Abstract
Sarcoidosis is a noncaseating granulomatous disease, likely of autoimmune etiology, that causes inflammation and tissue damage in multiple organs, most commonly the lung, but also skin, and lymph nodes. Reduced dendritic cell (DC) function in sarcoidosis peripheral blood compared with peripheral blood from control subjects suggests that blunted end organ cellular immunity may contribute to sarcoidosis pathogenesis. Successful treatment of sarcoidosis with tumor necrosis factor (TNF) inhibitors, which modulate DC maturation and migration, has also been reported. Together, these observations suggest that DCs may be important mediators of sarcoidosis immunology. This review focuses on the phenotype and function of DCs in the lung, skin, blood, and lymph node of patients with sarcoidosis. We conclude that DCs in end organs are phenotypically and functionally immature (anergic), while DCs in the lymph node are mature and polarize pathogenic Th1 T cells. The success of TNF inhibitors is thus likely secondary to inhibition of DC-mediated Th1 polarization in the lymph node.
Keywords: sarcoidosis, granuloma, dendritic cell, macrophage, inflammation
CLINICAL RELEVANCE.
This review focuses on dendritic cells as integral players in sarcoidosis pathogenesis. As such, these cells become potential therapeutic targets.
Sarcoidosis was first described in 1877 as a skin disease by British physician Sir Jonathan Hutchinson (1). It was later discovered that only 20% of patients with sarcoidosis have skin lesions, whereas over 90% have interstitial lung disease (2). In the United States, the incidence rate of sarcoidosis is 10.9 and 35.5 per 100,000 in persons of European and African descent, respectively (3).
Although the pathogenesis of sarcoidosis remains undetermined, many observations, including the presence of oligoclonal T cells in sarcoidosis bronchoalveolar lavage (BAL) fluid, blood, and skin granulomas, are suggestive of an antigen-driven autoimmune disease (4, 5). However, despite multiple proposed causative agents, a single agent has not yet been identified.
T cell subsetting studies have identified IFN-γ–producing Th1 cells as the dominant subtype in both sarcoidosis BAL fluid and skin lesions (6–8), although studies have not yet been done to rule out involvement of the recently described Th17 cell subset. The working model for antigen-driven inflammation involves peripheral organ dendritic cells (DCs) digesting antigenic material, migrating to the lymph node, presenting that antigen to naïve resting T cells and activating a clonal T cell response. T cells then migrate back to the end organ, where they produce a disease-specific profile of inflammatory cytokines and chemokines. Despite the necessity of DCs for induction of naïve T cell clonal expansion (9), to date sarcoidosis research has focused on alveolar macrophages as the causative antigen-presenting cell (APC). This review focuses on evidence supporting an important role for DCs in directing sarcoidosis Th1 polarization and immunopathogenesis.
DENDRITIC CELL DEFINITION
In 1973, Steinman and Cohn first described the dendritic cell as having three essential characteristics: (1) Ability to potently induce T cell proliferation (immunostimulatory capacity); (2) high expression of MHC class II peptides; and (3) ability to migrate in and out of tissue, blood, and lymph nodes (migratory capacity) (10). Subsequent work has shown that there are three major lineages of DCs—myeloid DCs (mDC), plasmacytoid DCs (pDC), and Langerhans cells (LC).
Myeloid DCs are descendents of blood myeloid hematopoetic precursor cells (11–13). They respond to innate bacterial stimuli (14) through ligation of Toll-like receptors (TLR) 2 and TLR4 that recognize components of the bacterial cell wall peptidoglycan/lipoteichoiec acid and lipopolysaccharide (LPS), respectively (15, 16). One proposed causative agent for sarciodosis is Mycobacterium tuberculosis, which contains ligands for both TLR2 and TLR4 (17–20). TLR2/4 ligation induces up-regulation of MHC-II and co-stimulatory molecules that promote T cell activation (21), and increases expression of inflammatory cytokines TNF and iNOS (14).
Plasmacytoid DCs are phylogenetically most similar to lymphocytes (22) and produce large quantities of interferon-α (IFN-α) in response to viral invasion (23). Specifically, pDCs express TLR9, which recognizes viral DNA and CpG oligodeoxynucleotides, and TLR7, which recognizes single-stranded viral RNA (24, 25).
Langerhans cells are derived from multipotent bone marrow stem cells and monocytes (26–30) and are primarily located in the suprabasal layer of the epidermis and other stratified epithelium, where they are thought to both present foreign antigen (31) and tolerize to self-peptides (32, 33). Although LCs and pDCs have the capacity to induce T cell proliferation, only mDCs are found in abundance in sarcoidosis BAL fluid (34).
Like mDCs, macrophages are also descendents of a myeloid pluripotent precursor (35), but differ phenotypically and functionally. Despite similar stellate “dendritic” shapes and a common monocyte precursor, macrophages are much less immunostimulatory, express lower (mid) levels of MHC-II, and are less migratory than DCs (36, 37). As their name suggests, macrophages engulf large particles, such as a bacteria, virus, or necrotic tissue and “digest” these antigens in large lysosomal compartments (38). In the lung, macrophages also digest extra surfactant and dirt. Much of sarcoidosis research focuses on macrophages (39, 40), but in this article, we present data suggesting that the pathogenic cells are in fact immunostimulatory, inflammatory myeloid DCs that drive the adaptive Th1 immune response (41).
MYELOID DENDRITIC CELLS IN SARCOIDOSIS
DCs in BAL Fluid Are Representative of a Large Interstitial Lung DC Population
The lung is the most commonly affected organ in sarcoidosis (95% of patients), compared with 20% with cutaneous disease (2). Lung biopsy and BAL are frequently performed during the diagnosis of sarcoidosis to rule out respiratory infection and malignancy (2). Thus immunology studies on sarcoidosis consist of mostly in situ immunohistochemistry on lung biopsies, and functional studies using BAL fluid as the source of cellular material.
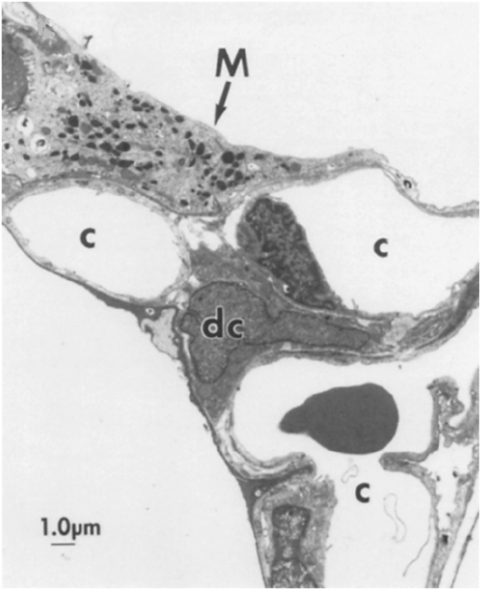
BAL fluid contains 85% alveolar macrophages as defined by high forward (FSC) and side scatter (SSC) flow cytometry, indicating large cell size and high cellular complexity, respectively (42). DCs only constitute approximately 1% of BAL fluid, as defined by lower FSC and SSC, and high expression of both MHC class II receptor HLA-DR and myeloid lineage integrin CD11c (34, 43). This relative paucity of DCs in the BAL does not, however, reflect a lack of DCs in the airway. An electron micrograph of a rat alveolus (Figure 1) shows a large alveolar macrophage filled with characteristic electron-dense cytoplasmic inclusions (lysosomes, phagosomes) nestled within the alveolar space, and a smaller interstitial DC (44). Quantitative analysis of the interstitial lung DC population density and distribution in rats using in situ immunohistochemistry demonstrates an approximate 1:1 ratio of interstitial DCs to alveolar macrophages (45). Interstitial DCs are functionally active and increase in number by 50% after inhalation of LPS antigen (46). Therefore, although relatively few DCs extravasate into the alveolar space, the BAL DC population is a reflection of a much more abundant interstitial DC population that has the capacity to expand during inflammation.
