Abstract
Chronic use of marijuana impairs synaptic plasticity and cognitive function. However, the molecular mechanisms by which marijuana alters long-term synaptic plasticity are largely unknown. Here we show that repeated in vivo exposures to Δ9-THC for 7 consecutive days significantly impaired hippocampal long-term potentiation (LTP) of excitatory glutamatergic synaptic transmission. The Δ9-THC exposure-induced decrease in LTP was prevented by pharmacological inhibition or deletion of the CB1 receptor. To determine the molecular mechanisms underlying Δ9-THC-altered LTP, we targeted expression and function of the glutamate receptors and phosphorylation status of cAMP response element binding protein (CREB). Chronic in vivo exposure to Δ9-THC produced CB1 receptor-dependent decreases in expression of hippocampal glutamate receptor subunits GluR1, NR2A and NR2B, the ratio of AMPA/NMDA receptor-gated currents, and phosphorylation of CREB. Our results suggest that reduced expression and function of the glutamate receptor subunits and phosphorylation of CREB may underlie the impaired long-term synaptic plasticity induced by repeated in vivo exposure to Δ9-THC.
Keywords: Marijuana, long-term potentiation, phosphorylation, cannabinoids, CB1 receptor, AMPA receptors, NMDA receptors
Introduction
Marijuana is the most commonly used illicit drug of abuse in the United States. The use of marijuana is also associated with taking other addictive substances such as alcohol, cocaine, morphine or nicotine (Maldonado et al., 2006). It has been long known that chronic use of marijuana induces cannabis-related disorders (CRDs) and impairs cognitive function. These cannabis-related disorders also include tolerance, dependence and possibly addiction (Heishman et al., 1997; Hampson and Deadwyler, 1999; Pope et al., 2001; 2002; Bolla et al., 2002; Solowij et al., 2002; Sim-Selley, 2003; Martin et al., 2004; Kelly and Thayer, 2004; Lupica et al., 2004; Martin, 2005; Lichtman and Martin, 2005). Currently, there are no effective medications available for the treatment of CRDs. This is largely due to our limited understanding of the mechanisms by which marijuana alters synaptic function and induces adaptive changes in neural circuitry.
It is now recognized that marijuana-induced effects are mainly mediated by Δ9-tetrahydrocannabinol (Δ9-THC), the major psychoactive ingredient in marijuana (Gaoni & Mechoulam, 1964; 1971). The actions of Δ9-THC on synaptic activity result likely from its binding to the cannabinoid receptor CB1 (CB1), which was molecularly cloned from rat brain in 1990 (Matsuda et al., 1990), and is predominantly expressed in the central nervous system. The discovery of the cannabinoid receptors prompted the search for endogenous cannabinoids (eCBs). Several endogenous ligands have been identified, which are involved in a variety of physiological and pathological processes (Wilson & Nicoll, 2002; Alger, 2002; Piomelli, 2003; Freund et al., 2003; Sugiura et al., 2006; Mackie, 2006; Howlett et al., 2004; Di Marzo et al., 2004; Walter & Stella, 2004, Bisogno et al., 2005; Mackie, 2006; Chevaleyre et al., 2006; Frazier, 2007; Lovinger, 2008). It has been proposed that the downregulation, desensitization, and altered trafficking of the CB1 receptor, the decoupling the CB1 receptor and its associated G protein (Gi/o), and the changes in eCB content are likely responsible for tolerance and dependence to cannabinoids after prolonged administration of marijuana (Breivogel et al., 1999; Bass and Martin, 2000; Rubino et al., 2000; Sim-Selley, 2003; Martin et al., 2004; Mato et al., 2004; Lupica et al., 2004; Piomelli, 2004; Martin, 2005; Lichtman and Martin, 2005; González et al., 2005; Childers, 2006). Since tolerance or dependence reflects reduced synaptic responsiveness to cannabinoids, the Δ9-THC-altered synaptic plasticity and tolerance to cannabinoids may likely share the same signaling mechanisms. Recent evidence shows that single in vivo exposure to Δ9-THC blocks eCB-mediated long-term depression of excitatory synaptic transmission (eCB-LTD) in the nucleus accumbens (NAc) and eCB-mediated LTD of inhibitory synaptic transmission (I-LTD) in the hippocampus (Mato et al., 2004). Repeated in vivo exposures to Δ9-THC for 7 days have been shown to eliminate LTD in the nucleus accumbens (Hoffman et al., 2003; Mato et al., 2005) and LTP in the hippocampal CA1 area (Hoffman et al., 2007). However, the mechanisms underlying the marijuana- or Δ9-THC-induced alterations in long-term synaptic plasticity are still not well understood.
In this report, we show that repeated in vivo exposures to Δ9-THC induced a CB1 receptor-dependent decrease in hippocampal LTP. The reduced expression and function of the glutamate receptors and phosphorylation of CREB may be molecular mechanisms underlying the Δ9-THC-altered long-term synaptic plasticity in the hippocampus.
Materials and Methods
Animals
C57BL/6 (Charles River, Wilmington, MA) and CB1 knockout (KO) mice (cnr1(−/−), NIMH transgenic core, NIH, Bethesda, MD) at ages of 6 to 9 weeks were used in the present study. CB1R KO mice were backcrossed for more than ten generations onto the C57BL/6 background strain. Breeding of heterozygous mice produced homozygous CB1R wild type (WT) and KO mice. Age-matched littermates (either sex) were used in all the studies. The care and use of the animals reported in this study were approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center. Mice were intraperitoneally (i.p.) injected with vehicle, Δ9-THC (10 mg/kg, provided by NIDA Drug Supply Program, NIH), and SR141716 (5 mg/kg, provided by NIMH Chemical Synthesis and Drug Supply Program, NIH). Animals received repeated administrations of Δ9-THC once a day for 7 consecutive days. Δ9-THC was prepared from a solution at concentration of 200 mg/ml in ethanol, and suspended in an equivalent volume of DMSO by evaporating ethanol under N2 gas and diluted to 2 mg/ml in Tween 80 (10%), DMSO (20%), and saline (70%) as described by Hoffman et al. ((2003; 2007).
Hippocampal slice preparation
Hippocampal slices were prepared from mice as described previously (Chen et al., 2002; Chen, 2004; Yang et al., 2008). Briefly, after decapitation, brains were rapidly removed and placed in cold oxygenated (95% O2, 5% CO2) low-Ca2+/high-Mg2+ slice medium composed of (in mM) 2.5 KCl, 7.0 MgCl2, 28.0 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 7.0 glucose, 3.0 pyruvic acid, 1.0 ascorbic acid, and 234 sucrose. Slices were cut at a thickness of 350–400 µm and transferred to a holding chamber in an incubator containing oxygenated artificial cerebrospinal fluid (ACSF) composed of (in mM) 125.0 NaCl, 2.5 KCl, 1.0 MgCl2, 25.0 NaHCO3, 1.25 NaH2PO4, 2.0 CaCl2, 25.0 glucose, 3 pyruvic acid, and 1 ascorbic acid at 36 °C for 0.5 to 1 hour, and maintained in an incubator containing oxygenated ACSF at room temperature (~22–24 °C) for >1.5 h before recordings. Slices were then transferred to a recording chamber where they were continuously perfused with 95% O2, 5% CO2-saturated standard ACSF at ~32–34 °C. Individual dentate granule neurons were viewed with an upright microscope (Olympus BX51WI) fitted with a 60×water-immersion objective and differential interference contrast (DIC) optics.
Electrophysiological recordings
Field EPSP (fEPSP) recordings in response to stimulation of the perforant path at a frequency of 0.05 Hz were made using an Axoclamp-2B patch-clamp 6 amplifier (Molecular Devices, CA) in bridge mode. Recording pipettes were pulled from borosilicate glass with a micropipette puller (Sutter Instrument), filled with artificial ACSF (2–4 MΩ) and placed at the middle one third of the molecular layer of the dentate gyrus. Hippocampal perforant path LTP was induced by a theta-burst stimulation (TBS), consisting of a series of 10 bursts of 5 stimuli at 100 Hz (200 ms interburst interval, which was repeated three time (Chevaleyre & Castillo, 2004; Hoffman et al., 2007; Yang et al., 2009). The input-output function was tested before recording of LTP, and the baseline stimulation strength was set to provide fEPSP with an amplitude of ~30% from the subthreshold maximum derived from the input-output function. The bath-perfused solutions contained 10 µM bicuculline to block ionotropic GABA receptors.
