Abstract
ATP-gated P2X4 receptors (P2X4R) are abundantly expressed in the CNS. However, little is known about the molecular targets for ethanol action in P2X4Rs. The current investigation tested the hypothesis that the ectodomain-TM interface contains residues that are important for the action of ethanol in P2X4Rs. Wildtype (WT) and mutant P2X4R were expressed in Xenopus oocytes. ATP concentration-response curves and ethanol (10–200 mM)-induced changes in ATP EC10-gated currents were determined using two-electrode voltage clamp (−70 mV). Alanine substitution at the ectodomain-TM1 interface (positions 50–61) resulted in minimal changes in ethanol response. On the other hand, alanine substitution at the ectodomain-TM2 interface (positions 321–337) identified two key residues (D331 and M336) that the alanine mutation significantly reduced ethanol inhibition of ATP-gated currents without causing marked changes in ATP Imax, EC50 or Hill slope. Other amino acid substitutions at positions 331 and 336 significantly altered or eliminated the modulatory effects of ethanol. Linear regression analyses revealed a significant relationship between hydropathy and polarity, but not molecular volume/molecular weight of the residues at these two positions. The results support the proposed hypothesis and represent an important step towards developing ethanol-insensitive receptors for investigating the role of P2X4Rs in mediating behavioral effects of ethanol.
Keywords: Purinergic P2XRs, ion channels, sites of ethanol action, alcohol, Xenopus oocytes, two-electrode voltage clamp
INTRODUCTION
Alcohol abuse and dependence are major problems in our society with approximately 14 million people in the United States being affected (McGinnis J.M. and Foege WH. 1999;Volpicelli J.R. 2001;Grant et al. 2004). The development of effective treatments for alcohol related disorders faces a number of challenges due to a limited knowledge of the sites and mechanisms of ethanol action in the central nervous system (CNS). A better understanding of how and where alcohol acts to cause its behavioral effects is a primary goal of the alcohol research community.
P2XRs constitute a superfamily of LGICs that are becoming a focus of interest by the alcohol community (for review see (Xiong et al. 2000;Davies et al. 2002;Davies et al. 2005;Asatryan et al. 2008)). P2XRs are fast acting, cation-permeable LGICs that are gated by synaptically released extracellular ATP and are broadly distributed in the central and peripheral nervous systems (Khakh 2001;North 2002;Burnstock 2008). Currently, seven genes of the P2X family of LGICs have been identified (P2X1-P2X7). P2XRs form homomeric (e.g., P2X2, P2X4), as well as heteromeric (e.g., P2X2/3, P2X4/6) channels with a functional P2XR resulting from the assembly of three subunits (Stoop et al. 1999;Torres et al. 1999;Jiang et al. 2003). Each P2X subunit consists of two transmembrane (TM) domains, a large extracellular domain (ectodomain) and intracellular amino- (N) and carboxy (C)-terminals (for review see (Khakh 2001;North 2002;Burnstock 2008)) (see Supporting Information, Fig. S1). A crystal structure of zebrafish P2X4Rs was recently reported at 3.1A resolution which verified the trimeric construction of P2X4Rs (Kawate et al. 2009).
Building evidence supports the notion that P2XRs play a role in mediating and/or modulating at least a subset of the cellular and behavioral effects of ethanol. First, native P2XR channels are sensitive to ethanol at intoxicating concentrations (Li et al. 1998;Li et al. 2000). Therefore, ethanol could exert behavioral changes by directly modifying P2XR function. Second, presynaptic P2XRs have been shown to facilitate the release of neurotransmitters (e.g., GABA, glycine and glutamate) (Chizh and Illes 2001;Khakh 2001;Deuchars et al. 2002;Hugel and Schlichter 2002;Mori et al. 2001;Papp et al. 2004) already reported to play important roles in ethanol-induced behavioral effects. Hence, ethanol-induced changes in presynaptic ATP-activated P2XR function could alter the strength of the neurochemical signal from GABAergic, glycinergic and/or glutamatergic terminals. In support of this hypothesis, we have recently shown that P2Rs can modulate ethanol’s effect on GABAergic synaptic transmission of dopamine neurons in the ventral tegmental area supporting a role for P2Rs in alcohol addiction (Xiao et al. 2008).
Studies using heterologous expression systems have begun to investigate the molecular sites of ethanol action in P2XRs. These studies found that P2X2R, P2X3R and P2X4R expressed in Xenopus oocytes are sensitive to ethanol at intoxicating concentrations (Davies et al. 2002;Davies et al. 2005;Xiong et al. 2000). Further studies using a chimeric strategy exploited the opposite effects of ethanol on P2X2R (ethanol inhibition) and P2X3R (ethanol potentiation) to identify key regions mediating the effects of ethanol (Asatryan et al. 2008). Performing an ectodomain swap between P2X2R and P2X3R reversed the modulatory effects of ethanol in the chimeric receptors compared to that of the WT P2X2R and P2X3R (Asatryan et al. 2008). Further chimeric studies followed by point mutations identified positions in the ectodomain-TM interfaces that reversed the direction (L304) or changed the magnitude (D53, A55 and N313) of ethanol response in P2X3Rs (Asatryan et al. 2008). Taken together, these studies indicate that residues near the ectodomain-TM domain interfaces are important for causing or modulating the qualitative and quantitative effects of ethanol in P2X3Rs (Asatryan et al. 2008).
P2X4Rs are the most abundant P2XR subtype expressed in the CNS (Buell et al. 1996;Soto et al. 1996); are inhibited by pharmacologically relevant concentrations of ethanol when expressed in recombinant expression systems (Xiong et al. 1999;Xiong et al. 2000;Davies et al. 2002;Xiong et al. 2005;Davies et al. 2005;Asatryan et al. 2008) and P2X4Rs show the greatest magnitude of response to ethanol (Davies et al. 2002;Davies et al. 2005).
Investigations focusing on the identification of ethanol sensitive sites in P2X4Rs were first undertaken by the Weight laboratory. This initial work found that substitution of a histidine residue for alanine at position 241 markedly decreases the EC50 value of the ATP concentration-response curve in the presence of ethanol (Xiong et al. 2005). Follow-up work in which the cysteine residues in the ectodomain region of P2X4Rs were individually mutated found that cysteines and disulfide bonds between cysteines are differentially involved in ethanol inhibition of P2X4Rs (Yi et al. 2009). Taken together, this work provided supporting evidence regarding a potential role for the ectodomain region of P2X4Rs in ethanol action.