Figure 1.
Interstitial dendritic cells (DCs) and alveolar macrophages in the alveolus. Electron micrograph of rat alveolar septal junction showing a large macrophage (M) containing many electron-dense vacuoles, spread upon the type I epithelial lining of an alveolus, and a smaller DC (dc) with an irregularly shaped indented nucleus, located in the interstitial space. c, capillary. Reprinted with permission from Reference 45.
Phenotypically Immature Myeloid DCs Are Enriched in Sarcoidosis BAL Fluid and Cutaneous Lesions, while Mature DCs Are Located in the Lymph Node
In sarcoidosis BAL fluid, there is a 2-fold enrichment of myeloid DCs compared with normal, a finding that was specific to sarcoidosis but not to other inflammatory lung diseases, including idiopathic pulmonary fibrosis and pneumonia. These inflammatory DCs have decreased expression of DC maturation marker CD83 and co-stimulatory molecule CD86 compared with normal, indicating a predominance of immature DCs in sarcoidosis BAL fluid (34).
As in the lung, immature DCs accumulate in cutaneous sarcoidosis granulomas (47). Subcutaneous injection of sarcoidosis granuloma distillate (Kveim reagent) into patients with sarcoidosis induces granulomas containing 2-fold more DCs than in foreign body reactions (48), suggesting that sarcoidosis granulomas are more DC trophic than other granulomatous reactions. These infiltrating DCs are immature, as evidenced by their lack of DC maturation markers CD83 and DC-lysosomal-associated membrane protein (DC-LAMP). However, after treatment with thalidomide, the number of DCs expressing CD83 and DC-LAMP increases, suggesting an inverse relationship between DCs maturity in the skin and disease activity (49). This seemingly paradoxical observation may result from a thalidomide-dependent interruption of mature DC migration to the lymph node, where they would otherwise present antigen to T cells and promote disease.
In contrast to immature DCs found in the lung and skin, many mature DC-LAMP+ DCs surround granulomas in the lymph nodes. Double label immunohistochemistry shows mature DCs and T cells situated in close proximity, suggesting that the DCs are actively presenting antigen and stimulating T cell proliferation (8). Thus, although DCs in sarcoidosis do not mature in the lung and skin, they do mature in the lymph node. This pattern deviates from other inflammatory skin diseases, such as psoriasis, where DC-LAMP and CD83+ cells collect in the cutaneous lesions (50), and may explain why patients with sarcoidosis are anergic and patients with psoriasis are not. Alternative explanations include the observation that CD4+CD25++FoxP3+ cells proliferate in patients with sarcoidosis both in the peripheral blood and at the periphery of granulomas (51). These cells exert an antiproliferative effect on naïve T cells, which may also be used to explain the presence of anergy in patients with sarcoidosis.
Sarcoidosis Lung and Peripheral Blood DCs Are Less Immunostimulatory than Normal DCs
Consistent with their immature phenotype, sarcoidosis lung DCs are less able to induce T cell proliferation than normal lung DCs. Autologous mixed leukocyte reactions (MLR) using unfractionated BAL fluid indicates that sarcoidosis BAL cells are less immunostimulatory (52) than normal, reflecting the decreased immunostimulatory capacity of sarcoidosis alveolar DCs and macrophages (42, 53). Using histoincompatibility-insensitive Jurkat indicator cells, however, one study showed increased IL-2 secretion after co-culture with sarcoidosis compared with normal alveolar macrophages (54). The immunostimulatory capacity of sarcoidosis BAL fluid is inhibited with anti-CD86, suggesting that their ability to induce T cell proliferation is dependent on CD86 co-stimulatory molecule (42). Myeloid DCs from sarcoidosis peripheral blood are also less immunostimulatory than normal blood DCs, and immunostimulatory capacity is directly correlated with ability to mount a delayed-type hypersensitivity (DTH) response (55). Decreased DC function is a potential mechanism for sarcoidosis-induced anergy, and may suggest that reduced cellular immunity at the end organs is responsible for disease perpetuation rather than resolution.
Proposed Hypotheses for Lack of Sarcoidosis DC Maturation
Although no data are available to explain the lack of maturation in cutaneous sarcoidosis DCs, two theories exist to explain the lack of lung DC maturation. These include short lung DC half-life, and DC suppression from alveolar macrophage anti-inflammatory cytokines. Mouse models using adoptive precursor cell transfer and conditional cell ablation techniques have shown that blood monocytes can replenish lung DCs during inflammation (56, 57). Moreover, in sarcoidosis, morphologic studies showed monocytes migrating from capillaries of the alveolar interstitium toward lung granulomas (58). However, inflammatory lung DCs have a relatively short half-life of only 2 days (59). This rapid transit time for development from blood monocytes into inflammatory lung DCs and subsequent cell death may explain the relatively immature state of lung DCs in sarcoidosis. Alternatively, sarcoidosis alveolar macrophages secrete anti-inflammatory diffusible factors, such as IL-10, prostaglandin E2 (PGE2), and inducible nitric oxide synthase (iNOS) (45, 60, 61). These mediators inhibit DC maturation and production of IL-12, a Th1 T cell–polarizing cytokine involved in sarcoidosis immunopathogenesis (6, 62). Therefore, although myeloid DCs accumulate in sarcoidosis lung, the DCs are phenotypically and functionally immature secondary to either intrinsic sarcoidosis DC characteristics or macrophage-mediated inhibition of maturation.
DCs Are More Immunostimulatory than Macrophages
Despite the somewhat blunted immunostimulatory capacity of DCs in sarcoidosis lung and blood, DCs are still more effective inducers of T cell proliferation than macrophages. DCs purified from normal BAL induce 5 to 10 times more T cell proliferation than alveolar macrophages. This result has been replicated using multiple DC purification techniques including “high-tech” bead selection and cell sorting (63), and “low-tech” density gradient fractionation (43, 64, 65). While DCs have not yet been isolated from sarcoidosis BAL fluid for functional studies, it is likely that they are more immunostimulatory than sarcoidosis alveolar macrophages.
Although alveolar macrophages have a limited capacity to stimulate naïve T cells, they are moderately more effective at stimulating primed T cell clones with corresponding HLA-DR type (66). These findings may be explained by the less stringent requirements for clonal T cell activation compared with naïve T cell activation. Considering the high proportion of clonal T cells in sarcoidosis BAL fluid (67), alveolar macrophages may selectively induce these memory cells to proliferate, although DCs are still necessary to initiate clonal expansion of naïve T cells in the lymph node (68, 69).
The Role of Cytokines Released by DCs in T Cell Activation and Leukocyte Trafficking
Apart from the DC's ability to stimulate T cell proliferation through direct T cell receptor and co-stimulatory molecule engagement, DCs secrete potent inflammatory mediators that polarize Th1 T cells and potentiate T cell proliferation and leukocyte chemotactic factors that promote granuloma formation. Most of the studies assessing cytokines and chemokines involved in sarcoidosis pathogenesis have used unfractionated BAL fluid containing both DCs and macrophages, or use plastic adherence purification that isolates both pre-DCs and pre-macrophages (70). Therefore, we now describe inflammatory mediators overexpressed in sarcoidosis BAL that may be produced by either DCs, macrophages, or both.