Whole cell patch-clamp recordings were made under voltage clamp using an Axopatch-200B amplifier to determine excitatory postsynaptic currents (EPSCs) in dentate granule neurons in response to stimulation of the perforant path. The membrane potential was held at +40 mV to eliminate Mg2+-induced blockade of the NMDA receptors. Stimuli were elicited via a bipolar tungsten electrode placed in the molecular layer of the dentate gyrus. Recording pipettes (4–5 MΩ) were filled with the internal solution containing (in mM) 115.0 Cs gluconate, 15.0 CsCl, 5.0 NaCl, 0.28 CaCl2, 10.0 HEPES, 0.5 EGTA, 4.0 Mg2ATP, 0.2 Na2GTP and 3.0 QX-314 (pH 7.25 with CsOH). Bicuculline (10 µM) was included in the bath solution to block ionotropic GABA receptors. D-AP5 (50 µM, provided by NIH NIMH Drug Supply Program) was applied via the bath perfusion to block the glutamate NMDA receptor-gated current. The remaining AMAP-gated current could be eliminated by DNQX (10 µM). A paired-pulse protocol with varying interpulse intervals was used to determine paired-pulse ratio (PPR, calculated as P2/P1: P1, the amplitude of the first EPSC; P2, the amplitude of the second EPSC).
Spontaneous EPSCs (sEPSCs) were recorded in granule neurons in hippocampal slices of animals that chronically received vehicle or Δ9-THC for 7 days. The frequency, amplitude, and kinetics of sEPSCs were analyzed using the MiniAnalysis program (Synaptosoft, Fort Lee, NJ). The bath solution contained bicuculline (10 µM).
RNA isolation and DNase treatment
Total RNA was prepared from harvested hippocampal tissue with the RNeasy Mini Kit (Qiagen) and treated with RNase-free DNase (Qiagen) according to the manufacturer’s instructions. The RNA concentration was measured by spectrophotometer (DU 640; BECKMAN). RNA integrity was verified by electrophoresis in a 1% agarose gel.
Reverse transcription and real-time RT-PCR
The iscript cDNA synthesis kit (BioRad) was used for the reverse transcription reaction. We used 1 µg total RNA, with 4 µl 5× iscript reaction mix and 1 µl iscript reverse transcriptase. The total volume was 20 µl. Samples were incubated for 5 min at 25 °C. All samples were then heated to 42 °C for 30 min, and reactions were stopped by heating to 85 °C for 5 min.
Real-time RT-PCR specific primers for GluR1, GluR2, NR1, NR2A, NR2B, and GAPDH were selected using Beacon Designer Software (BioRad) and synthesized by IDT (Coralville, IA). They are listed as follows: Name: forward primer, reverse primer (amplicon size), genebank number: GluR1: 5’-CGAGAAGAACTGGCAGGTGAC-3’, 5’-CAGGTTGGCGAGGATGTAGTG-3’ (202 bp, BC056397), GluR2: 5’- CATCTCCTCCTACACGGCTAAC-3’, 5’-TCCTGACTCTGGCTACTCCTTC-3’ (248bp, AB111957), NR1: 5’-GCGACACGGCTCTTGGAAG-3’, 5’- GTGGTACGGTGCGAAGGAAG-3’ (275 bp, BC039157), NR2A: 5’- GGGAGGGATGAAGGCTGTAAAC-3’, 5’-GATGAAGGTGATGAGGCTGAGG-3’ (291 bp, NM_008170), NR2B: 5 ’-TCCGCAGCACTATTGAGAACAG-3’, 5’-GAAGGCACCGTGTCCGTATC-3’ (256 bp, NM_008171), and GAPDH: 5-ACCACAGTCCATGCCATCAC-3’, 5-ACCTTGCCCACAGCCTTG-3’ (134 bp, M32599). The PCR amplification of each product was further assessed using 10-fold dilutions of mouse brain cDNA library as a template and found to be linear over five orders of magnitude and at greater than 95% efficiency. All the PCR products were verified by sequencing. The reactions were set up in duplicate in total volumes of 25 µl containing 12.5 µl 2× iQSYBR green Supermix (BioRad) and 5 µl template (1:10 dilution from RT product) with a final concentration of 400 nM of the primer. The PCR cycle was as follows: 95 °C/3 min, 45 cycles of 95 °C/30 sec, 58 °C/45 sec and 95 °C/1 min, and the melt-curve analysis was performed at the end of each experiment to verify that a single product per primer pair was amplified. Furthermore, the sizes of the amplified DNA fragments were verified by gel electrophoresis on a 3% agarose gel. The amplification and analysis were performed using an iCycler iQ Multicolor Real-Time PCR Detection System (BioRad). Samples were compared using the relative CT method. The fold increase or decrease was determined relative to a vehicle-treated control after normalizing to a housekeeping gene using 2−ΔΔCT, where ΔCT is (gene of interest CT) - (GAPDH CT), and ΔΔCT is (ΔCT treated) - (ΔCT control), as described previously (Sang et al., 2005; 2007; Zhang & Chen, 2008). The ranges of CT for GAPDH were from 17.6 to 18.1 (17.9 ± 0.1, n=6) for the vehicle control and 17.6 to 17.9 (17.8 ± 0.1, n=6) for the treatment with Δ9-THC.
Surface biotinylation assay
Surface biotinylation assays were performed in hippocampal slices as described previously (Yu et al., 2008). Briefly, hippocampal slices were cut at thickness of 400 µm using a vibratome (St. Louis, MO) from mice that received vehicle or Δ9-THC for 7 consecutive days, and then transferred to a six-well plate and incubated on ice for 1 h in carbogenated ACSF containing 500 µM Sulfo-NHS-SS-biotin (Thermo-Pierce, Rockford, IL). Collected tissue was then washed three times for 5 min with ice-cold ACSF containing 10 mM glycine, and was immediately homogenized in 800 µL ice-cold lysis buffer containing 20 mM Tris–HCl, pH 7.5, 1% Triton X-100, 50 mM NaCl, 1 mM EDTA, 0.1% sodium dodecyl sulfate, and a cocktail of protease inhibitors (Sigma, St. Louis, MO). After incubation on ice for 30 min, the homogenate was centrifuged at 18000 g at 4°C for 10 min. Supernatants were collected. The biotinylated proteins from 300 µg of total protein in the lysate were precipitated with 60 µL of Ultra-link immobilized Streptavidin beads (Thermo-Pierce, Rockford, IL), diluted with the addition of 800 µL lysis buffer, on a rotator overnight at 4°C. Precipitates were collected by centrifuging at 3500 g for 1 min, washed by lysis buffer for three times, and then boiled for 5 min in 30 µL 2x sample buffer. 30 µg tissue lysates were used as controls for the total protein.
Cross-linking
Extracelluar cross-linking of proteins was performed as described previously (Chen et al., 2006) by removing the brain from vehicle or Δ9-THC exposed mice. Slices (400 µm) were cut and placed in oxygenated ACSF buffer. Slices were then removed and placed in 2 ml buffer on ice to which 2 mg/ml of BS3 (Bis (Sulfosuccinimidyl) suberate, Thermo-Pierce, Rockford, IL) was added, and incubated for 45 minutes. The reaction was stopped by removing the buffer and replacing with cold 20 mM Tris pH 7.6. The samples were washed three times and placed in lysis buffer. The samples were sonicated and aliquots taken for protein analysis.