The current investigation tests the hypothesis that the ectodomain-TM interface contains residues that are important for the action of ethanol in P2X4Rs. Overall, using an alanine scanning strategy followed by a large scale mutagenesis approach, we identified two key residues in the ectodomain-TM2 region that are critical for the modulatory action of ethanol in P2X4Rs.
EXPERIMENTAL PROCEDURES
Isolation of Xenopus Laevis Oocytes and capped RNA injections
Xenopus oocytes were isolated from Xenopus laevis frogs (Nasco, Fort Atkinson, WI) and maintained as described previously (Davies et al. 2002;Davies et al. 2005) (for details see S1. Experimental Procedures, Supporting Information).
Site-directed Mutagenesis
Mutations were generated in vitro by using QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and sequence-verified at the DNA Core Facility (University of Southern California, Los Angeles, CA). Primers were purchased from Integrated DNA Technologies (Coralville, IA).
Capped RNA synthesis
The DNA of WT or mutant receptors was linearized and transcribed using the mMESSAGE mMACHINE kit (Ambion, Austin, TX) to result in capped RNA, which was stored at −70 °C until injection.
Whole-Cell Voltage Clamp Recording
Two-electrode voltage-clamp recordings were performed using a Warner Instruments Model OC-725C oocyte clamp amplifier (Hamden, CT) at Vh= −70 mV following previously described procedures ((Asatryan et al. 2008)).
Experimental Procedures
Ethanol modulation was tested by the application of ethanol in combination with ATP EC10 for 20 sec. Prior studies found that the degree of ethanol inhibition of ATP-gated currents in P2X4Rs was similar regardless of whether ethanol was applied before or in combination with ATP (Davies et al. 2005). ATP activated EC10 responses were measured before and after each ethanol application to take into account possible shifts in the baseline current values. Importantly, ethanol did not affect the resting membrane currents in oocytes expressing P2X4Rs in the absence of agonist or in uninjected oocytes.
Data Analysis
Results are presented as percentage change in ATP EC10 -activated currents (nA) after normalizing them to the response obtained with agonist alone. All results are expressed as mean ± SEM. Data were obtained from oocytes from at least two different frogs. All experiments testing mutant P2X4Rs included WT control receptors expressed in the same batch of oocytes as the respective mutant P2X4R. The n refers to the number of different oocytes tested. The ATP/ethanol concentration response data were fitted to a concentration-response curve using the logistic equation: I=Imax*[drug]n/([drug]n+(EC50)n), where I/Imax is the percentage of the maximum obtainable response, EC50 is the concentration producing a half-maximal response and n is the Hill coefficient. Significant differences were determined by either one-way analysis of variance (ANOVA) or unpaired Student’s t-test. Prism (GraphPAD Prism Software, San Diego, CA) was used to perform statistical analyses and curve fitting. Statistical significance was defined as p< 0.05 and unless noted, the * indicates a significant difference in the modulatory effects of ethanol compared to WT P2X4Rs.
We used linear regression analyses (Prism) to test for possible relationships between the responses to ethanol and the physical-chemical properties (hydropathy, polarity, hydrophilicity and molecular volume) of the substituted residues at ethanol sensitive receptors. Hydropathy values were assigned using the Kyte-Doolittle hydropathy scale (Kyte and Doolittle 1982). Polarity values were assigned to amino acid residues using the Zimmerman polarity scale (Zimmerman et al. 1968). Hydrophilicity values were assigned using the Parker hydrophilicity scale (Parker et al. 1986). Molecular volumes were calculated using Peptide Property Calculator (Northwestern University Medical School).
Materials
Adenosine 5′-triphosphate disodium salt, ethanol (190 proof, USP), collagenase were purchased from Sigma Co. (St. Louis, MO, USA). All other chemicals were of reagent grade.
RESULTS
Alanine scan of non-conserved residues at the ectodomain-TM interfaces of P2X4Rs
The current study investigated the importance of agonist and ethanol responses of residues contained within the ectodomain-TM interface of P2X4Rs (see Supporting Information, Fig. S1). This work focused on amino acids at the ectodomain-TM interfaces that are non-conserved across P2X subtypes (P2X1 to P2X6) in order to avoid residues that are critical for ATP-binding and channel gating in P2X4Rs. Of these non-conserved residues, we focused on those that differed between P2X3R and P2X4R subtypes because these likely are critical for the qualitative differences (potentiation vs. inhibition) between P2X3R and P2X4R (see Supporting Information, Table S1). The residues that were replaced with alanine were W50, G53, E56, T57, S59, V60 and V61 near the TM1 domain and I321, I322, F324, K326, D331 and M336 near the TM2 domain.
Agonist Response
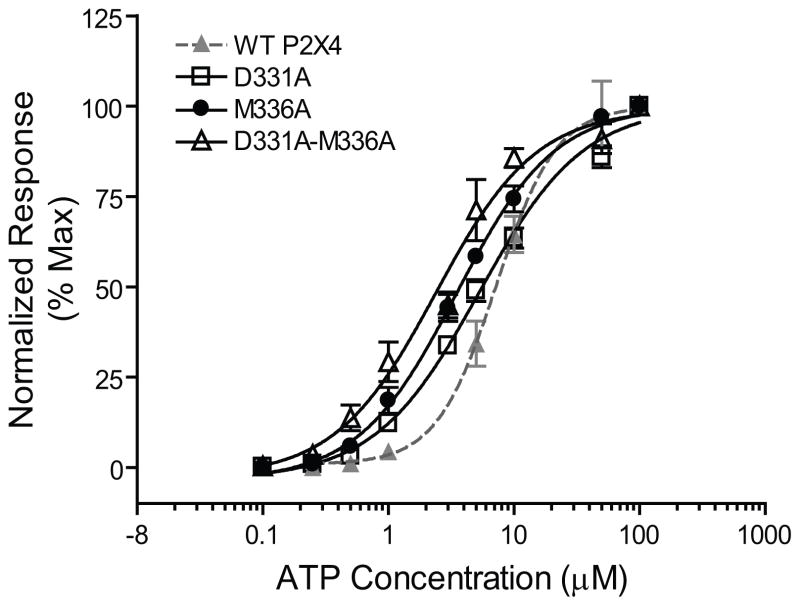
ATP concentration-response curves for WT and selected mutant P2X4Rs (D331A, M336A and D331A-M336A) are shown in Fig. 1. The mutant receptors illustrated in Fig 1 were selected because they showed the greatest amount of change in the magnitude of ethanol inhibition (see Fig. 3). As shown, the overall concentration-response curves for the mutant receptors were similar to the concentration-response curve of the WT P2X4R. All mutant P2X4Rs were functional and all but one mutant receptor had Imax, EC50 and Hill slope values that were similar to WT P2X4Rs. The Imax for the F324A mutant was significantly lower compared to WT, but its EC50 and Hill slope values were similar to those of WT P2X4R (see Supporting Information, Table S2). Overall, these results suggest that the non-conserved residues located at the channel interfaces are not critical for P2X4R agonist binding or channel gating.