Th1 T Cell Polarizing Cytokines
IL-12 is a Th1 T cell–polarizing cytokine (71, 72) constitutively expressed by DCs (9) and found at high levels in sarcoidosis BAL supernatant and at sites of Kveim skin reactions (6). DCs and macrophages in sarcoidosis BAL fluid spontaneously produce IL-12 (73–75), augmented by co-culture stimulation with IFN-γ, Staphylococcus aureus extract, or LPS (6). IL-12 amplifies its own response through induction of more IL-12 release from APCs and up-regulation of IL-12β expression on activated T cells, further polarizing them toward the Th1 lineage. Moreover, Th2 T cells do not develop in the presence of IFN-γ (76). A similar self-amplifying scenario exists for the cytokine IL-18, whereby IL-18 released by DCs/macrophages acts on T cells through the IL-18R to up-regulate IL-12Rβ2 and IFN-γ expression. IL-12 also induces IL-18R expression on Th1 T cells, creating a synergistic interaction between IL-12 and IL-18 that is thought to promote sarcoidosis pathogenesis (74, 77).
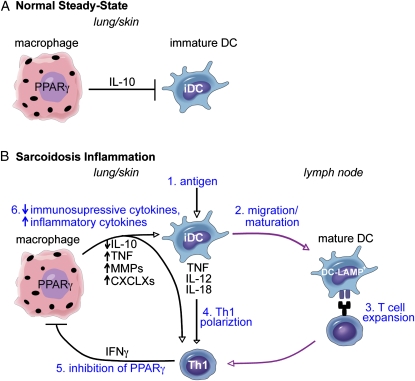
The functional importance of IFN-γ–producing Th1 T cells for sarcoidosis pathology was first established in IFN-γ knockout mouse models of hypersensitivity pneumonitis that lack granulomas (78). IFN-γ is also the most highly expressed cytokine in sarcoidosis BAL fluid (6). One proposed mechanism for involvement of IFN-γ in sarcoidosis pathogenesis is through inhibition of macrophage peroxisome proliferators–activated receptor γ (PPARγ) expression (79)—an important negative regulator of inflammation (80). During normal steady-state conditions, PPARγ is constitutively expressed by alveolar macrophages, promotes phagocytosis and IL-10 production (81), and inhibits DCs from releasing TNF, IL-12, and matrix metalloproteases (MMPs) (82) (Figure 2A). In sarcoidosis, normally immunosuppressive alveolar macrophages may not be able to control inflammatory DCs, contributing to excessive cellular and adaptive immune system activation (Figure 2B). MMP-8 and MMP-9 produced by activated alveolar macrophages are increased in sarcoidosis BAL fluid without compensatory increase in levels of tissue inhibitor of metalloproteinase. These MMPs then induce lung damage and subsequent fibrosis (83, 84). IFN-γ is also a direct inducer of DC/macrophage chemokines CXCL9, CXCL10, and CXCL11 that induce T cell chemotaxis by ligating the T cell receptor CXCR3 (85, 86). As expected, these IFN-γ–induced chemokines are elevated in sarcoidosis BAL fluid (87, 88).
Figure 2.
Critical role of DCs in sarcoidosis pathogenesis. (A) During normal steady-state, macrophages constitutively express peroxisome proliferator–activated receptor (PPAR)γ, a transcription factor that induces macrophage IL-10 production and inhibition of myeloid DCs. (B) During sarcoidosis antigen-driven inflammation (1), DCs are activated to mature and migrate to the lymph node (2), where they induce T cell expansion (3). They also release Th1 polarizing inflammatory mediators TNF, IL-12, and IL-18 (4). Th1 T cells express IFN-γ, which directly inhibits macrophage PPARγ (5), resulting in decreased expression of immunosuppressive cytokine IL-10 and increased expression of inflammatory cytokines TNF, MMPs, and CXCLX chemokines (6). Decreased IL-10 and increased TNF expression releases DCs from macrophage inhibition, completing the self-amplifying inflammatory loop of sarcoidosis. In addition, MMPs damage lung tissue and promote fibrosis, and chemokines attract more myeloid cells and T cells promoting granuloma formation. Purple lines indicate cell migration to and from the lymph node; black lines indicate cytokines released from one cell acting upon another; arrowheads indicate cell activation; and flat lines indicate cell inhibition.
Cytokines Promoting T Cell Proliferation and Survival
TNF produced by inflammatory DCs and macrophages (89–91) promotes T cell proliferation and survival, both indirectly by inducing DC maturation into potent antigen-presenting cells (70) and directly through induction of IL-2R on T cells (92, 93). IL-15 is also a trophic factor for T cells and, like IL-2, binds to the IL-2R to promote T cell survival (94).
The importance of TNF to sarcoidosis pathology is shown by the effectiveness of TNF inhibitors, particularly the anti-TNF chimeric monoclonal antibody infliximab/Remicade (95). Other effective treatments whose primary mechanism of action may be through TNF inhibition include phosphodiesterase inhibitor pentoxifylline/Trental (96, 97), and thalidomide (98, 99). Patients with sarcoidosis who continue to have elevated TNF production from alveolar macrophages during steroid treatment are 1.8 times more likely to relapse into active disease during a steroid taper, also suggesting that TNF production may be a critical cytokine for disease pathogenesis (100).
In summary, during sarcoidosis inflammation, a self-amplifying cycle is created where antigen-driven DCs migrate to the lymph node where they mature and polarize Th1 T cells that release IFN-γ, which activates alveolar macrophages to secrete TNF and other mediators of fibrosis, DC maturation, T cell survival and proliferation, and leukocyte chemotaxis (Figure 2B).
LANGERHANS CELLS AND PLASMACYTOID DCs IN SARCOIDOSIS
Langerhans Cells Do Not Play a Large Role in Sarcoidosis Pathogenesis
Apart from myeloid DCs, other DC subsets potentially involved in sarcoidosis pathogenesis are Langerhans cells (LCs), and plasmacytoid DC. LCs are primarily located in the basal layer of human epidermis, and were the first DC subset identifiable both by ultrastructural features and using monoclonal antibody labeling techniques for immunohistochemistry (101, 102). Their presence in sarcoidosis granulomas was probed by Munro and coworkers in 1987 (103). Using the NA1-34/OKT6 antibody later known as CD1a, an MHC-I–like molecule that presents microbial lipids to T cells (104), Munro and colleagues identified peri-granuloma LC infiltrate in only 1/6 lung and 1/3 lymph node granuloma samples. Using a more specific LC antibody, Langerin/CD207 C-type lectin (105), Smetana and coworkers found almost no Langerin+ cells in sarcoidosis BAL fluid (106), similar to results from normal BAL fluid (63). The paucity of LCs in sarcoidosis lung granulomas suggests that LCs do not play an active role in sarcoidosis lung pathology.
In contrast, there is an enrichment in NA1-34/OKT6/CD1a+ cells in the epidermis overlaying dermal granulomas and within the granulomas themselves (103, 107). However, as NA1-34/OKT6/CD1a is also a marker for dermal myeloid DCs (108), without immunohistochemistry using Langerin/CD207 LC-specific antibody, it is impossible to definitively conclude that the dermal cells infiltrating sarcoidosis granulomas are LCs. It is particularly interesting that epidermal LCs accumulate in the epidermis above the granuloma, but not in the draining lymph nodes, considering that the standard model of skin inflammation involves LCs migrating from the epidermis to the lymph node to present antigen (109, 110). This typically results in a sparseness of epidermal LCs at the sight of inflammation with an increased abundance in draining lymph nodes (111). The inverse pattern in cutaneous sarcoidosis may suggest that epidermal LCs are not acting as antigen presenters at the lymph node. As previously described, myeloid DCs have an immature phenotype in sarcoidosis granulomas, and are abundant and mature in draining lymph nodes, supporting the role for mDC, not LCs in lymph node antigen presentation.