Western blots
Western blot assay was conducted to determine expression of the glutamate receptor subunits and phosphorylation of CREB in the hippocampus as described previously (Chen et al., 2006; Sang et al., 2007; Zhang and Chen, 2008). Hippocampal tissue was extracted and immediately homogenized in RIPA lysis buffer and protease inhibitors, and incubated on ice for 30 min, then centrifuged for 10 min at 10,000 rpm at 4°C. Supernatants were fractionated on 4–15% SDS-PAGE gels (Bio-Rad) and transferred onto PVDF membranes (Bio-Rad). The membrane was incubated with, anti-GluR1 (1:200), GluR2 (1:1,000), NR1 (1:500), NR2A and NR2B (1:1,000, Millipore, Temecula, Ca) and anti-CREB and anti-phospho-CREB antibodies (1:1,000, Cell Signaling, Danvers, MA) at 4°C overnight. The blots were washed and incubated with a secondary antibody (goat anti-rabbit 1:2,000, Cell Signaling, Danvers, MA) at room temperature for 1 hr. Proteins were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences, UK). The densities of specific bands were quantified by densitometry using FUJIFILM Multi Gauge software (version 3.0). Band densities were normalized to the total amount of protein loaded in each well as determined by mouse anti β-actin (1:4000, Sigma).
Data analysis
Data are presented as mean ± S.E.M. Unless stated otherwise, Student’s t-test and analysis of variance (ANOVA) with Fisher’s PLSD test or Student-Newman-Keuls test were used for statistical comparison when appropriate. Differences were considered significant when P< 0.05.
Results
Chronic in vivo exposure to Δ9-THC impairs hippocampal LTP
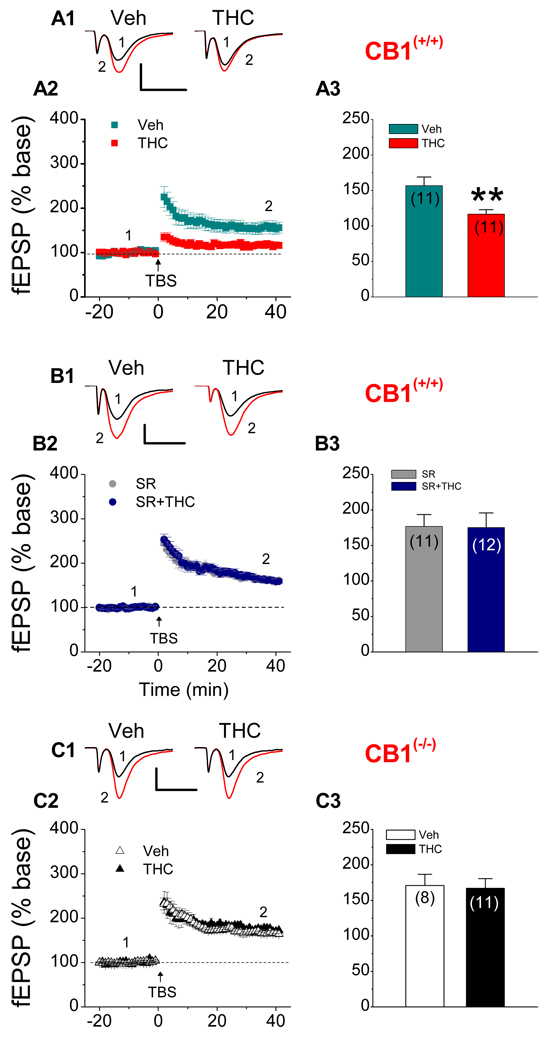
Marijuana or Δ9-THC has been demonstrated to induce adverse mental, psychotic and cognitive consequence (Heishman et al., 1997; Hampson and Deadwyler, 1999; Pope et al., 2001; 2002; Bolla et al., 2002; Solowij et al., 2002). However, very few studies were carried out to determine the effect of marijuana or Δ9-THC on hippocampal long-term synaptic plasticity from in vivo exposed animals. Recent evidence shows that repeated in vivo exposure to Δ9-THC eliminates hippocampal LTP at Schaffer-collateral synapses in the CA1 region (Hoffman et al., 2007). However, the mechanisms by which Δ9-THC impairs synaptic plasticity are largely unknown. Thus, we decided to determine LTP of glutamatergic excitatory synaptic transmission at hippocampal perforant path synapses in hippocampal slices from mice that received repeated in vivo exposure to Δ9-THC for 7 consecutive days. Field extracellular EPSPs (fEPSPs) were recorded 24 hrs after cessation of last injection, and LTP was induced by a theta-burst stimulation (TBS) protocol in the presence of bicuculline (10 µM). We found that repeated in vivo exposure to Δ9-THC in WT mice at dosage of 10 mg/kg, which is adopted by others (Zhuang et al., 1998; Bass & Martin, 2000; Hoffman et al., 2003; 2007), led to a significant reduction of perforant path LTP when compared that of the vehicle control (Figure 1A). This is consistent with the observations that repeated in vivo exposure to Δ9-THC eliminates LTP in the hippocampal CA1 region (Hoffman et al., 2007).
Figure 1.
Repeated in vivo exposures to Δ9-THC induce a CB1 receptor-dependent decrease in hippocampal LTP. A1. Representative traces of fEPSPs recorded before (1) and after (2) TBS in hippocampal slices from CB1R wild-type (WT) animals that received vehicle or Δ9-THC (10 mg/kg) once a day for 7 consecutive days. LTP was measured 24 hrs after cessation of last injection. A2. Time course of the Δ9-THC-induced changes in fEPSP slopes. A3. Mean values of the potentiation of fEPSPs averaged from 36 to 40 min following TBS (Veh: 11 recordings/6 animals, THC: 11 recordings/6 animals). **P<0.01 compared with the vehicle control. B1. Representative traces of fEPSPs recorded before (1) and after (2) TBS in hippocampal slices from CB1R WT animals that received repeated injections with SR141716 (SR, 5 mg/kg) or SR plus Δ9-THC (10 mg/kg) for 7 consecutive days. B2. Time course of the Δ9-THC-induced changes in fEPSP slopes. B3. Mean values of the potentiation of fEPSPs averaged from 36 to 40 min following TBS (SR: 11 recordings/5 animals, SR+THC: 12 recordings/6 animals). C1. Representative traces of fEPSPs recorded before (1) and after (2) TBS in hippocampal slices from CB1R knockout (KO) mice that received vehicle or Δ9-THC (10 mg/kg) once a day for 7 consecutive days. LTP was measured 24 h after cessation of injections. C2. Time course of the Δ9-THC-induced changes in fEPSP slopes. C3. Mean values of the potentiation of fEPSPs averaged from 36 to 40 min following TBS (Veh: 8 recordings/6 animals, THC: 11 recordings/6 animals). Scale bar: 0.3 mV/10 msec.
Δ9-THC-induced decrease in LTP is mediated via the CB1 receptor
It has been well established that the action of Δ9-THC on synaptic activity is mediated via the CB1 receptor. To determine whether the Δ9-THC-induced reduction of LTP is mediated via the CB1 receptor, we first administrated SR141716 (SR), a selective CB1 antagonist, in WT animals that received repeated injections of Δ9-THC. As shown in Figure 1B, while SR slightly elevated LTP, the Δ9-THC-induced decrease in hippocampal LTP was prevented in animals that received Δ9-THC + SR (5 mg/kg, i.p.). To further confirm the mediation of the CB1 receptor in the Δ9-THC-induced impairment in long-term synaptic plasticity, we employed mice deficient in the CB1 receptor. As expected, the Δ9-THC-induced decrease in LTP was also absent in CB1 KO animals (Figure 1C). These results indicate that chronic in vivo Δ9-THC exposure-reduced LTP is mediated via the CB1 receptors.
