] [Google Scholar]
- Logroscino G. The role of early life environmental risk factors in Parkinson disease: what is the evidence? Environmental health perspectives. 2005;113:1234–1238. doi: 10.1289/ehp.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Albini E, Benedetti L, et al. High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am J Ind Med. 2007;50:788–800. doi: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, Langston JW. Model fusion, the next phase in developing animal models for Parkinson’s disease. Neurotox Res. 2007;11:219–240. doi: 10.1007/BF03033569. [DOI] [PubMed] [Google Scholar]
- Marsh GM, Gula MJ. Employment as a welder and Parkinson disease among heavy equipment manufacturing workers. J Occup Environ Med. 2006;48:1031–1046. doi: 10.1097/01.jom.0000232547.74802.d8. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- Mergler D, Huel G, Bowler R, Iregren A, Belanger S, Baldwin M, Tardif R, Smargiassi A, Martin L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ Res. 1994;64:151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicology and applied pharmacology. 2009 doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Sullivan KA, Carbone DL, Hanneman WH, Tjalkens RB. Manganese potentiates nuclear factor-kappaB-dependent expression of nitric oxide synthase 2 in astrocytes by activating soluble guanylate cyclase and extracellular responsive kinase signaling pathways. J Neurosci Res. 2008;86:2028–2038. doi: 10.1002/jnr.21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. Journal of neuropathology and experimental neurology. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Powers KM, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD, Checkoway H. Parkinson’s disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology. 2003;60:1761–1766. doi: 10.1212/01.wnl.0000068021.13945.7f. [DOI] [PubMed] [Google Scholar]
- Przedborski S. Neuroinflammation and Parkinson’s disease. Handb Clin Neurol. 2007;83:535–551. doi: 10.1016/S0072-9752(07)83026-0. [DOI] [PubMed] [Google Scholar]
- Qin L, Block ML, Liu Y, et al. Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress. Faseb J. 2005;19:550–557. doi: 10.1096/fj.04-2857com. [DOI] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B. Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology. 2005;64:230–235. doi: 10.1212/01.WNL.0000149511.19487.44. [DOI] [PubMed] [Google Scholar]
- Rempe S, Hayden JM, Robbins RA, Hoyt JC. Tetracyclines and pulmonary inflammation. Endocr Metab Immune Disord Drug Targets. 2007;7:232–236. doi: 10.2174/187153007782794344. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. Faseb J. 2006;20:1695–1697. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- Spranger M, Schwab S, Desiderato S, Bonmann E, Krieger D, Fandrey J. Manganese augments nitric oxide synthesis in murine astrocytes: a new pathogenetic mechanism in manganism? Exp Neurol. 1998;149:277–283. doi: 10.1006/exnr.1997.6666. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Leitch DB, Savchenko V, Wu J, Fitsanakis VA, Anderson DJ, Stankowski JN, Aschner M, McLaughlin B. Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. Journal of neurochemistry. 2009 doi: 10.1111/j.1471-4159.2009.06145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Tan EK, Tan C, Fook-Chong SM, et al. Dose-dependent protective effect of coffee, tea, and smoking in Parkinson’s disease: a study in ethnic Chinese. J Neurol Sci. 2003;216:163–167. doi: 10.1016/j.jns.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158:1062–1073. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Hatter A, Liu B. Manganese chloride stimulates rat microglia to release hydrogen peroxide. Toxicology Letters. 2007;173:88–100. doi: 10.1016/j.toxlet.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wong TA, Lokuta KM, Turner DE, Vujisic K, Liu B. Microglia enhance manganese chloride-induced dopaminergic neurodegeneration: Role of free radical generation. Exp Neurol. 2009;217:219–230. doi: 10.1016/j.expneurol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Ren S, Graziano JH. Manganese inhibits mitochondrial aconitase: a mechanism of manganese neurotoxicity. Brain Res. 1998;799:334–342. doi: 10.1016/s0006-8993(98)00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.