Plasmacytoid DCs Do Not Play a Large Role in Sarcoidosis Pathogenesis
Like Langerhans cells, plasmacytoid DCs do not appear to play a crucial role in sarcoidosis pathogenesis. In sarcoidosis BAL fluid (34) and in blood (55), pDCs appear in similar frequency and absolute numbers as in normals. Stimulation of sarcoidosis and normal blood pDCs with TLR9 ligand CpG-A showed no difference in IFN-α protein expression.
Despite the lack of evidence for increased pDC frequency or activation in sarcoidosis, IFN-α therapy is a well documented risk factor for development of IFN-induced sarcoidosis (IIS) (112, 113), occurring in 5% of patients with chronic hepatitis C treated with IFN-α (114). Also, an IFN-α17 polymorphism, that shows increased IFN-α secretion when whole blood is activated with Sendal virus, has been documented in a cohort of Japanese patients with sarcoidosis (115). However, exogenous IFN-α–induced autoimmunity is not specific to sarcoidosis and has been implicated in inducing systemic lupus erythematosus, thyroiditis, and psoriasis vulgaris (116, 117). The mechanism by which IFN-α induces autoimmunity is still unclear, although IFN-α induces both MHC class II and co-stimulatory molecules on blood monocytes (118) and may enhance DC and macrophage antigen presentation to Th1 T cells (119). Th1 T cells are important mediators of sarcoidosis (62, 120), lupus (121), and psoriasis immunopathogenesis (36, 50). IFN-α also directly increases IFN-γ and IL-2 production from T cells, thus promoting granuloma formation (122). Therefore, although pDCs are likely not causal to most patients' sarcoidosis, the IFN-α they secrete may potentiate the disease.
Genomic Signature of Myeloid DCs in Sarcoidosis
While a comprehensive review of sarcoidosis genetics has been recently published (123), we now focus on genetic associations that may be linked to myeloid DCs. Two genome wide scans have identified candidate genes within genomic loci linked to sarcoidosis. One study in 63 white German families with sarcoidosis found the greatest linkage on the short arm of chromosome 6 (6p21 to 6p22), a 16cM region containing both the MHC genes and TNF. Other minor loci included 3p21, 1p22, 9q33, Xq22, 7q22, and 7q36 (124). As previously described in this review, TNF is both a product and an activator of myeloid DCs. The second study was conducted in 229 African-American families, and identified a major linkage on 5q11 with minor loci at 3p21-14, 1p22, 9q34, 2p25, 5p15-13, 5q35, 11p15, and 20q13 (125). Both the white German and the African-American groups shared allele 3p21 containing chemokine receptors CCR2 and CCR5, which bind macrophage chemotactic protein 1 (MCP-1), promoting monocyte, macrophage, and DC recruitment into the lungs (64). CCR2−/− mice are unable to control tuberculosis infection in the lungs secondary to impaired early recruitment of macrophages and later DCs (126). CCR5−/− mice, however, retain the capacity to control tuberculosis infection, form granulomas, and induce a Th1 T cell response, making CCR2 the more likely sarcoidosis candidate gene (127).
Smaller studies probing individual candidate genes have identified TNF polymorphisms conferring sarcoidosis disease risk in British and Dutch (128), Japanese (129), and Indian patients (130). However, there is also evidence that TNF promoter polymorphisms may be irrelevant, or may predispose patients to variant forms of sarcoidosis, such as Lofgren syndrome (131–134). Other DC-related candidate genes include innate bacterial antigen receptors TLR2 (135) and TLR4 (136), although these genes have not yet been confirmed using unbiased genome-wide scans.
DISCUSSION AND FUTURE DIRECTIONS
DCs in sarcoidosis have paradoxical function. Although they have the capacity to initiate an antigen-driven, inflammatory oligoclonal T cell response (4), they are also anergic and less immunostimulatory than normal DCs (55). In this review of the literature, we show that DCs in sarcoidosis lymph nodes are mature, while those in the peripheral blood, lung, and skin are immature, suggesting a spatial segregation of DC function in sarcoidosis and a proposed resolution of the anergy paradox (8, 34, 47, 55). It is still unclear, however, what immunosuppressive factors in these end-organ sites are preventing DC maturation.
TNF is clearly an important mediator of sarcoidosis pathogenesis, as evidenced by high TNF concentrations in sarcoidosis BAL fluid that correlate with disease prognosis, genetic linkage, and the newly discovered therapeutic effects of TNF inhibitors (100, 137, 138). While TNF is produced by many cells, including DCs, macrophages, and T cells, TNF receptors are found in high abundance on DCs, making them a likely target for this inflammatory cytokine (139). Recent studies using TNF inhibitors to treat psoriasis, another autoimmune disease of the skin, identified DCs as the primary target of these drugs (50).
Fundamentally, sarcoidosis causation can be organized into two schools of thought: (1) extrinsic antigen-driven immune activation, and (2) intrinsic genetically determined overactivation of inflammatory pathways. Recently TLR2/4 ligand Mycobacterium tuberculosis (MTB) has been suggested as a causative agent of antigen-driven immune activation in sarcoidosis based on MTB nucleic acid identification within granulomas (140) and enhancement of proinflammatory cytokine release from sarcoidosis peripheral blood monocytes when incubated with MTB ligands (18) and antigens (141). In this review, we suggest that myeloid DCs are involved in pathogenesis, regardless of the initiating factor(s).
Although there is much evidence supporting the importance of DCs for the pathogenesis of sarcoidosis, many experiments must still be performed. In the lung BAL fluid, DCs can be separated from macrophages and tested for their immunostimulatory capacity. Similarly, immunostimulatory capacity of DC émigrés from sarcoidosis skin lesions could be tested against DCs from both normal skin and from other granulomatous skin diseases to determine if their decreased phenotypic maturity translates into decreased function. While most research proposes that macrophages are the major antigen-presenting cells in sarcoidosis, the relative immunostimulatory capacity of DCs versus alveolar macrophages has not yet been tested. A clinical trial treating patients with sarcoidosis with TNF inhibitors could be performed to determine if DCs are indeed the target cell for these drugs in sarcoidosis. Indeed, DCs in sarcoidosis will be an interesting field to study in the future.
Acknowledgments
The authors thank Dr. Ralph Steinman, Dr. Michelle Lowes, and Dr. Mark Judson for discussion and editorial comments.
This work was supported by National Institutes of Health MSTP grant GM07739 (to L.Z.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0033TR on April 16, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hutchinson J. Case of livid papillary psoriasis: illustrations of clinical surgery. London, UK: J'A Churchill; 1877.