Repeated exposures to Δ9-THC reduce expression and function of the glutamate receptors
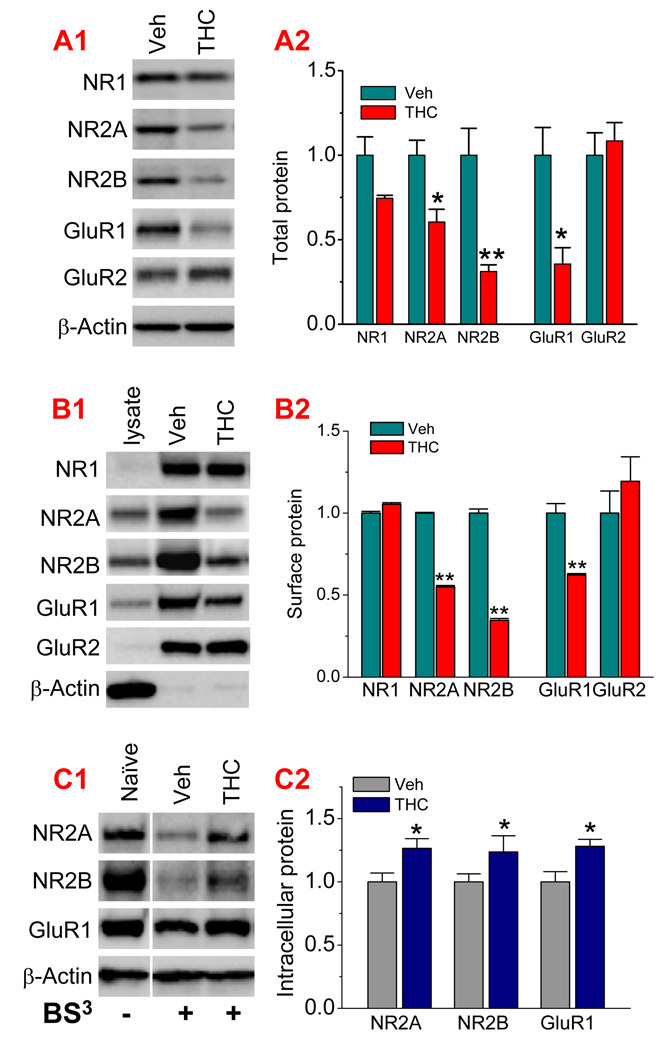
To explore the molecular mechanisms responsible for the Δ9-THC-induced reduction of LTP, we thought that glutamate receptors might be the target of Δ9-THC in Δ9-THC-induced adaptive changes in synaptic circuitry. This idea occurred as a result from the observations that exposure to addictive drugs produces dynamic changes in AMPA receptors (Ungless et al., 2001; Suárez et al., 2003; Saal et al., 2004; Boundreau et al., 2005; 2007; Kauer and Malenka, 2007). To this end, we used the Western blot analysis to determine hippocampal expression of AMPA receptor subunits GluR1 and GluR2, and NMDA receptor subunits NR1, NR2A and NR2B in WT animals that received repeated in vivo exposures to Δ9-THC. As indicated in Figure 2A, repeated in vivo exposures to Δ9-THC for 7 days significantly reduced expression of GluR1, NR2A and NR2B subunits when compared to that of vehicle controls, while NR1 and GluR2 remained unchanged. This is the first report of chronic in vivo exposure to Δ9-THC inducing a down-regulation of hippocampal expression of both AMPA and NMDA receptors. It is likely that decreased GluR1, NR2A and NR2B subunits are responsible for the reduced LTP in animals that were exposed to Δ9-THC for 7 consecutive days.
Figure 2.
Chronic in vivo exposure to Δ9-THC reduces expression of AMPA and NMDA receptor subunits. A1. Immunoblot analysis of NMDA subunits NR1, NR2A, NR2B, and AMPA subunits GluR1 and GluR2 in hippocampal tissues from CB1 wild type mice that received repeated exposures to Δ9-THC (10 mg/kg) for 7 days. Proteins were detected 24 hrs after cessation of last injection. A2. Quantification of the total subunits in vehicle- and Δ9-THC-treated animals (6 animals per group). B1. Surface expression of NR1, NR2A, NR2B, GluR1, and GluR2 subunits is reduced in animals that were chronically exposed to Δ9-THC for 7 days. Surface proteins were biotinylated with EZ-link sulfo-NHS-LC biotin. B2. Quantification of the surface NR1, NR2A, NR2B, GluR1, and GluR2 subunits (3 animals per group). C1. Intracellular NR2A, NR2B and GluR1 subunits in hippocampal tissues from vehicle- or Δ9-THC-treated animals. Extracellular proteins were cross-linked using BS3 (Bis [Sulfosuccinimidyl] suberate). C2. Quantification of the intracellular NR2A, NR2B and GluR1 subunits (3 animals per group). *P<0.05; **P<0.01 compared with vehicle controls.
The down-regulated GluR1, NR2A and NR2B subunits detected in chronically Δ9-THC-exposed animals are the total protein. Since the function of glutamate receptors relies on their membrane surface expression, we decided to determine whether Δ9-THC exposure also alters the trafficking of these subunits. Surface biotinylation assay was performed in hippocampal slices from animals that received Δ9-THC (Yu et al., 2008). As shown in Figure 2B, the surface expression of NR1 and GluR2 was not significantly altered, but the expression of NR2A, NR2B and GluR1 subunits was greatly reduced in Δ9-THC-treated animals when compared to that in vehicle controls. To further determine alterations in glutamate receptor trafficking in Δ9-THC-exposed animals, we measured intracellular pools of these subunits. Extracellular proteins were cross-linked as we previously described (Chen et al., 2006). As shown in Figure 2C, chronic in vivo exposure to Δ9-THC resulted in increases in intracellular NR2A, NR2B and GluR1 subunits, despite the fact that total protein levels of these subunits were reduced (Fig. 2A). This information indicates that repeated exposures to Δ9-THC not only down-regulate these glutamate receptor subunits, but also induce their internalization or endocytosis.
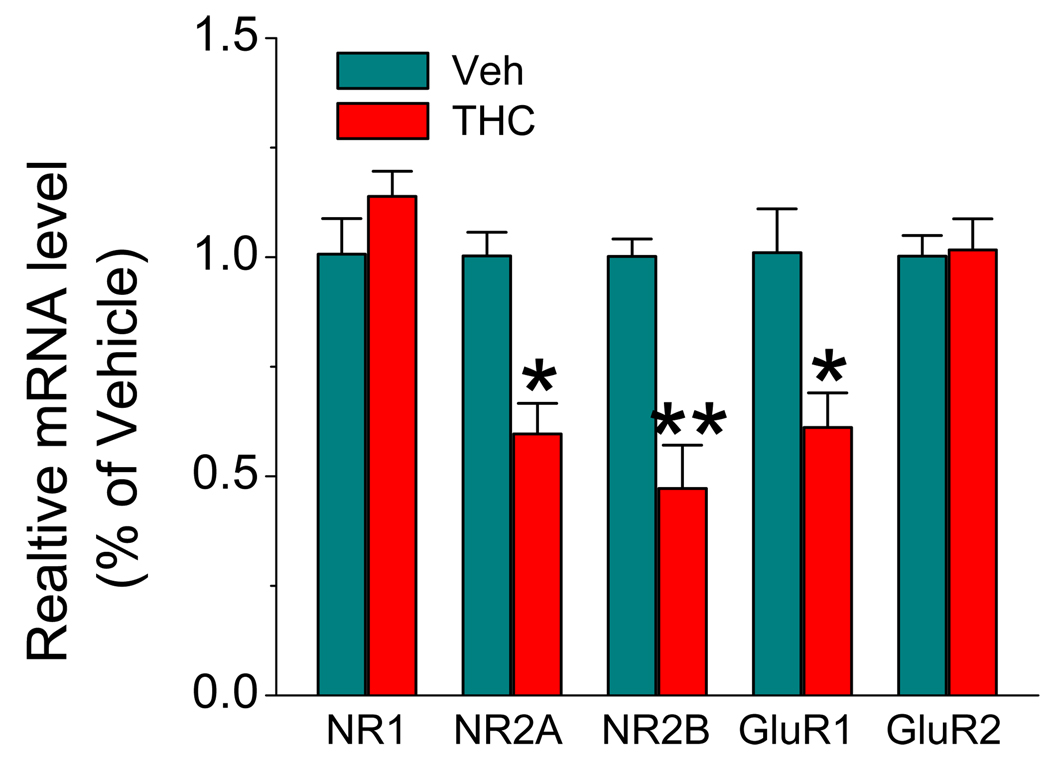
To determine whether the reduced protein expressions of these subunits result from changes of the transcription, we employed the quantitative real-time PCR analysis, as described previously (Chen et al., 2006; Zhang & Chen, 2008), to detect changes in mRNAs of these subunits in hippocampal tissue from animals that received vehicle or Δ9-THC for 7 days. As shown in Figure 3, mRNAs of GluR1, NR2A and NR2B subunits in animals that received THC for 7 days are significantly reduced when compared to those of vehicle controls. However, mRNAs of NR1 and GluR2 subunits remained unchanged. This is consistent with the immunoblot data, as showed in Figure 2.