Fig. 1. Concentration-response curves for ATP (0.1–100 μM)-activated currents in Xenopus oocytes expressing WT and mutant (D331A, M336A and D331A-M336A) receptors.
ATP-activated currents were normalized to the maximal current activated by a saturating concentration of ATP (100 μM). The curves represent non-linear regression analysis of the ATP concentration-responses in the WT and mutant P2X4Rs. Details of maximal current amplitude (Imax), EC50 and Hill Slope and are provided in Table 2. The similarity in the ATP concentration-response curves for WT vs. mutant receptors suggesting that the mutants did not markedly alter normal receptor function. Each data point represents the mean ± SEM from at least five different oocytes. Note: the symbol legends for the bottom and top points of the concentration response curves are lying on top of each other and thus appear as a black box at each of these positions.
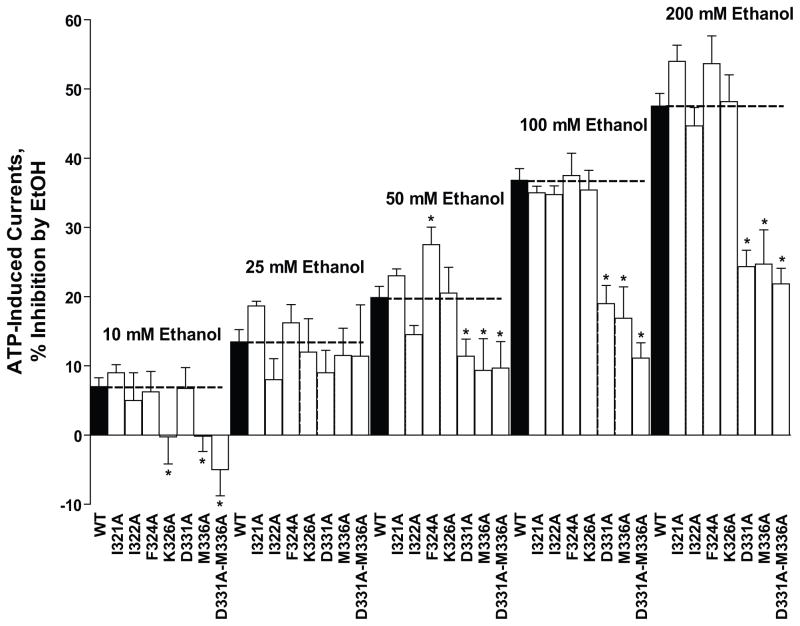
Fig. 3. Effects of ethanol (10–200 mM) in WT and mutant P2X4Rs in the ectodomain-TM2 interface.
Alanine substitution at positions 331 and 336 reduced the degree of ethanol inhibition in a concentration dependent manner starting at 50 mM. Data are presented as mean ± SEM, n= 3–14.
Ethanol Response
Ethanol (10–200 mM) induced a significant, reversible, concentration-dependent inhibition of EC10 ATP-gated currents in WT P2X4Rs (Fig. 2/Fig. 3 –black bars). Ethanol inhibition ranged from approximately 7% at 10 mM up to approximately 50% when tested at 200 mM in WT P2X4Rs and was similar to previously reported findings (Davies et al. 2002;Xiong et al. 2000).
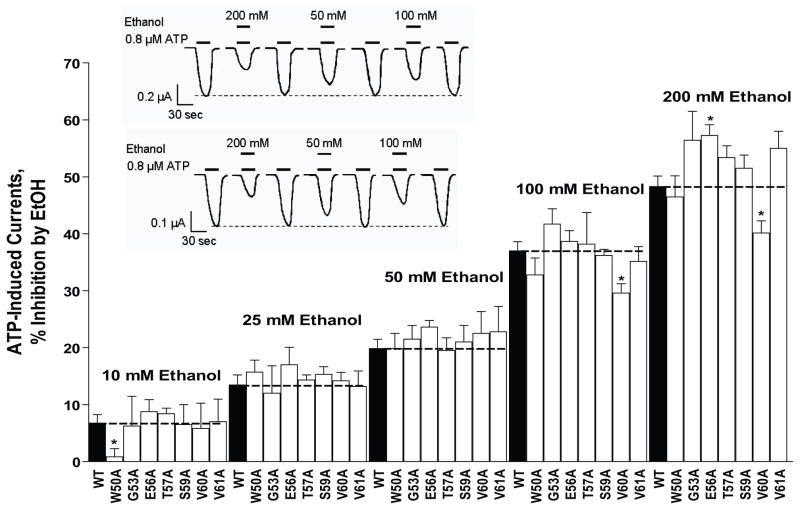
Fig. 2. Effects of ethanol (10–200 mM) in WT and mutant P2X4Rs in the ectodomain- TM1 interface.
In most instances, alanine substitution of residues in the ectodomain-TM1 interface region did not significantly alter ethanol inhibition. The bar graph illustrates the response to ethanol (10–200mM) of WT and mutant P2X4Rs. Data are presented as mean ± SEM, n= 3–8. Inset: Representative tracings of ATP EC10-induced currents and effect of 200 mM, 50 mM and 100 mM ethanol on WT (upper trace) and mutant ([S59A] lower trace) P2X4Rs. The horizontal bars depict when ATP (lower bar) and ethanol (upper bar) were applied to the oocyte. The dashed line illustrates the relative stability of the ATP EC10 over the duration of the experiment. Vertical scale bars are 0.2 and 0.1 μA respectively; horizontal scale bars represent 30 sec.
Ectodomain-TM1 interface
Ethanol significantly inhibited ATP-gated currents on all mutant receptors (Fig. 2 - open bars). As in WT P2X4Rs, the effect of ethanol in the mutants was reversible and concentration-dependent. In general, the degree of ethanol inhibition was similar to that of WT P2X4Rs with the exception of three mutants. The degree of ethanol inhibition was significantly reduced in W50A (10 mM) and V60A (100 and 200 mM) but significantly increased in E56A (200 mM) (Fig. 2).