- 2.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001;164:1885–1889. [DOI] [PubMed] [Google Scholar]
- 3.Rybicki BA, Major M, Popovich J Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997;145:234–241. [DOI] [PubMed] [Google Scholar]
- 4.Forman JD, Klein JT, Silver RF, Liu MC, Greenlee BM, Moller DR. Selective activation and accumulation of oligoclonal v beta-specific T cells in active pulmonary sarcoidosis. J Clin Invest 1994;94:1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mempel M, Flageul B, Suarez F, Ronet C, Dubertret L, Kourilsky P, Gachelin G, Musette P. Comparison of the T cell patterns in leprous and cutaneous sarcoid granulomas: presence of valpha24-invariant natural killer T cells in T-cell-reactive leprosy together with a highly biased T cell receptor valpha repertoire. Am J Pathol 2000;157:509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P, Holden DA, Forrester JM, Lazarus A, Wysocka M, et al. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol 1996;156:4952–4960. [PubMed] [Google Scholar]
- 7.Wahlstrom J, Berlin M, Skold CM, Wigzell H, Eklund A, Grunewald J. Phenotypic analysis of lymphocytes and monocytes/macrophages in peripheral blood and bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Thorax 1999;54:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ota M, Amakawa R, Uehira K, Ito T, Yagi Y, Oshiro A, Date Y, Oyaizu H, Shigeki T, Ozaki Y, et al. Involvement of dendritic cells in sarcoidosis. Thorax 2004;59:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol 1995;154:5071–5079. [PubMed] [Google Scholar]
- 10.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice: I. Morphology, quantitation, tissue distribution. J Exp Med 1973;137:1142–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 2007;131:994–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, Bardelli M, Montagna D, Locatelli F, Wack A. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood 2007;109:5371–5379. [DOI] [PubMed] [Google Scholar]
- 13.Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (FCgammariii(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med 2002;196:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003;19:59–70. [DOI] [PubMed] [Google Scholar]
- 15.Uehori J, Matsumoto M, Tsuji S, Akazawa T, Takeuchi O, Akira S, Kawata T, Azuma I, Toyoshima K, Seya T. Simultaneous blocking of human toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus calmette-guerin peptidoglycan. Infect Immun 2003;71:4238–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres D, Barrier M, Bihl F, Quesniaux VJ, Maillet I, Akira S, Ryffel B, Erard F. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect Immun 2004;72:2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krutzik SR, Modlin RL. The role of Toll-like receptors in combating mycobacteria. Semin Immunol 2004;16:35–41. [DOI] [PubMed] [Google Scholar]
- 18.Wiken M, Grunewald J, Eklund A, Wahlstrom J. Higher monocyte expression of tlr2 and tlr4, and enhanced pro-inflammatory synergy of tlr2 with nod2 stimulation in sarcoidosis. J Clin Immunol 2009;29:78–89. [DOI] [PubMed] [Google Scholar]
- 19.Jang S, Uzelac A, Salgame P. Distinct chemokine and cytokine gene expression pattern of murine dendritic cells and macrophages in response to mycobacterium tuberculosis infection. J Leukoc Biol 2008;84:1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moller DR. Potential etiologic agents in sarcoidosis. Proc Am Thorac Soc 2007;4:465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anis MM, Fulton SA, Reba SM, Liu Y, Harding CV, Boom WH. Modulation of pulmonary dendritic cell function during mycobacterial infection. Infect Immun 2008;76:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rissoan MC, Duhen T, Bridon JM, Bendriss-Vermare N, Peronne C, de Saint Vis B, Briere F, Bates EE. Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, eph-b1, granzyme b, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood 2002;100:3295–3303. [DOI] [PubMed] [Google Scholar]
- 23.Liu YJ. Ipc: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 2005;23:275–306. [DOI] [PubMed] [Google Scholar]
- 24.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human tlr9 confers responsiveness to bacterial DNA via species-specific cpg motif recognition. Proc Natl Acad Sci USA 2001;98:9237–9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 2004;303:1526–1529. [DOI] [PubMed] [Google Scholar]
- 26.Katz SI, Tamaki K, Sachs DH. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature 1979;282:324–326. [DOI] [PubMed] [Google Scholar]
- 27.Perreault C, Pelletier M, Landry D, Gyger M. Study of Langerhans cells after allogeneic bone marrow transplantation. Blood 1984;63:807–811. [PubMed] [Google Scholar]
- 28.Strunk D, Egger C, Leitner G, Hanau D, Stingl G. A skin homing molecule defines the Langerhans cell progenitor in human peripheral blood. J Exp Med 1997;185:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larregina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, Thomson AW, Falo LD Jr. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol 2001;2:1151–1158. [DOI] [PubMed] [Google Scholar]
- 30.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol 2006;7:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nestle FO, Filgueira L, Nickoloff BJ, Burg G. Human dermal dendritic cells process and present soluble protein antigens. J Invest Dermatol 1998;110:762–766. [DOI] [PubMed] [Google Scholar]
- 32.Simon JC, Tigelaar RE, Bergstresser PR, Edelbaum D, Cruz PD Jr. Ultraviolet b radiation converts Langerhans cells from immunogenic to tolerogenic antigen-presenting cells: induction of specific clonal anergy in CD4+ T helper 1 cells. J Immunol 1991;146:485–491. [PubMed] [Google Scholar]
- 33.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003;21:685–711. [DOI] [PubMed] [Google Scholar]
- 34.Lommatzsch M, Bratke K, Bier A, Julius P, Kuepper M, Luttmann W, Virchow JC. Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur Respir J 2007;30:878–886. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Naito M, Takeya M. Development and heterogeneity of macrophages and their related cells through their differentiation pathways. Pathol Int 1996;46:473–485. [DOI] [PubMed] [Google Scholar]
- 36.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, Gonzalez J, Krueger JG, Lowes MA. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol 2009;129:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of cd11c+bdca-1+ dendritic cells and cd163+fxiiia+ macrophages. J Clin Invest 2007;117:2517–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol 2005;23:901–944. [DOI] [PubMed] [Google Scholar]
- 39.Lohmann-Matthes ML, Steinmuller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J 1994;7:1678–1689. [PubMed] [Google Scholar]
- 40.Reddy RC. Immunomodulatory role of PPAR-gamma in alveolar macrophages. J Investig Med 2008;56:522–527. [DOI] [PubMed] [Google Scholar]
- 41.Mentzer SJ. Dendritic cells in the pathophysiology of sarcoidal reactions. In Vivo 2000;14:209–212. [PubMed] [Google Scholar]