Figure 3.
Real-time PCR analysis of NR1, NR2A, NR2B, GluR1, and GluR2 expressions in hippocampal tissue from mice treated with vehicle or Δ9-THC (10 mg/kg) for 7 days. Results are from six independent observations (6 animals per group) with duplicate wells. *P<0.05, **P<0.01 compared with vehicle controls.
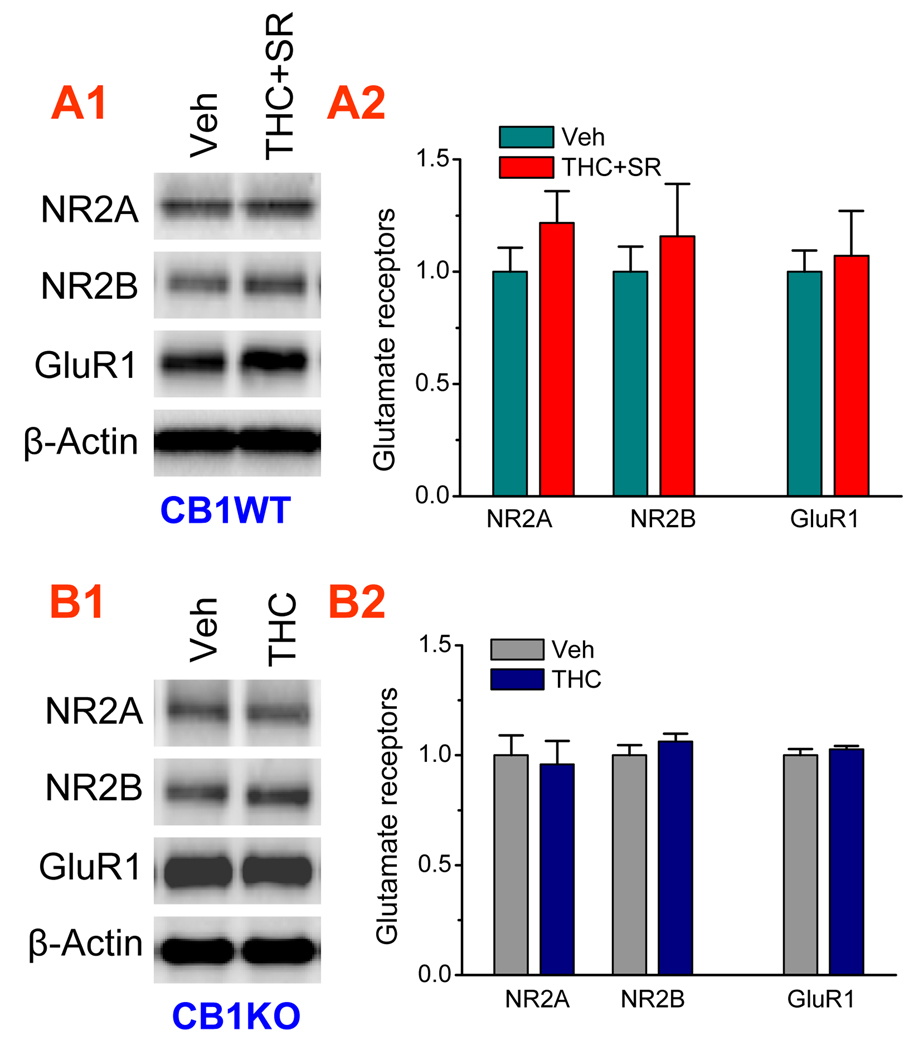
To determine whether the Δ9-THC-induced decreases in the AMPA and NMDA receptor subunits are mediated via the CB1 receptor, the CB1 receptor was pharmacologically blocked with a selective CB1 receptor antagonist, and genetically deleted using mice deficient in the CB1 receptor. As shown in Figure 4, Δ9-THC exposure-induced reduction of the AMPA and NMDA receptor subunits was prevented in wild type animals that received SR and in CB1 knockout mice, indicating that the Δ9-THC-induced decreases in the expression of the glutamate receptors are mediated via the CB1 receptor.
Figure 4.
The CB1 receptor mediates Δ9-THC-reduced expression of AMPA and NMDA receptor subunits. A1–A2. Δ9-THC-reduced expression of NR2A, NR2B and GluR1 subunits is prevented by pharmacological inhibition of the CB1 receptor. CB1R wild-type (WT) animals were administrated with vehicle or Δ9-THC (10 mg/kg) plus SR (5 mg/kg) for 7 days. Subunit proteins were analyzed 24 hrs after cessation of last injection (3 animals per group). B1–B2. Δ9-THC-reduced expression of NR2A, NR2B and GluR1 subunits is prevented by genetic deletion of the CB1 receptor. CB1R knockout (KO) mice were injected with vehicle or Δ9-THC (10 mg/kg) for 7 days (3 animals per group).
To functional characterize the glutamate receptors in animals exposed to Δ9-THC, we employed the whole-cell voltage clamp recordings of the AMPA- and NMDA-gated currents in dentate granule neurons in response to perforant path stimuli. Membrane potential was held at +40 mV to relieve the voltage-dependent Ma2+ blockage. D-AP5 (50 µM) was applied via the bath perfusion after obtaining the total whole cell currents in the presence of bicuculline (10 µM). The remaining AMPA current component was eliminated by DNQX (10 µM). The NMDA current component was obtained by subtraction of the AMPA current from the total currents. As shown in Figure 5A, repeated exposures to Δ9-THC in WT animals reduced the ratio of AMPA/NMDA-mediated currents, but the change was prevented in CB1 KO mice (Fig. 5B). These data indicate that the ratio of AMPA/NMDA receptor-gated currents is still reduced even though the expression of the NMDA subunits is also decreased in animals that received Δ9-THC, suggesting that the AMPA receptor-gated current is the predominant component of glutamate receptor-gated currents.
Figure 5.
Exposure to Δ9-THC induces changes in the ratio of AMPA/NMDA receptor-gated currents. A1. Representative traces of the AMPA- and NMDA-gated currents at hippocampal perforant path synapses in CB1R WT animals that received repeated injections with vehicle or Δ9-THC for 7 days. Membrane potential was held at +40 mV, and the AMPA-mediated current was isolated by AP5 (50 µM) and bicuculline (10 µM). The NMDA-mediated current was obtained by subtraction of the AMPA component from the total currents. Scale bar: 50 pA/100 msec. A2. Chronic in vivo exposure to Δ9-THC reduces the ratio of AMPA/NMDA-mediated currents (Veh: 12 recordings/5 animals, THC: 15 recordings/5 animals). *P<0.05 compared with vehicle controls. B1. Representative traces of the AMPA- and NMDA-gated currents at hippocampal perforant path synapses in CB1R KO animals that received repeated injections with vehicle or Δ9-THC for 7 days. B2. Repeated in vivo exposures to Δ9-THC fail to change the ratio of AMPA/NMDA-mediated currents in CB1KO mice (Veh: 13 recordings/5 animals, THC: 12 recordings/5 animals).