Ectodomain-TM2 interface
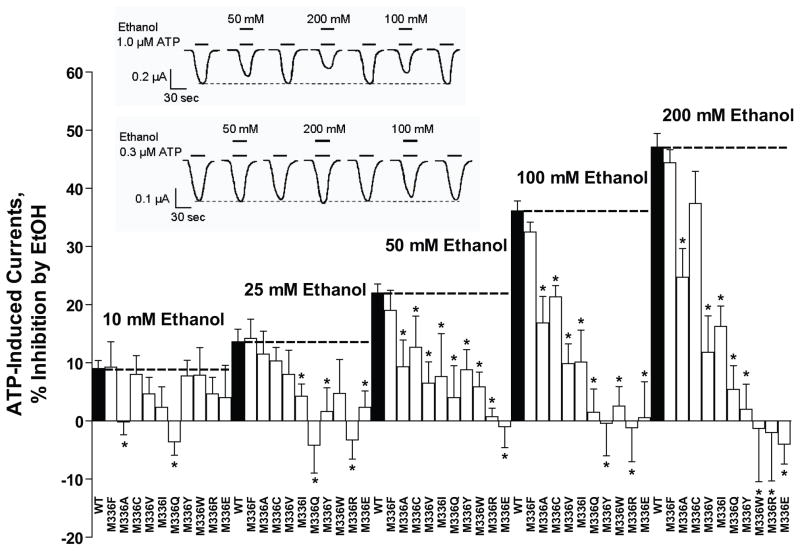
In contrast to the results for the ectodomain-TM1 interface, the magnitude and pattern of the effects of alanine substitutions in the ectodomain-TM2 interface indicate that this region contains residues that are important for ethanol action (Fig. 3). Alanine substitutions at position 326 and 336 eliminated ethanol inhibition at 10 mM, whereas at 25 mM ethanol, alanine mutations at these positions did not significantly affect the degree of ethanol inhibition. Alanine substitutions at positions 331 and 336 markedly decreased the degree of ethanol inhibition produced by 50, 100 and 200 mM ethanol. In addition, alanine substitution at position 324 significantly increased the degree of ethanol inhibition produced by 50 mM ethanol, but did not affect ethanol modulation at other concentrations.
Since alanine substitutions at positions 331 and 336 resulted in mutant receptors with differential changes in ethanol sensitivities that were both residue specific and ethanol concentration dependent we created a D331A-M336A double mutant and tested the receptor’s response to ethanol. As presented in Fig. 3, substitution of alanine at both positions 331 and 336 (D331A-M336A) resulted in a significant reduction in ethanol inhibition compared to WT P2X4Rs. The magnitude of ethanol inhibition in this double mutant when tested from 50 – 200 mM ethanol did not significantly differ from that of either single mutant P2X4R (i.e., D331A or M336A). The magnitude of ethanol inhibition in the double mutant when tested at 10 mM ethanol did not significantly differ from that of the single M336A mutant receptor. But, the double mutant did significantly differ in its sensitivity to 10 mM ethanol compared to that of the single D331A mutant receptor.
Physical-chemical properties of residues D331 and M336 are important determinants of ethanol sensitivity
Prior studies have shown that amino acid substitutions with different physical-chemical properties at key positions can affect the response to ethanol in other LGICs (Ye et al. 1998;Yamakura et al. 1999;Ronald et al. 2001;Ren et al. 2003;Perkins D.I. et al. 2008). Based on the studies above, we individually mutated D331 and M336 to residues with different physical-chemical properties to determine whether polarity or size of the mutated amino acids or their individual side-chain properties play a role in the 1) affinity and function of the mutant receptor to ATP or 2) ethanol sensitivity.
Agonist response
All mutations at positions 331 and 336 in P2X4Rs yielded measurable ATP-gated currents (see Supportive Information, Table S3). Moreover, the mutations appeared to be well tolerated as shown by minimal changes in Imax, EC50 and Hill slope values compared to those of WT P2X4Rs. We note that several mutations did cause a more marked change in their respective EC50 (D331V, M336I and M336R), but these mutations did not cause significant changes in either their respective Imax or Hill slope values. Overall, these findings add to the evidence presented above that the non-conserved residues located at the channel interfaces are not critical for P2X4R agonist binding or channel gating.
Ethanol response
Position 331 mutations
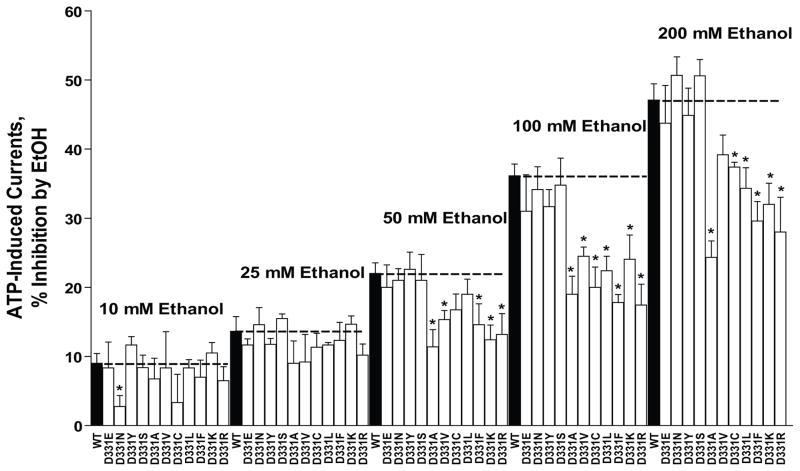
Ethanol (10–200 mM) significantly inhibited ATP-induced currents in WT and mutant P2X4Rs in a reversible and concentration-dependent manner (Fig. 4). Substituting the polar, negatively charged aspartic acid residue at position 331 (D331) in WT P2X4Rs, with another polar, negatively charged (D331E) or a polar non-charged residue (D331N, D331S or D331Y) did not significantly alter ethanol inhibition compared to WT (Fig. 4). Therefore, negative charge, per se, at position 331, does not appear to be a key factor in determining the magnitude and direction of ethanol response. On the other hand, substituting D331 (polar, charged) with non-polar residues (i.e., D331A, D331C, D331L, D331V or D331F) significantly reduced the magnitude of ethanol inhibition compared to WT P2X4Rs. Thus, polarity, but not charge, appears to play an important role in determining the threshold and magnitude of ethanol action
Fig. 4. Effects of ethanol (10–200mM) in mutant receptors at position 331 (D, aspartic acid).
Amino acid substitutions with different physical-chemical properties at position 331 significantly reduce the inhibitory effect of ethanol. There were no significant differences among the polar mutants with respect to ethanol sensitivity compared to WT P2X4R. However, substitution of D331 to non-polar residues significantly altered the ethanol sensitivity compared to the WT P2X4R. Data are expressed as mean ± SEM, n= 3–15.