- 42.Nicod LP, Isler P. Alveolar macrophages in sarcoidosis coexpress high levels of CD86 (b7.2), CD40, and CD30l. Am J Respir Cell Mol Biol 1997;17:91–96. [DOI] [PubMed] [Google Scholar]
- 43.Havenith CE, van Haarst JM, Breedijk AJ, Betjes MG, Hoogsteden HC, Beelen RH, Hoefsmit EC. Enrichment and characterization of dendritic cells from human bronchoalveolar lavages. Clin Exp Immunol 1994;96:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holt, et al. Originally published in the Journal of Experimental Medicine. 1993;177:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med 1993;177:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schon-Hegrad MA, Oliver J, McMenamin PG, Holt PG. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med 1991;173:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra BB, Poulter LW, Janossy G, James DG. The distribution of lymphoid and macrophage like cell subsets of sarcoid and kveim granulomata: possible mechanism of negative PPD reaction in sarcoidosis. Clin Exp Immunol 1983;54:705–715. [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw PA, Fletcher A. Counts of s-100 positive epidermal dendritic cells in kveim test skin biopsies. Histopathology 1991;18:149–153. [DOI] [PubMed] [Google Scholar]
- 49.Oliver SJ, Kikuchi T, Krueger JG, Kaplan G. Thalidomide induces granuloma differentiation in sarcoid skin lesions associated with disease improvement. Clin Immunol 2002;102:225–236. [DOI] [PubMed] [Google Scholar]
- 50.Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez Farinas M, Fuentes-Duculan J, Novitskaya I, Khatcherian A, Bluth MJ, Lowes MA, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med 2007;204:3183–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, Kambouchner M, Valeyre D, Chapelon-Abric C, Debre P, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med 2006;203:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spiteri MA, Clarke SW, Poulter LW. Phenotypic and functional changes in alveolar macrophages contribute to the pathogenesis of pulmonary sarcoidosis. Clin Exp Immunol 1988;74:359–364. [PMC free article] [PubMed] [Google Scholar]
- 53.Krombach F, Gerlach JT, Padovan C, Burges A, Behr J, Beinert T, Vogelmeier C. Characterization and quantification of alveolar monocyte-like cells in human chronic inflammatory lung disease. Eur Respir J 1996;9:984–991. [DOI] [PubMed] [Google Scholar]
- 54.Zissel G, Ernst M, Schlaak M, Muller-Quernheim J. Accessory function of alveolar macrophages from patients with sarcoidosis and other granulomatous and nongranulomatous lung diseases. J Investig Med 1997;45:75–86. [PubMed] [Google Scholar]
- 55.Mathew S, Bauer KL, Fischoeder A, Bhardwaj N, Oliver SJ. The anergic state in sarcoidosis is associated with diminished dendritic cell function. J Immunol 2008;181:746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol 2007;179:3488–3494. [DOI] [PubMed] [Google Scholar]
- 57.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med 2007;204:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soler P, Basset F. Morphology and distribution of the cells of a sarcoid granuloma: ultrastructural study of serial sections. Ann N Y Acad Sci 1976;278:147–160. [DOI] [PubMed] [Google Scholar]
- 59.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol 1994;153:256–261. [PubMed] [Google Scholar]
- 60.Metzger Z, Hoffeld JT, Oppenheim JJ. Macrophage-mediated suppression: I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol 1980;124:983–988. [PubMed] [Google Scholar]
- 61.Tailleux L, Pham-Thi N, Bergeron-Lafaurie A, Herrmann JL, Charles P, Schwartz O, Scheinmann P, Lagrange PH, de Blic J, Tazi A, et al. DC-sign induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med 2005;2:e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antoniou KM, Tzouvelekis A, Alexandrakis MG, Tsiligianni I, Tzanakis N, Sfiridaki K, Rachiotis G, Bouros D, Siafakas NM. Upregulation of Th1 cytokine profile (IL-12, IL-18) in bronchoalveolar lavage fluid in patients with pulmonary sarcoidosis. J Interferon Cytokine Res 2006;26:400–405. [DOI] [PubMed] [Google Scholar]
- 63.Demedts IK, Brusselle GG, Vermaelen KY, Pauwels RA. Identification and characterization of human pulmonary dendritic cells. Am J Respir Cell Mol Biol 2005;32:177–184. [DOI] [PubMed] [Google Scholar]
- 64.Cochand L, Isler P, Songeon F, Nicod LP. Human lung dendritic cells have an immature phenotype with efficient mannose receptors. Am J Respir Cell Mol Biol 1999;21:547–554. [DOI] [PubMed] [Google Scholar]
- 65.Nicod LP, Lipscomb MF, Toews GB, Weissler JC. Separation of potent and poorly functional human lung accessory cells based on autofluorescence. J Leukoc Biol 1989;45:458–465. [DOI] [PubMed] [Google Scholar]
- 66.Lipscomb MF, Lyons CR, Nunez G, Ball EJ, Stastny P, Vial W, Lem V, Weissler J, Miller LM. Human alveolar macrophages: Hla-dr-positive macrophages that are poor stimulators of a primary mixed leukocyte reaction. J Immunol 1986;136:497–504. [PubMed] [Google Scholar]
- 67.Zissel G, Baumer I, Fleischer B, Schlaak M, Muller-Quernheim J. Tcr v beta families in T cell clones from sarcoid lung parenchyma, BAL, and blood. Am J Respir Crit Care Med 1997;156:1593–1600. [DOI] [PubMed] [Google Scholar]
- 68.Xia W, Pinto CE, Kradin RL. The antigen-presenting activities of ia+ dendritic cells shift dynamically from lung to lymph node after an airway challenge with soluble antigen. J Exp Med 1995;181:1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med 1994;179:1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood: an improved method with special regard to clinical applicability. J Immunol Methods 1996;196:137–151. [DOI] [PubMed] [Google Scholar]
- 71.Jacobson NG, Szabo SJ, Guler ML, Gorham JD, Murphy KM. Regulation of interleukin-12 signalling during T helper phenotype development. Adv Exp Med Biol 1996;409:61–73. [DOI] [PubMed] [Google Scholar]
- 72.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of Th1 CD4+ T cells through IL-12 produced by listeria-induced macrophages. Science 1993;260:547–549. [DOI] [PubMed] [Google Scholar]
- 73.Shigehara K, Shijubo N, Ohmichi M, Kon S, Shibuya Y, Takahashi R, Morita-Ichimura S, Tatsuno T, Hiraga Y, Abe S, et al. Enhanced mRNA expression of Th1 cytokines and IL-12 in active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2000;17:151–157. [PubMed] [Google Scholar]
- 74.Shigehara K, Shijubo N, Ohmichi M, Takahashi R, Kon S, Okamura H, Kurimoto M, Hiraga Y, Tatsuno T, Abe S, et al. IL-12 and IL-18 are increased and stimulate IFN-gamma production in sarcoid lungs. J Immunol 2001;166:642–649. [DOI] [PubMed] [Google Scholar]
- 75.Taha RA, Minshall EM, Olivenstein R, Ihaku D, Wallaert B, Tsicopoulos A, Tonnel AB, Damia R, Menzies D, Hamid QA. Increased expression of IL-12 receptor mrna in active pulmonary tuberculosis and sarcoidosis. Am J Respir Crit Care Med 1999;160:1119–1123. [DOI] [PubMed] [Google Scholar]