Repeated exposures to Δ9-THC elevate synaptic release of glutamate
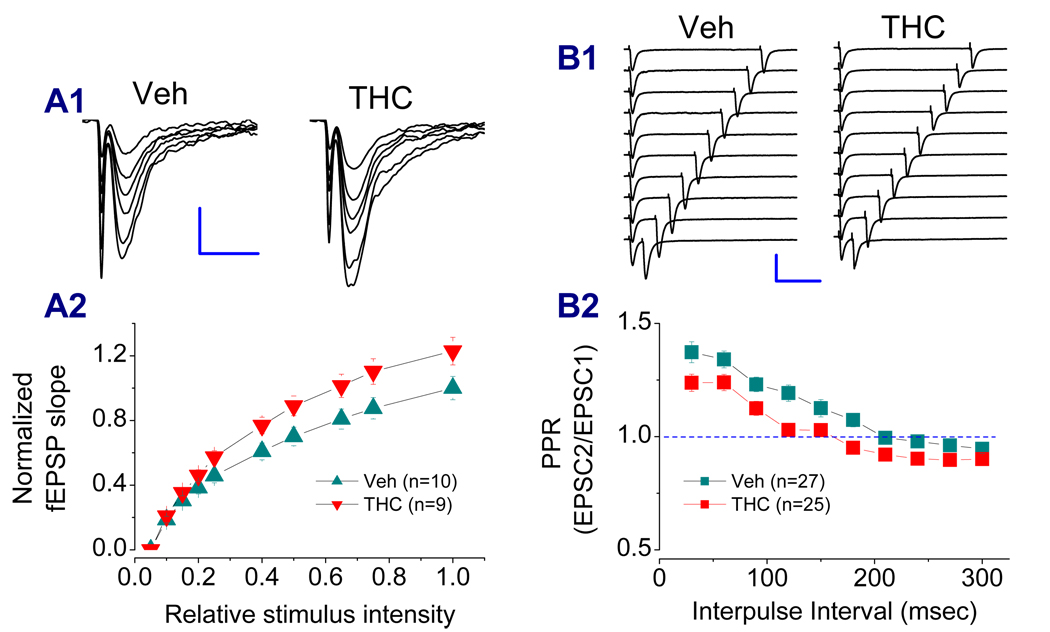
Earlier studies demonstrated that synthetic cannabinoid WIN55,212-2 (WIN) and Δ9-THC produce a CB1 receptor-dependent elevation of extracellular glutamate levels in vitro and in vivo (Cristina et al., 2002; Ferraro et al., 2001). The increased basal release probability of glutamatergic synaptic transmission has also been seen in the cerebellum after repeated injections of Δ9-THC (Tonini et al., 2006). Recent evidence indicates that chronic Δ9-THC exposure reduces the ratio of paired-pulse facilitation of EPSCs at hippocampal Schaffer-collateral synapses, indicating an elevated probability of synaptic glutamate release (Hoffman et al., 2007). Thus, we hypothesized that elevated synaptic release of glutamate in Δ9-THC-exposed animals may lead to down-regulation or internalization of the glutamate receptors as a mechanism of homeostatic plasticity (Turrigiano and Nelson, 2004; Turrigiano, 2008; Perez-Otano and Ehlers, 2005). To this end, we measured input-output function in hippocampal slices of animals that received Δ9-THC. As shown in Figure 6A, chronic in vivo exposure to Δ9-THC increased input-output function, suggesting that basal synaptic release is elevated in Δ9-THC-treated animals. We also used a paired-pulse protocol to determine the paired-pulse ratio (PPR) of EPSCs at perforant synapses in animals that received Δ9-THC for 7 days. As indicated in Figure 6B, chronic exposure to Δ9-THC reduced PPR at perforant path synapses when compared to that of vehicle controls.
Figure 6.
Chronic in vivo Δ9-THC exposure enhances basal synaptic transmission. A1. Representative fEPSP waveforms recorded at hippocampal perforant path synapses in the dentate gyrus of animals injected with vehicle and Δ9-THC (10 mg/kg) for 7 days. A2. Input-output function curves (Veh: 10 recordings/5 animals, THC: 9 recordings/5 animals). Stimulus intensity was normalized to the maximum intensity. Scale bar: 0.3 m V/10 msec. B1. Representative EPSC traces recorded in granule neurons in response to perforant path stimuli from animals injected with vehicle and Δ9-THC (10 mg/kg) for 7 days. EPSCs were induced by a paired-pulse protocol with varying interpulse intervals. B2. Paired-pulse ratios (EPSC2/EPSC1) in vehicle- and Δ9-THC-treated animals (Veh: 27 recordings/6 animals, THC: 25 recordings/6 animals). Scale bar: 300 pA/100 msec
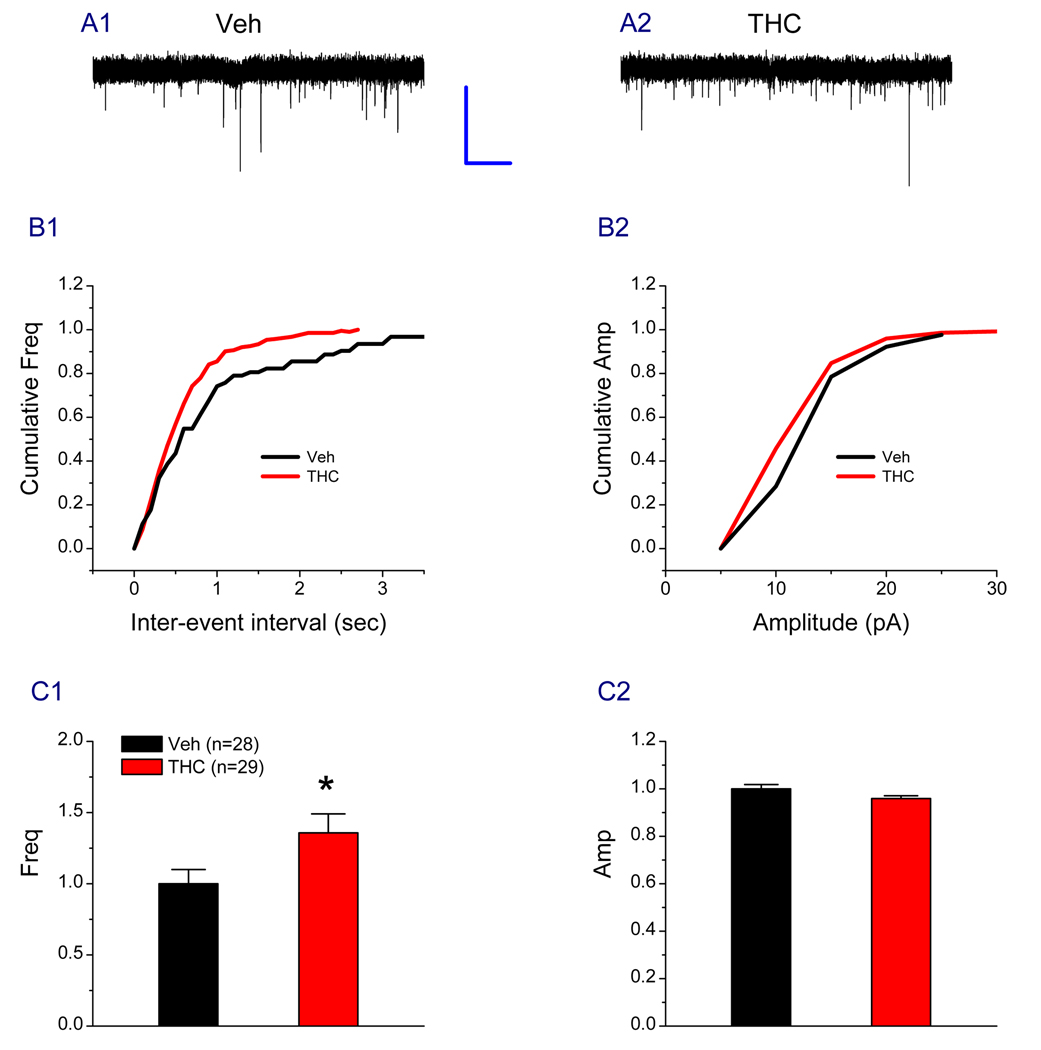
To further determine the effect of Δ9-THC on synaptic transmission, we determined spontaneous EPSCs. As shown in Figure 7, the frequency of sPESCs was elevated from vehicle: 1.00 ± 0.10 to Δ9-THC: 1.36 ± 0.13 (P<0.05) in Δ9-THC-exposed animals, but not the amplitude (Veh: 1.00 ± 0.01, Δ9-THC: 0.96 ± 0.01, P>0.05). Also we did not detect significant changes in decay time of sEPSCs (Veh: 1.00 ± 0.06, Δ9-THC: 0.92 ± 0.05, P>0.05). These results suggest that the probability of synaptic glutamate release is elevated in Δ9-THC-exposed animals. Persistent elevation of glutamate release may lead to desensitization and down-regulation of the postsynaptic glutamate receptors. Thus, the alterations in the glutamate receptor expression upon chronically exposed to Δ9-THC likely result from such neural adaptive changes in synapses.