Based on the finding that polar substitutions at position 331 behaved like the WT P2X4R, we reasoned that D331 could play a key role as a hydrogen bond acceptor. If so, one would predict that mutating the negatively charged aspartic acid to positively charged arginine (D331R) or lysine (D331K) should produce a response similar to the non-polar substitutions, since neither of these residues are good hydrogen bond acceptors. In support of this prediction, we found that: 1) Replacing the aspartic acid with a positively charged residue (D331R or D331K) significantly reduced the magnitude of ethanol inhibition in these mutant P2X4Rs (Fig. 4) and 2) There was no significant difference in the reduction of magnitude of ethanol inhibition compared to the non-polar substitutions presented above (e.g., approximately 15% inhibition by 50 mM ethanol) (Fig. 4). These latter results suggest that side-chain properties are important determinants of ethanol action and/or modulation at position 331.
Interestingly, substituting N for D at position 331 eliminated the response of the receptor to 10 mM ethanol, but did not have an effect at higher ethanol concentrations. In contrast, other substitutions at position 331 had no effect on the response to 10 mM ethanol. Overall, this minimal and isolated effect suggests that D331 does not appear to play an important role at low ethanol concentrations.
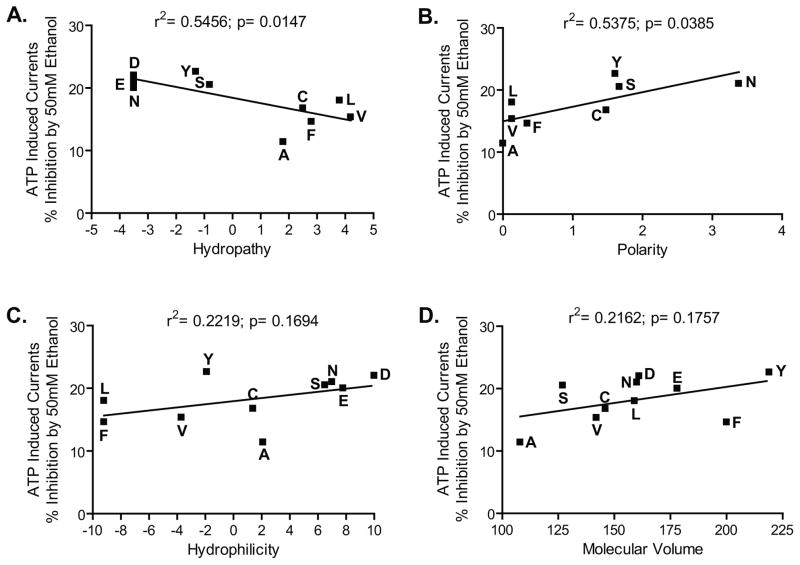
We used linear regression analyses to test for possible relationships between the responses to ethanol and the physical-chemical properties of the residue at position 331. For these analyses, we elected to use the data collected at 50 mM ethanol which represents a behaviorally intoxicating concentration. As illustrated in Fig. 5A, the analysis revealed a significant inverse correlation between hydropathy of the residue at position 331 and the % inhibition by ethanol. The slope of the linear regression was significantly different from 0, with r2 value of 0.55, indicating that hydropathy accounted for approximately 55% of the variance in ethanol inhibition. On the other hand, there was no significant relationship between hydrophilicity, molecular volume (Fig. 5C and 5D), polarity and the degree of ethanol inhibition at position 331.
Fig. 5. Correlation analyses of physico-chemical properties of the residues at position 331 and ethanol sensitivity.
The hydropathy and polarity of substitutions at position 331 are significantly correlated with sensitivity of P2X4Rs to ethanol. The physical-chemical properties of the residues substituted at position 331 A) Hydropathy, B) Polarity, C) Hydrophilicity and D) Molecular volume, respectively, were correlated against sensitivity to 50 mM ethanol.
Although the data suggests an obvious distinction between polar and non-polar substitutions at position 331, the linear regression analysis using a polarity scale did not reflect this effect. The lack of correlation may be due to the inclusion of charged residues which have a disproportionally greater separation on most polarity scales (Perkins D.I. et al. 2008), thereby skewing polarity correlations. To test this notion, we excluded the charged residues from the analysis, which then spreads out the scale, as shown in Fig. 5B. Excluding the charged residues led to a significant direct correlation between polarity and the % inhibition by ethanol. The r2 value from this analysis indicated that polarity accounted for approximately 54% of the variance in ethanol inhibition.
Ethanol response
Position 336 mutations
The majority of the amino acid substitutions at position 336 significantly reduced or eliminated the inhibitory effects of ethanol compared to WT at all ethanol concentrations (Fig. 6). To determine whether the mutations altered the mechanism of ethanol action in P2X4Rs (Yi et al. 2009), we performed ATP (0.5 – 100μM) concentration–response (C/R) studies in the absence and presence of 200 mM ethanol in WT and in the M336Q (ethanol insensitive) and M336A (ethanol sensitivity reduced) mutant P2X4Rs. As expected (Xiong et al. 2000), ethanol caused a parallel rightshift in the ATP concentration-response curve, significantly increasing the EC50 for ATP in WT P2X4Rs (Supplementary Fig. S2A). On the other hand, ethanol did not cause a significant change in the ATP concentration-response curve nor did it significantly alter the EC50 for ATP in the mutant receptors (Supplementary Fig. S2B and C). Moreover, there was no significant difference in Hill coefficient or maximal current response between the WT and mutant P2X4Rs. Taken together, the data suggest that these mutations reduce or eliminate the action of ethanol without altering the mechanism of ethanol action (Yi et al. 2009).
Fig. 6. Effects of ethanol (10–200mM) in mutant receptors at position 336 (M, methionine).
Amino acid substitutions with different physical-chemical properties at position 336 significantly reduce or eliminate the inhibitory effect of ethanol (10–200 mM). The bar graph illustrates the response to ethanol (10–200mM) of WT and mutant P2X4Rs. Polar substitutions at position 336 eliminated the effect of ethanol. Data are presented as mean ± SEM, n= 3–11. Inset: Representative tracings of ATP EC10-induced currents and effect of 50 mM, 200 mM and 100 mM ethanol on WT (upper trace) and mutant ([M336W] lower trace) P2X4Rs (note the lack of ethanol effect on ATP-gated currents in the mutant receptor). The horizontal bars depict when ATP (lower bar) and ethanol (upper bar) were applied to the oocyte. The dashed line illustrates the relative stability of the ATP EC10 over the duration of the experiment. Vertical scale bars are 0.2 and 0.1 μA respectively; horizontal scale bars represent 30 sec.