- 76.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12r beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med 1997;185:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu D, Chan WL, Leung BP, Hunter D, Schulz K, Carter RW, McInnes IB, Robinson JH, Liew FY. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med 1998;188:1485–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gudmundsson G, Hunninghake GW. Interferon-gamma is necessary for the expression of hypersensitivity pneumonitis. J Clin Invest 1997;99:2386–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barna BP, Culver DA, Abraham S, Malur A, Bonfield TL, John N, Farver CF, Drazba JA, Raychaudhuri B, Kavuru MS, et al. Depressed peroxisome proliferator-activated receptor gamma (PPARgamma) is indicative of severe pulmonary sarcoidosis: possible involvement of interferon gamma (IFN-gamma). Sarcoidosis Vasc Diffuse Lung Dis 2006;23:93–100. [PubMed] [Google Scholar]
- 80.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998;391:79–82. [DOI] [PubMed] [Google Scholar]
- 81.Thompson PW, Bayliffe AI, Warren AP, Lamb JR. Interleukin-10 is upregulated by nanomolar rosiglitazone treatment of mature dendritic cells and human CD4+ T cells. Cytokine 2007;39:184–191. [DOI] [PubMed] [Google Scholar]
- 82.Reddy RC, Keshamouni VG, Jaigirdar SH, Zeng X, Leff T, Thannickal VJ, Standiford TJ. Deactivation of murine alveolar macrophages by peroxisome proliferator-activated receptor-gamma ligands. Am J Physiol Lung Cell Mol Physiol 2004;286:L613–L619. [DOI] [PubMed] [Google Scholar]
- 83.Fireman E, Kraiem Z, Sade O, Greif J, Fireman Z. Induced sputum-retrieved matrix metalloproteinase 9 and tissue metalloproteinase inhibitor 1 in granulomatous diseases. Clin Exp Immunol 2002;130:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henry MT, McMahon K, Mackarel AJ, Prikk K, Sorsa T, Maisi P, Sepper R, Fitzgerald MX, O'Connor CM. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-1 in sarcoidosis and IPF. Eur Respir J 2002;20:1220–1227. [DOI] [PubMed] [Google Scholar]
- 85.Agostini C, Cassatella M, Zambello R, Trentin L, Gasperini S, Perin A, Piazza F, Siviero M, Facco M, Dziejman M, et al. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol 1998;161:6413–6420. [PubMed] [Google Scholar]
- 86.Agostini C, Facco M, Chilosi M, Semenzato G. Alveolar macrophage-T cell interactions during Th1-type sarcoid inflammation. Microsc Res Tech 2001;53:278–287. [DOI] [PubMed] [Google Scholar]
- 87.Nishioka Y, Manabe K, Kishi J, Wang W, Inayama M, Azuma M, Sone S. CXCl9 and 11 in patients with pulmonary sarcoidosis: a role of alveolar macrophages. Clin Exp Immunol 2007;149:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gibejova A, Mrazek F, Subrtova D, Sekerova V, Szotkowska J, Kolek V, du Bois RM, Petrek M. Expression of macrophage inflammatory protein-3 beta/ccl19 in pulmonary sarcoidosis. Am J Respir Crit Care Med 2003;167:1695–1703. [DOI] [PubMed] [Google Scholar]
- 89.Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, Novitskaya I, Carbonaro H, Cardinale I, Kikuchi T, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc Natl Acad Sci USA 2005;102:19057–19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nicod LP, Galve-de Rochemonteix B, Dayer JM. Dissociation between allogeneic T cell stimulation and interleukin-1 or tumor necrosis factor production by human lung dendritic cells. Am J Respir Cell Mol Biol 1990;2:515–522. [DOI] [PubMed] [Google Scholar]
- 91.Fehrenbach H, Zissel G, Goldmann T, Tschernig T, Vollmer E, Pabst R, Muller-Quernheim J. Alveolar macrophages are the main source for tumour necrosis factor-alpha in patients with sarcoidosis. Eur Respir J 2003;21:421–428. [DOI] [PubMed] [Google Scholar]
- 92.Plaetinck G, Combe MC, Corthesy P, Sperisen P, Kanamori H, Honjo T, Nabholz M. Control of IL-2 receptor-alpha expression by IL-1, tumor necrosis factor, and IL-2: complex regulation via elements in the 5′ flanking region. J Immunol 1990;145:3340–3347. [PubMed] [Google Scholar]
- 93.Tartaglia LA, Goeddel DV, Reynolds C, Figari IS, Weber RF, Fendly BM, Palladino MA Jr. Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J Immunol 1993;151:4637–4641. [PubMed] [Google Scholar]
- 94.Agostini C, Trentin L, Facco M, Sancetta R, Cerutti A, Tassinari C, Cimarosto L, Adami F, Cipriani A, Zambello R, et al. Role of IL-15, IL-2, and their receptors in the development of T cell alveolitis in pulmonary sarcoidosis. J Immunol 1996;157:910–918. [PubMed] [Google Scholar]
- 95.Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, Albera C, Brutsche M, Davis G, Donohue JF, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174:795–802. [DOI] [PubMed] [Google Scholar]
- 96.Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U. Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages. Am J Respir Crit Care Med 1999;159:508–511. [DOI] [PubMed] [Google Scholar]
- 97.Zabel P, Entzian P, Dalhoff K, Schlaak M. Pentoxifylline in treatment of sarcoidosis. Am J Respir Crit Care Med 1997;155:1665–1669. [DOI] [PubMed] [Google Scholar]
- 98.Nguyen YT, Dupuy A, Cordoliani F, Vignon-Pennamen MD, Lebbe C, Morel P, Rybojad M. Treatment of cutaneous sarcoidosis with thalidomide. J Am Acad Dermatol 2004;50:235–241. [DOI] [PubMed] [Google Scholar]
- 99.Tavares JL, Wangoo A, Dilworth P, Marshall B, Kotecha S, Shaw RJ. Thalidomide reduces tumour necrosis factor-alpha production by human alveolar macrophages. Respir Med 1997;91:31–39. [DOI] [PubMed] [Google Scholar]
- 100.Ziegenhagen MW, Muller-Quernheim J. The cytokine network in sarcoidosis and its clinical relevance. J Intern Med 2003;253:18–30. [DOI] [PubMed] [Google Scholar]
- 101.Mc Dermott R, Ziylan U, Spehner D, Bausinger H, Lipsker D, Mommaas M, Cazenave JP, Raposo G, Goud B, de la Salle H, et al. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal langerhans cells, which form where langerin accumulates. Mol Biol Cell 2002;13:317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Langerhans P. Uber die Nerven der menschlichen Haut. Virchows Arch 1868;A(44):325–337.
- 103.Munro CS, Campbell DA, Du Bois RM, Mitchell DN, Cole PJ, Poulter LW. Dendritic cells in cutaneous, lymph node and pulmonary lesions of sarcoidosis. Scand J Immunol 1987;25:461–467. [DOI] [PubMed] [Google Scholar]
- 104.Braathen LR, Thorsby E. Studies on human epidermal Langerhans cells: I. Allo-activating and antigen-presenting capacity. Scand J Immunol 1980;11:401–408. [DOI] [PubMed] [Google Scholar]
- 105.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol 2002;2:77–84. [DOI] [PubMed] [Google Scholar]
- 106.Smetana K Jr, Mericka O, Saeland S, Homolka J, Brabec J, Gabius HJ. Diagnostic relevance of langerin detection in cells from bronchoalveolar lavage of patients with pulmonary Langerhans cell histiocytosis, sarcoidosis and idiopathic pulmonary fibrosis. Virchows Arch 2004;444:171–174. [DOI] [PubMed] [Google Scholar]
- 107.Martin AG, Kleinhenz ME, Elmets CA. Immunohistologic identification of antigen-presenting cells in cutaneous sarcoidosis. J Invest Dermatol 1986;86:625–629. [DOI] [PubMed] [Google Scholar]
- 108.Angel CE, George E, Brooks AE, Ostrovsky LL, Brown TL, Dunbar PR. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J Immunol 2006;176:5730–5734. [DOI] [PubMed] [Google Scholar]
- 109.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 2005;22:643–654. [DOI] [PubMed] [Google Scholar]