Figure 7.
Repeated in vivo exposures to Δ9-THC enhance probability of synaptic glutamate release. A1–A2. Representative sweeps of spontaneous excitatory postsynaptic currents (sEPSCs) recorded in granule neurons in hippocampal slices from animals injected with vehicle or Δ9-THC (10 mg/kg) for 7 days. The membrane potential was held at −70 mV. Bicuculline (10 µM) was included in the external solution. The synaptic events were analyzed using the MiniAnalysis program. B1–B2. Cumulative probability of sEPSC frequency and amplitude recorded in neurons from animals treated with vehicle or Δ9-THC. C1–C2. Mean percentage changes in the frequency and amplitude of sEPSCs (Veh: 28 recrodings/5 animals, THC: 29 recordings/5 animals). *P<0.05 compared with the vehicle control (The Kolmogorov-Smirnov test). Scale bar: 20 pA/1 sec.
Chronic exposure to Δ9-THC decreases phosphorylation of CREB
cAMP-response-element-binding protein (CREB) is a transcription factor that is crucial for the transmission of activities occurring at membranes to triggering gene expression, and in turn, regulating the function of neurons. A typical example is the functional role of CREB in learning and long-term plasticity (Yin and Tully, 1996; Mayr and Montminy, 2001; Carezon et al., 2005). It is also believed to be involved in addictive drugs of abuse (Casu et al., 2005; Kauer and Malenka, 2007). To determine whether CREB plays a role in the Δ9-THC-induced changes in LTP, we measured CREB and its phosphorylated form (p-CREB) in the hippocampus from animals that received repeated injections of Δ9-THC. We observed that chronic exposure to Δ9-THC decreased both total and phosphorylation of CREB (Figure 8A). The Δ9-THC-induced changes in phosphorylation of CREB are CB1 receptor-dependent because the changes were eliminated by administration of a selective CB1 antagonist or by deletion of the CB1 gene (Figure 8B & 8C). We need to mention here that repeated injections of SR alone did not induce significant changes in CREB and its phosphorylation (data not shown). These results indicates that repeated in vivo exposures to Δ9-THC induce reductions of expression and phosphorylation of CREB, which regulate expression and function of the glutamate receptors, resulting in altered synaptic efficacy.
Figure 8.
Repeated in vivo exposures to Δ9-THC produce a CB1 receptor-dependent reduction of CREB phosphorylation. A1–A2. Western blot analysis of hippocampal CREB and its phosphorylation in CB1WT animals that received repeated exposures to Δ9-THC for 7 days (6 animals per group). B1–B2. Repeated Δ9-THC exposure-induced decreases in hippocampal CREB and its phosphorylation are blocked by inhibition of the CB1 receptor in CB1R WT mice (Veh: 3 animals, THC+SR: 3 animals). C1–C2. Chronic in vivo exposure to Δ9-THC fails to induce decreases in hippocampal CREB and its phosphorylation in CB1R KO animals (3 animals per group).
Discussion
Chronic use of marijuana induces cannabis-related disorders including cognitive impairment (Heishman et al., 1997; Hampson and Deadwyler, 1999; Pope et al., 2001; 2002; Bolla et al., 2002; Solowij et al., 2002; Ranganathan & D’Souza, 2006). Its psychoactive ingredient Δ9-THC has been shown to alter long-term synaptic plasticity (Mato et al., 2004; 2005; Hoffman et al., 2007). However, the molecular mechanisms underlying the Δ9-THC-induced alterations in synaptic plasticity are largely unknown. In this study, we provide the molecular evidence that the changes in expression and function the glutamate receptors and phosphorylation of CREB in animals chronically exposed to Δ9-THC are likely responsible for the Δ9-THC-altered long-term synaptic plasticity.
Most previous work on Δ9-THC-induced changes in hippocampal long-term synaptic activity focuses mainly in the CA1 region, and very few studies have been done in the dentate gyrus, which is the first gate of the hippocampus for information flow from the entorhinal cortex through the CA1–CA3 regions to the subiculum. The CB1 receptor is expressed in presynaptic terminals in the dentate gyrus, meaning that the synapses in this region are the target of endogenous and exogenous cannabinoids (Herkenham et al., 1991; Tsou et al., 1998). Chronic in vivo exposure to Δ9-THC suppresses hippocampal LTP at Schaffer-collateral synapses in the CA1 region (Mato et al., 2005; Hoffman et al., 2007). We confirmed in the present study that repeated exposure to Δ9-THC also significantly reduces hippocampal LTP at perforant path synapses in the dentate gyrus. This information indicates that chronic in vivo exposure to Δ9-THC impairs both synapses in the areas of hippocampal CA1 and dentate gyrus.
It is now believed that cannabinoid-induced modulation of behavioral, cognitive and synaptic functions is primarily mediated via the CB1 receptor, which is predominantly expressed in the CNS. In the present study, we confirm that in vivo Δ9-THC exposure-induced decrease in LTP is mediated via the CB1R. Importantly, we reveal in the present study that Δ9-THC exposure-induced down-regulation and endocytosis of the glutamate receptors are mediated via the CB1R. Drugs of abuse such as cocaine, morphine and amphetamine have been shown to induce changes in the glutamate receptor expression and function in the brain (Ungless et al., 2001; Saal et al., 2003; Boudreau and Wolf, 2005; Boudreau et al., 2007; Kauer and Malenka, 2007). To explore possible mechanisms and factors contributing to the Δ9-THC-altered LTP, we targeted expression and function of the glutamate receptors. We found that repeated in vivo exposure to Δ9-THC reduces expression of GluR1, NR2A and NR2B subunits. We provided information that in vivo exposure to Δ9-THC not only reduces total protein of these subunits but also their trafficking. It is possible that altered expression, trafficking, and function of the glutamate receptors, exhibiting as adaptive changes in neural circuitry, are a common mechanism of addictive drugs of abuse. The downregulation and internalization of the glutamate receptors are likely results of elevated probability of glutamate release. This assumption is supported by evidence that WIN and Δ9-THC induce a CB1 receptor-dependent elevation of extracellular glutamate levels in vitro and in vivo (Cristna et al., 2002; Ferraro et al., 2001). Prenatal exposure to WIN increases glutamate uptake in rat front cerebral cortex (Castaldo et al., 2007), indicating a long-lasting increase in the release of glutamate. The increased basal release probability of glutamatergic synaptic transmission has been observed in the cerebellum after repeated injections of Δ9-THC (Tonini et al., 2006). Recent evidence also indicates that chronic Δ9-THC exposure reduces the ratio of paired-pulse facilitation of EPSCs at hippocampal Schaffer-collateral synapses, indicating an elevated probability of synaptic release of glutamate (Hoffman et al., 2007). The results obtained in the present study showing that input-output function and the frequency of sEPSCs are enhanced and PPR is reduced in chronically Δ9-THC-exposed animals support this speculation. The Δ9-THC-reduced expression of glutamate receptors may a result of homeostatic plasticity when synaptic release of glutamate is elevated (Turrigiano and Nelson, 2004; Turrigiano, 2008; Perez- Otano and Ehlers, 2005).