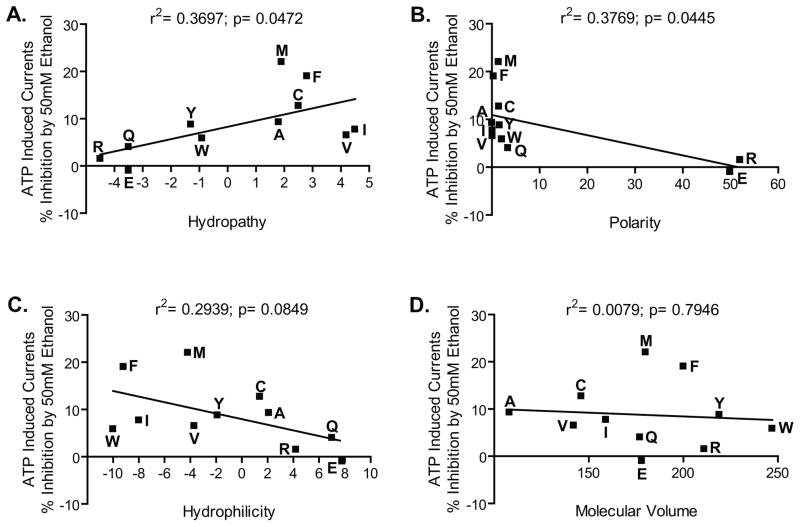
Using linear regression analyses to test for possible relationships between the responses to ethanol and the physical-chemical properties of the residue at position 336, revealed a significant correlation between hydropathy, polarity and the % inhibition by 50 mM ethanol (Fig. 7A and 7B). The r2 value from these analyses indicated that hydropathy accounted for approximately 37% while polarity accounted for approximately 38% of the variance in ethanol inhibition. On the other hand, there was no significant correlation between hydrophilicity and the degree of ethanol inhibition at position 336; however, a trend towards a notable relationship was observed (Fig. 7C). In addition, there was no significant correlation between the degree of ethanol inhibition and molecular volume at this position (Fig. 7D).
Fig. 7. Correlation analyses of physico-chemical properties of the residues at position 336 and ethanol sensitivity.
The hydropathy and polarity of substitutions at position 336 are significantly correlated with sensitivity of P2X4Rs to ethanol. The physical-chemical properties of the residues substituted at position 331 A) Hydropathy, B) Polarity, C) Hydrophilicity and D) Molecular volume, respectively, were correlated against sensitivity to 50 mM ethanol.
Substituting the large, non-polar methionine in WT P2X4Rs (M336), with a small, non-polar residue (M336A, M336V or M336C) significantly, and similarly, reduced the magnitude of ethanol inhibition (Fig. 6). This result indicates that the size of the residue at position 336 plays an important role in determining the magnitude of the ethanol effect. Further support for this conclusion is provided by the finding in which the substitution of the WT methionine with another large, non-polar residue, phenylalanine (M336F), did not significantly alter the response to ethanol. Interestingly, substituting the medium sized non-polar isoleucine at position 336 (M336I) reduced the response to ethanol in a similar fashion to substituting the small, non-polar residues (M336A, M336V and M336C). This latter finding indicates that position 336 requires a large residue for full response to ethanol and that this requirement does not appear to be graded.
Substituting the non-polar, large methionine in the WT receptor (M336), with a polar, large (non-charged) residue (M336Q or M336Y) significantly reduced the response to ethanol at 50 mM and eliminated the response to ethanol at higher concentrations. In addition, substituting methionine with polar, large (charged) residues (M336E or M336R) appeared to eliminate the sensitivity of the receptor to ethanol at all concentrations. It did not matter whether the substitution was positively or negatively charged.
DISCUSSION
P2X4Rs are widely distributed in the CNS and have been shown to be sensitive to ethanol when expressed in recombinant expression systems (Xiong et al. 2000;Davies et al. 2002;Xiong et al. 2005;Davies et al. 2005;Asatryan et al. 2008). However, little is known regarding their molecular targets for ethanol action. Findings from the current study significantly advance our knowledge on this issue by identifying two residues that when mutated reduced or eliminated the inhibitory effects of ethanol in P2X4Rs. Importantly, this work is the first to identify amino acid positions within P2X4Rs that are critical for ethanol action. Moreover, analyses of the data revealed a significant correlation between the physical-chemical properties of residues at positions 331 and 336 and the action of ethanol in P2X4Rs. Taken together, the results support the hypothesis that the ectodomain-TM2 interface contains residues that are important for the action of ethanol in P2X4Rs.
Alanine mutagenesis in the ectodomain-TM1 interface region resulted in minimal changes in ethanol response in these mutant P2X4Rs suggesting that this region may not be important for the action of ethanol. On the other hand, alanine mutagenesis in the ectodomain-TM2 interface region identified two residues, D331 and M336 that when mutated significantly reduced the effects of ethanol suggesting that this region is important for the action of ethanol in P2X4Rs.
Changes in ethanol response observed with the D331A and M336A mutant P2X4Rs were concentration-dependent and similar in magnitude at higher ethanol concentrations. However, between the two sites, there were subtle differences in sensitivity at lower ethanol concentrations. At 10 mM ethanol, the M336A mutant receptor was insensitive to ethanol, whereas the D331A mutant receptor was WT-like. Overall, these initial findings suggest that position 336, but not position 331, is important for the modulatory effects of ethanol at lower ethanol concentrations. The results from the D331A-M336A double mutant P2X4R add support for this notion as it was shown that the response of the double mutant to 10 mM ethanol was similar to that of the single M336A mutant.
In contrast, the modulatory effects of ethanol at higher ethanol concentrations on the D331A-M336A double mutant were similar to those of either of the single D331A and M336A mutant P2X4Rs. The lack of significant difference in the modulatory effects of ethanol between the double mutant and either single mutant at higher ethanol concentrations suggests that these sites do not act independently from each other (i.e., not additive) and that either site can produce a similar effect of ethanol. Taken together, the differences in ethanol modulation between low and high ethanol concentrations suggest that there may be multiple sites of ethanol action within the ectodomain-TM2 region of P2X4Rs.
Ethanol studies focusing on other LGICs support the hypothesis that there can be multiple sites of ethanol action within a protein structure. In glycine receptors, sites important for the action of ethanol have been reported in the TM domain (Mihic et al. 1997;Wick et al. 1998;Ye et al. 1998;Yamakura et al. 1999;Lobo et al. 2008) and in loop 2 of the extracellular domain (Mascia et al. 1996;Davies and Alkana 2003;Davies et al. 2004;Crawford et al. 2007;Perkins D.I. et al. 2008;Perkins et al. 2009). Further studies using a sulfhydryl-specific reagent demonstrated that the two sites are part of the same alcohol binding pocket (Crawford et al. 2007). Ethanol studies in other LGICs, such as GABAAR (Wick et al. 1998;Ueno et al. 2000) and nAChsR (Borghese et al. 2003), also support the hypothesis that ethanol may have multiple targets with different responses in the same receptor protein.