- 110.Silberberg-Sinakin I, Thorbecke GJ. Contact hypersensitivity and Langerhans cells. J Invest Dermatol 1980;75:61–67. [DOI] [PubMed] [Google Scholar]
- 111.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol 2002;3:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alazemi S, Campos MA. Interferon-induced sarcoidosis. Int J Clin Pract 2006;60:201–211. [DOI] [PubMed] [Google Scholar]
- 113.Doyle MK, Berggren R, Magnus JH. Interferon-induced sarcoidosis. J Clin Rheumatol 2006;12:241–248. [DOI] [PubMed] [Google Scholar]
- 114.Hoffmann RM, Jung MC, Motz R, Gossl C, Emslander HP, Zachoval R, Pape GR. Sarcoidosis associated with interferon-alpha therapy for chronic hepatitis C. J Hepatol 1998;28:1058–1063. [DOI] [PubMed] [Google Scholar]
- 115.Akahoshi M, Ishihara M, Remus N, Uno K, Miyake K, Hirota T, Nakashima K, Matsuda A, Kanda M, Enomoto T, et al. Association between ifna genotype and the risk of sarcoidosis. Hum Genet 2004;114:503–509. [DOI] [PubMed] [Google Scholar]
- 116.Ronnblom LE, Alm GV, Oberg KE. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med 1991;115:178–183. [DOI] [PubMed] [Google Scholar]
- 117.Ketikoglou I, Karatapanis S, Elefsiniotis I, Kafiri G, Moulakakis A. Extensive psoriasis induced by pegylated interferon alpha-2b treatment for chronic hepatitis b. Eur J Dermatol 2005;15:107–109. [PubMed] [Google Scholar]
- 118.Cheng PN, Wei YL, Chang TT, Chen JS, Young KC. Therapy with interferon-alpha and ribavirin for chronic hepatitis C virus infection upregulates membrane hla-abc, CD86, and CD28 on peripheral blood mononuclear cells. J Med Virol 2008;80:989–996. [DOI] [PubMed] [Google Scholar]
- 119.Mohty M, Vialle-Castellano A, Nunes JA, Isnardon D, Olive D, Gaugler B. IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities. J Immunol 2003;171:3385–3393. [DOI] [PubMed] [Google Scholar]
- 120.Hata M, Sugisaki K, Miyazaki E, Kumamoto T, Tsuda T. Circulating IL-12 p40 is increased in the patients with sarcoidosis, correlation with clinical markers. Intern Med 2007;46:1387–1393. [DOI] [PubMed] [Google Scholar]
- 121.Tucci M, Lombardi L, Richards HB, Dammacco F, Silvestris F. Overexpression of interleukin-12 and T helper 1 predominance in lupus nephritis. Clin Exp Immunol 2008;154:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kikawada M, Ichinose Y, Kunisawa A, Yanagisawa N, Minemura K, Kasuga I, Yonemaru M, Kawanishi K, Takasaki M, Toyama K. Sarcoidosis induced by interferon therapy for chronic myelogenous leukaemia. Respirology 1998;3:41–44. [DOI] [PubMed] [Google Scholar]
- 123.Smith G, Brownell I, Sanchez M, Prystowsky S. Advances in the genetics of sarcoidosis. Clin Genet 2008;73:401–412. [DOI] [PubMed] [Google Scholar]
- 124.Schurmann M, Reichel P, Muller-Myhsok B, Schlaak M, Muller-Quernheim J, Schwinger E. Results from a genome-wide search for predisposing genes in sarcoidosis. Am J Respir Crit Care Med 2001;164:840–846. [DOI] [PubMed] [Google Scholar]
- 125.Rybicki BA, Hirst K, Iyengar SK, Barnard JG, Judson MA, Rose CS, Donohue JF, Kavuru MS, Rabin DL, Rossman MD, et al. A sarcoidosis genetic linkage consortium: the sarcoidosis genetic analysis (saga) study. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:115–122. [PubMed] [Google Scholar]
- 126.Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA 2001;98:7958–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Algood HM, Flynn JL. CCR5-deficient mice control Mycobacterium tuberculosis infection despite increased pulmonary lymphocytic infiltration. J Immunol 2004;173:3287–3296. [DOI] [PubMed] [Google Scholar]
- 128.Grutters JC, Sato H, Pantelidis P, Lagan AL, McGrath DS, Lammers JW, van den Bosch JM, Wells AU, du Bois RM, Welsh KI. Increased frequency of the uncommon tumor necrosis factor -857t allele in British and Dutch patients with sarcoidosis. Am J Respir Crit Care Med 2002;165:1119–1124. [DOI] [PubMed] [Google Scholar]
- 129.Takashige N, Naruse TK, Matsumori A, Hara M, Nagai S, Morimoto S, Hiramitsu S, Sasayama S, Inoko H. Genetic polymorphisms at the tumour necrosis factor loci (TNFa and TNFb) in cardiac sarcoidosis. Tissue Antigens 1999;54:191–193. [DOI] [PubMed] [Google Scholar]
- 130.Sharma S, Ghosh B, Sharma SK. Association of TNF polymorphisms with sarcoidosis, its prognosis and tumour necrosis factor (TNF)-alpha levels in asian indians. Clin Exp Immunol 2008;151:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schurmann M, Kwiatkowski R, Albrecht M, Fischer A, Hampe J, Muller-Quernheim J, Schwinger E, Schreiber S. Study of Toll-like receptor gene loci in sarcoidosis. Clin Exp Immunol 2008;152:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seitzer U, Swider C, Stuber F, Suchnicki K, Lange A, Richter E, Zabel P, Muller-Quernheim J, Flad HD, Gerdes J. Tumour necrosis factor alpha promoter gene polymorphism in sarcoidosis. Cytokine 1997;9:787–790. [DOI] [PubMed] [Google Scholar]
- 133.Somoskovi A, Zissel G, Seitzer U, Gerdes J, Schlaak M, Muller Quernheim J. Polymorphisms at position -308 in the promoter region of the TNF-alpha and in the first intron of the TNF-beta genes and spontaneous and lipopolysaccharide-induced TNF-alpha release in sarcoidosis. Cytokine 1999;11:882–887. [DOI] [PubMed] [Google Scholar]
- 134.McDougal KE, Fallin MD, Moller DR, Song Z, Cutler DJ, Steiner LL, Cutting GR. Variation in the lymphotoxin-alpha/tumor necrosis factor locus modifies risk of erythema nodosum in sarcoidosis. J Invest Dermatol 2009;129:1921–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Veltkamp M, Wijnen PA, van Moorsel CH, Rijkers GT, Ruven HJ, Heron M, Bekers O, Claessen AM, Drent M, van den Bosch JM, et al. Linkage between Toll-like receptor (TLR) 2 promotor and intron polymorphisms: functional effects and relevance to sarcoidosis. Clin Exp Immunol 2007;149:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pabst S, Baumgarten G, Stremmel A, Lennarz M, Knufermann P, Gillissen A, Vetter H, Grohe C. Toll-like receptor (TLR) 4 polymorphisms are associated with a chronic course of sarcoidosis. Clin Exp Immunol 2006;143:420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest 2005;127:1064–1071. [DOI] [PubMed] [Google Scholar]
- 138.Schurmann M, Reichel P, Muller-Myhsok B, Dieringer T, Wurm K, Schlaak M, Muller-Quernheim J, Schwinger E. Angiotensin-converting enzyme (ACE) gene polymorphisms and familial occurrence of sarcoidosis. J Intern Med 2001;249:77–83. [DOI] [PubMed] [Google Scholar]
- 139.Funk JO, Walczak H, Voigtlander C, Berchtold S, Baumeister T, Rauch P, Rossner S, Steinkasserer A, Schuler G, Lutz MB. Cutting edge: resistance to apoptosis and continuous proliferation of dendritic cells deficient for TNF receptor-1. J Immunol 2000;165:4792–4796. [DOI] [PubMed] [Google Scholar]
- 140.Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J 2007;30:508–516. [DOI] [PubMed] [Google Scholar]
- 141.Chen ES, Wahlstrom J, Song Z, Willett MH, Wiken M, Yung RC, West EE, McDyer JF, Zhang Y, Eklund A, et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol 2008;181:8784–8796. [DOI] [PMC free article] [PubMed] [Google Scholar]