CREB, functioning as a transcription factor, is best known for its involvement in learning and memory (Yin and Tully, 1996; Mayr and Montminy, 2001; Carezon et al., 2005). It has also been implicated in drug addiction (Nestler, 2001). As revealed in the present study, repeated in vivo exposure to Δ9-THC results in decreases in CREB and its phosphorylation. The reduced phosphorylation of CREB may also be responsible for the Δ9-THC-induced down-regulation of the expression of glutamate receptors because CREB regulates gene expression as a transcript factor. Our results are consistent with reports that chronic administration of Δ9-THC markedly attenuates phosphorylation of CREB in rat cerebellum (Casu et al., 2005).
In this study, we provide electrophysiological and molecular evidence that chronic in vivo exposure to Δ9-THC produces alterations in hippocampal long-term synaptic plasticity, the expression and activities of the glutamate receptors, and phosphorylation of CREB. These Δ9-THC-induced adaptive synaptic changes may share common mechanisms with addictive drugs in modifying neural circuitry.
ACKNOWLEDGMENTS
The authors thank NIH NIMH transgenic core for providing CB1R knockout mice, NIMH Chemical Synthesis and Drug Supply Program for providing SR141716 and D-AP5, and NIDA Drug Supply Program for providing Δ9-THC. This work was supported by National Institutes of Health grants NS054886 and DA025971.
Abbreviations
- LTP
long-term potentiation
- eCBs
endocannabinoids
- Δ9-THC
Δ9-tetrahydrocannabinol
- CB1R
cannabinoid receptor 1
- SR
SR141716
- CRD
cannabis-related disorders
- CREB
cAMP response element binding protein
- TBS
theta-burst stimulation
- EPSCs
excitatory postsynaptic currents
- fEPSPs
field extracellular excitatory postsynaptic potentials
References
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Bass CE, Martin BR. Time course for the induction and maintenance of tolerance to Δ9- tetrahydrocannabinol in mice. Drug Alcohol Dependence. 2000;60:113–119. doi: 10.1016/s0376-8716(99)00150-7. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signaling system: biochemical aspects. Pharmacol Biochem Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurol. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine in associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in associated with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic Δ9- tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Castaldo P, Magi S, Gaetani S, Cassano T, Ferraro L, Antonelli T, et al. Prenatal exposure to the cannabinoid receptor agonist WIN55,212-2 increases glutamate uptake through overexpression of GLT1 and EAAC1 glutamate transporter subtypes in rat frontal cerebral cortex. Neuropharmacol. 2007;53:369–378. doi: 10.1016/j.neuropharm.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Casu MA, Pisu C, Sanna A, Tambaro S, Spada GP, Mongeau R, Pani L. Effect of Δ9-tetrahydrocannabinol on phosphorylated CREB in rat cerebellum: An immunohistochemical study. Brain Res. 2005;1048:41–47. doi: 10.1016/j.brainres.2005.04.053. [DOI] [PubMed] [Google Scholar]
- Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- Chen C. ZD7288 inhibits postsynaptic glutamate receptor-mediated responses at hippocampal perforant path-granule cell synapses. Eur J Neurosci. 2004;19:643–649. doi: 10.1111/j.0953-816x.2003.03174.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Hardy M, Zhang J, LaHoste JL, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophysi Res Comm. 2006;340:435–440. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Ann Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Childers SR. Activation of G-proteins in brain by endogenous and exogenous cannabinoids. AAPS J. 2006;8:E112–E117. doi: 10.1208/aapsj080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristina M, Ferraro L, Bebe BW, Cassano T, Cuomo V, Antonelli T. Δ9-Tetrahydrocannabinol increases endogenous extracellular glutamate levels in primary cultures of rat cerebral cortex neurons : involvement of CB1 receptors. J Neurosci Res. 2002;68:449–453. doi: 10.1002/jnr.10242. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Gessa GL, Bebe BW, Tanganelli S, Antonelli T. The cannabinoid receptor agonist WIN 55,212-2 regulates glutamate transmission in rat cerebral cortex: an in vivo and in vitro study. Cereb Cortex. 2001;11:728–733. doi: 10.1093/cercor/11.8.728. [DOI] [PubMed] [Google Scholar]
- Frazier CJ. Endocannabinoids in the dentate gyrus. Prog Brain Res. 2007;163:319–338. doi: 10.1016/S0079-6123(07)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93:217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- González S, Cebeira M, Fernández-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life Sci. 1999;65:715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacol Biochem Behav. 1997;58:93–101. doi: 10.1016/s0091-3057(96)00456-x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, DeCosta BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci. 2003;23:4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, yang R, Lichtman AH, Lupica CR. Opposing actions of chronic Δ9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem. 2007;14:63–74. doi: 10.1101/lm.439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelly BG, Thayer SA. Δ9-tetrahydrocannabinol antagonizes endocannabinoid modulation of synaptic transmission between hippocampal neurons in culture. Neuropharmacol. 2004;46:709–715. doi: 10.1016/j.neuropharm.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handb Exp Pharmacol. 2005;168:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008;184:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Martin BR, Sim-Selly LJ, Selley DE. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci. 2004;25:325–330. doi: 10.1016/j.tips.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Martin BR. Role of lipids and lipid signaling in the development of cannabinoid tolerance. Life Sci. 2005;77:1543–1558. doi: 10.1016/j.lfs.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo P, Manzoni OJ. A single in-vivo exposure to Δ9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci. 2004;6:585–586. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- Mato S, Robbe D, Puente N, Grandes P, Manzoni OJ. Presynaptic homeostatic plasticity rescues long-term depression after chronic Δ9-tetrahydrocannabinol exposure. J Neurosci. 2005;25:11619–11627. doi: 10.1523/JNEUROSCI.2294-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The endogenous cannabinoid system and the treatment of marijuana dependence. Neuropharmacol. 2004;47:359–367. doi: 10.1016/j.neuropharm.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber Aj, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42:41s–47s. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC. The acute effect of cannabinoids on memory in humans: a review. Psychopharmacol. 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Rubino T, Viganò D, Massi P, Parolaro D. Changes in the cannabinoid receptor binding, G protein coupling, and cyclic AMP cascade in the CNS of rats tolerance to and dependent on the synthetic cannabinoid compound CP55, 940. J Neurochem. 2000;75:2080–2086. doi: 10.1046/j.1471-4159.2000.0752080.x. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. J Neurochem. 2007;102:1966–1977. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Critical Rev Neurobiol. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. J Am Med Assoc. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Suárez I, Bodega G, Fernández-Ruiz J, Ramos JA, Rubio M, Fernández B. Down-regulation of the AMPA glutamate receptor subunits GluR1 and GluR2/3 in the rat cerebellum following pre- and perinatal Δ9-tetrahydrocannabinol exposure. Cerebellum. 2003;2:66–74. doi: 10.1080/14734220310017230. [DOI] [PubMed] [Google Scholar]
- Sugiura T, kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid.Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Tonini R, Ciardo S, Cerovic M, Rubino T, Parolaro D, Mazzanti M, Zippel R. ERK-dependent modulation of cerebellar synaptic plasticity after chronic Δ9-tetrahydrocannabinol exposure. J Neurosci. 2006;26:5810–5818. doi: 10.1523/JNEUROSCI.5469-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous ststem. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoids signaling in the brain. Science. 2002;296:678–681. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhang J, Andreasson K, Chen C. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Mol Cell Neurosci. 2008;37:682–695. doi: 10.1016/j.mcn.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang J, Breyer RM, Chen C. Altered hippocampal long-term synaptic plasticity in mice deficient in the PGE2 EP2 receptor. J Neurochem. 2009;108:295–304. doi: 10.1111/j.1471-4159.2008.05766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- Yu SY, Wu DC, Liu L, Ge Y, Wang YT. Role of AMPA receptor trafficking in NMDA receptor-dependent synaptic plasticity in the rat lateral amygdala. J Neurochem. 2008;106:889–899. doi: 10.1111/j.1471-4159.2008.05461.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J Biol Chem. 2008;283:22601–22611. doi: 10.1074/jbc.M800524200. [DOI] [PMC free article] [PubMed] [Google Scholar]