Substitutions of residues with different physical-chemical properties at positions 331 and 336 revealed a significant correlation between the properties of the residues and the action of ethanol in P2X4Rs. At position 331 the presence of a polar, negatively charged or neutral, amino acid residue was required to maintain the full response of ethanol. On the other hand, the presence of a polar, positively charged residue (e.g., arginine or lysine) or a non-polar residue (e.g., alanine or valine) caused a significant reduction in the degree of ethanol modulation compared to WT P2X4Rs. This latter finding suggests that the site forms a hydrogen bond with the ethanol molecule and the aspartic acid residue at position 331 may participate as a hydrogen bond acceptor.
At position 336 the presence of a large, non-polar residue appeared to be required to maintain a full response to ethanol in P2X4Rs, whereas substitution with polar residues at position 336 abolished the modulatory effect of ethanol. Interestingly, substituting tryptophan, which is generally considered to be a large, non-polar residue, reduced the response to ethanol in a similar manner to the large polar substitutions. On the surface, this finding appears to contradict the notion that a large non-polar substitution at position 336 should yield a receptor that responds to ethanol like a WT P2X4R. However, the electron pair on the indole nitrogen allows it to form a hydrogen bond and causes it to behave as a polar amino acid (Perkins D.I. et al. 2008). In the current study this appeared to be the case as it was shown that substituting tryptophan for methionine behaved similarly to the polar mutants in regards to ethanol sensitivity.
When using mutagenesis as a strategy to investigate potential sites of drug action, one should consider the possibility that the changes caused by the mutations could be a result of an effect on the protein conformation and/or a transduction pathway. Modeling work in P2X4Rs suggests that an ectodomain segment located before the TM2 domain (linker region) is organized as an α-helix and acts as an elastic spring that could operate as a signal transduction module (Yan et al. 2006).
Based on the recently published P2X4 crystal structure (Kawate et al. 2009), it appears that position 331 is located in the linker region close to the TM2 domain and that position 336 is close to that region. Therefore, one would expect to see significant changes in agonist EC50 or Imax if either position (331 or 336) were involved in the transduction of the agonist binding. Because mutations at position 331 or 336 reduced or eliminated the effects of ethanol without significantly altering ATP EC50 or Imax it is unlikely that these sites are located in the agonist transduction pathway. This logic is further supported by the fact that the amino acids found at positions 331 and 336 are not conserved across the family of P2XRs. Finally, the mutations did not alter the maximal response of ATP in the presence or absence of ethanol ruling out the possibility that ethanol insensitivity was due to changes in the mechanism of ethanol action (Yi et al. 2009). Taken together, the results suggest that positions 331 and 336 play a direct role in the modulatory effects of ethanol.
Although in the early stages, building evidence supports a role for purinergic P2XRs in the physiological and behavioral effects of ethanol. However, there remains a paucity of knowledge regarding the molecular targets and actions of ethanol in P2XRs. Due, in part to the lack of pharmacological antagonists, we have taken an alternative approach to this problem by focusing on the development of mutant P2XRs that do not respond to ethanol. Identification of mutant receptors that can abolish the effects of ethanol without markedly changing agonist response represents the first step in identifying the roles of P2X4Rs in the physiological and behavioral effects of ethanol. As a second step in this process we have recently begun to utilize a recombinant lentiviral delivery system and using this system we have preliminary evidence showing that ATP currents of P2X4Rs expressed in hippocampal neurons are inhibited by ethanol. Future investigations will utilize ethanol insensitive receptors in combination with the lentiviral delivery system and knock-in and knockout animals to delineate the role of P2X4Rs in ethanol-induced behaviors.
Supplementary Material
Acknowledgments
We thank Daya Perkins for her many helpful scientific discussions, Miriam Fine and Rachel Kelly for mutagenesis assistance. This work was supported by research grants NIAAA/NIH F31 AA017029 (M.P.), AA013890 (D.L.D), KO1 AA017243 (L.A.), AA03972 (R.L.A.) and the USC School of Pharmacy.
This work was conducted as partial fulfillment of the requirements for the PhD degree in Pharmaceutical Sciences, University of Southern California (M.P.)
Portions of these findings were presented at the Annual Meeting of the Research Society on Alcoholism in Washington, DC (Alcohol: Clin. Exp. Res., Vol. 32, Issue 6, p. 211A, 2008) and at the Purines Meeting in Copenhagen, Denmark (Purinergic Signaling, Vol. 4, Issue 5, p. 206, 2008).
Abbreviations
- LGIC
ligand-gated ion channel
- nACh receptor
nicotinic acetylcholine receptor
- WT
wild type
- TM
transmembrane
- EC
effective concentration
- P2XR
P2X receptor
References
- Asatryan L, Popova M, Woodward JJ, King BF, Alkana RL, Davies DL. Roles of ectodomain and transmembrane regions in ethanol and agonist action in purinergic P2X2 and P2X3 receptors. Neuropharmacology. 2008;55:835–843. doi: 10.1016/j.neuropharm.2008.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Henderson LA, Bleck V, Trudell JR, Harris RA. Sites of excitatory and inhibitory actions of alcohols on neuronal a2b4 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;307:42–52. doi: 10.1124/jpet.102.053710. [DOI] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Suprenant A. An antagonist insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Illes P. P2X receptors and Nociception. Pharmacol Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- Crawford DK, Trudell JR, Bertaccini E, Li KX, Davies DL, Alkana RL. Evidence that ethanol acts on a target in Loop 2 of the extracellular domain of alpha1 glycine receptors. J Neurochem. 2007;102:2097–2109. doi: 10.1111/j.1471-4159.2007.04680.x. [DOI] [PubMed] [Google Scholar]
- Davies DL, Alkana RL. Benzodiazepine agonist and inverse agonist coupling in GABAA receptors antagonized by increased atmospheric pressure. Eur J Pharmacol. 2003;469:37–45. doi: 10.1016/s0014-2999(03)01733-3. [DOI] [PubMed] [Google Scholar]
- Davies DL, Crawford DK, Trudell JR, Mihic SJ, Alkana RL. Multiple sites of ethanol action in a1 and a2 glycine receptors suggested by sensitivity to pressure antagonism. J Neurochem. 2004;89:1175–1185. doi: 10.1111/j.1471-4159.2004.02390.x. [DOI] [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology. 2005;49:243–253. doi: 10.1016/j.neuropharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Davies DL, Machu TK, Guo Y, Alkana RL. Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcohol Clin Exp Res. 2002;26:773–778. [PubMed] [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NJ, Parson SH, Deuchars J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2002;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou P, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991 – 1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci. 2002;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Kim M, Spelta V, Bo X, Surprenant A, North RA. Subunit arrangement in P2X receptors. J Neurosci. 2003;23:8903–8910. doi: 10.1523/JNEUROSCI.23-26-08903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Ethanol-induced inhibition of a neuronal P2X purinoceptor by an allosteric mechanism. Br J Pharmacol. 1998;123:1–3. doi: 10.1038/sj.bjp.0701599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiong K, Weight FF. Ethanol inhibition of adenosine 5′-triphosphate-activated current in freshly isolated adult rat hippocampal CA1 neurons. Neurosci Lett. 2000;295:77–80. doi: 10.1016/s0304-3940(00)01586-x. [DOI] [PubMed] [Google Scholar]
- Lobo IA, Harris RA, Trudell JR. Cross-linking of sites involved with alcohol action between transmembrane segments 1 and 3 of the glycine receptor following activation. J Neurochem. 2008;104:1649–1662. doi: 10.1111/j.1471-4159.2007.05090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996;50:402–406. [PubMed] [Google Scholar]
- McGinnis JM, Foege WH. Mortality and morbidity attributable to use of addictive substances in the United States. Proc Assoc Am Physicians. 1999;111:109–118. doi: 10.1046/j.1525-1381.1999.09256.x. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Mori M, Heuss C, Gahwiler BH, Gerber U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J Physiol. 2001;535:115–123. doi: 10.1111/j.1469-7793.2001.t01-1-00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Papp L, Vizi ES, Sperlagh B. Lack of ATP-evoked GABA and glutamate release in the hippocampus of P2X7 receptor−/− mice. Neuroreport. 2004;15:2387–2391. doi: 10.1097/00001756-200410250-00017. [DOI] [PubMed] [Google Scholar]
- Parker JMR, Guo D, Hodges RS. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and x-ray-derived accessible sites. Biochemistry. 1986;25:5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. Targets for ethanol action and antagonism in loop 2 of the extracellular domain of glycine receptors. J Neurochem. 2008;106:1337–1349. doi: 10.1111/j.1471-4159.2008.05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Asatryan L, Alkana RL, Davies DL. Loop 2 Structure in Glycine and GABAA Receptors Plays a Key Role in Determining Ethanol Sensitivity. J Biol Chem. 2009;284:27304–27314. doi: 10.1074/jbc.M109.023598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Honse Y, Peoples RW. A site of alcohol action in the fourth membrane- associated domain of the NMDA receptor. J Biol Chem. 2003;49:48815–48820. doi: 10.1074/jbc.M302097200. [DOI] [PubMed] [Google Scholar]
- Ronald KM, Mirshahi T, Woodward JJ. Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalaine residue. J Biol Chem. 2001;276:44729–44735. doi: 10.1074/jbc.M102800200. [DOI] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor clonned from rat brain. Proc Natl Acad Sci USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R, Thomas S, Rassendren F, Kawashima E, Buell G, Surprenant A, North R. Contribution of individual subunits to the multimeric P2X2 receptor: Estimates based on methanethiosulfonate block at T336C. Mol Pharmacol. 1999;56:973–981. doi: 10.1124/mol.56.5.973. [DOI] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. Hetero-oligomeric Assembly of P2X Receptor Subunits. SPECIFICITIES EXIST WITH REGARD TO POSSIBLE PARTNERS. J Biol Chem. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- Ueno S, Lin A, Nikolaeva N, Trudell JR, Mihic SJ, Harris RA, Harrison NL. Tryptophan scanning mutagenesis in TM2 of the GABAA receptor a subunit: effects on channel gating and regulation by ethanol. Br J Pharmacol. 2000;131:296–302. doi: 10.1038/sj.bjp.0703504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR. Alcohol abuse and alcoholism: an overview. J Clin Psychiatry. 2001;62:4–10. [PubMed] [Google Scholar]
- Wick MJ, Mihic SJ, Ueno S, Mascia MP, Trudell JR, Brozowski SJ, Ye Q, Harrison NL, Harris RA. Mutations of g-aminobutyric acid and glycine receptors change alcohol cutoff: Evidence for an alcohol receptor? Proc Natl Acad Sci USA. 1998;95:6504–6509. doi: 10.1073/pnas.95.11.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhou C, Li K, Davies DL, Ye JH. Purinergic Type 2 Receptors at GABAergic Synapses on Ventral Tegmental Area Dopamine Neurons Are Targets for Ethanol Action. J Pharmacol Exp Ther. 2008;327:196–205. doi: 10.1124/jpet.108.139766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Hu XQ, Stewart RR, Weight FF, Li C. The mechanism by which ethanol inhibits rat P2X4 receptors is altered by mutation of histidine 241. Br J Pharmacol. 2005;145:576–586. doi: 10.1038/sj.bjp.0706192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Li C, Weight FF. Inhibition by ethanol of rat P2X4 receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;130:1394–1398. doi: 10.1038/sj.bjp.0703439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J Neurophysiol. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Mihic SJ, Harris RA. Amino Acid Volume and Hydropathy of a Transmembrane Site Determine Glycine and Anesthetic Sensitivity of Glycine Receptors. J Biol Chem. 1999;274:23006–23012. doi: 10.1074/jbc.274.33.23006. [DOI] [PubMed] [Google Scholar]
- Yan Z, Liang Z, Obsil T, Stojilkovic SS. Participation of the Lys313-Ile333 Sequence of the Purinergic P2X4 Receptor in Agonist Binding and Transduction of Signals to the Channel Gate. J Biol Chem. 2006;281:32649–32659. doi: 10.1074/jbc.M512791200. [DOI] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, Harris RA. Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position a267. J Biol Chem. 1998;273:3314–3319. doi: 10.1074/jbc.273.6.3314. [DOI] [PubMed] [Google Scholar]
- Yi CL, Liu YW, Xiong KM, Stewart RR, Peoples RW, Tian X, Zhou L, Ai YX, Li ZW, Wang QW, Li C. Conserved extracellular cysteines differentially regulate the inhibitory effect of ethanol in rat P2X4 receptors. Biochem Biophys Res Commun. 2009;381:102–106. doi: 10.1016/j.bbrc.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Eliezer N, Simha R. The characterization of amino acid sequences in proteins by statistical methods. J Theor Biol. 1968;21:170–201. doi: 10.1016/0022-5193(68)90069-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.