Abstract
This study was designed to isolate, characterize, and culture human spermatogonia. Using immunohistochemistry on tubule sections, we localized GPR125 to the plasma membrane of a subset of the spermatogonia. Immunohistochemistry also showed that MAGEA4 was expressed in all spermatogonia (Adark, Apale, and type B) and possibly preleptotene spermatocytes. Notably, KIT was expressed in late spermatocytes and round spermatids, but apparently not in human spermatogonia. UCHL1 was found in the cytoplasm of spermatogonia, whereas POU5F1 was not detected in any of the human germ cells. GFRA1 and ITGA6 were localized to the plasma membrane of the spermatogonia. Next, we isolated GPR125-positive spermatogonia from adult human testes using a two-step enzymatic digestion followed by magnetic-activated cell sorting. The isolated GPR125-positive cells coexpressed GPR125, ITGA6, THY1, and GFRA1, and they could be cultured for short periods of time and exhibited a marked increase in cell numbers as shown by a proliferation assay. Immunocytochemistry of putative stem cell genes after 2 wk in culture revealed that the cells were maintained in an undifferentiated state. MAPK1/3 phosphorylation was increased after 2 wk of culture of the GPR125-positive spermatogonia compared to the freshly isolated cells. Taken together, these results indicate that human spermatogonia share some but not all phenotypes with spermatogonial stem cells (SSCs) and progenitors from other species. GPR125-positive spermatogonia are phenotypically putative human SSCs and retain an undifferentiated status in vitro. This study provides novel insights into the molecular characteristics, isolation, and culture of human SSCs and/or progenitors and suggests that the MAPK1/3 pathway is involved in their proliferation.
Keywords: culture, G protein-coupled receptor 125 (GPR125), human putative SSCs and progenitor cells, human spermatogonial stem cells, isolation, MAPK1/3 pathway, phenotypic characterization, spermatogenesis, testis
Human GPR125-positive spermatogonia can be cultured, have phenotypic characteristics of spermatogonial stem cells, and proliferate via MAPK1/3 pathway activation.
INTRODUCTION
Spermatogenesis is a complex process that involves the self-renewal and differentiation of spermatogonial stem cells (SSCs) into mature spermatids in the seminiferous tubules of mammals. It begins at 5–7 days after birth in rodents and 10–13 yr after birth in man. A great deal of progress has been made on the biochemical characterization and culture of spermatogonia and SSCs in rodents. We and others have demonstrated that GFRA1 (GDNF family receptor alpha 1) is a marker for mouse SSCs and progenitor cells [1–4] and that KIT (CD117), the receptor for stem cell factor (SCF), is a hallmark for differentiating spermatogonia including type A1–A4 spermatogonia [4, 5]. POU5F1, also known as OCT-4, is regarded as a nuclear marker for spermatogonial stem and progenitor cells [2, 6, 7] and it is required for the self-renewal of mouse SSCs [8]. In addition, ZBTB16, zinc finger and BTB domain-containing 16, also known as PLZF (promyelocytic leukemia zinc finger protein), has been demonstrated to be a nuclear transcription factor for mouse SSCs and progenitors [9, 10], whereas ITGA6 (CD49f; also known as integrin, alpha 6) and THY1 (CD90) are surface markers for mouse SSCs and progenitors [11, 12]. Recently, G protein-coupled receptor 125 (GPR125) has been shown to be a marker for mouse SSCs and their progeny [13]. Furthermore, rodent spermatogonia and SSCs could be isolated efficiently and cultured for short and long periods [2, 14, 15], which makes it feasible to examine factors that control the fate determination of the cells and explore molecular mechanisms regulating the early phases of spermatogenesis. However, this type of research on human spermatogonia and SSCs is extremely limited. Clermont [16–19] first characterized the Adark and Apale spermatogonia in human testis and suggested that the Adark were reserve stem cells whereas the Apale were renewing stem cells. This spermatogonial renewal model for man has been challenged more recently in a review by Ehmcke and Schlatt [20], who suggested that Apale spermatogonia underwent additional mitotic divisions. Nevertheless, more than 40 yr after Clermont presented his initial findings, very little new information is available on the true identity of the human SSC and the pattern of renewal vs. differentiation [21]. The phenotypic characterization, isolation, and culture of human spermatogonia, particularly of the SSCs, may offer novel information on this topic. One major reason for so little progress on human spermatogonia and SSCs has been the difficulty in gaining access to sufficient quantities of normal human testis for research purposes. We identified a novel source of human testicular material, namely from recently (∼1–2 h from death) deceased organ donors, which allowed us to work efficiently on the spermatogonia.
In the current study, we used markers that have been identified for SSCs and progenitors in other species to characterize the phenotypes of human spermatogonia and more differentiated germ cells. For example, GPR125, THY1, ZBTB16, GFRA1, POU5F1, KIT, and PCNA have been shown to be expressed in mouse or monkey SSCs and progenitors [2–4, 13, 14, 22, 23]. We chose to examine UCHL1 (ubiquitin carboxyl-terminal esterase L1; also known as protein gene product 9.5) expression because it is expressed in pig spermatogonia [24] and may be involved in the asymmetric divisions [25]. We also chose to probe the expression of MAGEA4 in human testis because it is a potential specific marker for normal premeiotic germ cells [26].
The in vitro culture system is an effective approach to explore molecular mechanisms controlling the proliferation and differentiation of SSCs and progenitors, because it is difficult to investigate SSC/progenitor behavior in vivo. We have successfully isolated GPR125-positive spermatogonia from adult human testes using a two-step enzymatic digestion, followed by magnetic-activated cell sorting (MACS). The phenotype of the freshly isolated GPR125-positive spermatogonia was characterized by immunocytochemical analysis of expression of markers previously used for spermatogonial stem/progenitor cells in other species [2–4, 13, 14, 22, 23]. Human GPR125-postive spermatogonia could be cultured for a short period in StemPro-34 SFM medium supplemented with a number of growth factors.
The extracellular signal-regulated kinase (ERK), an important member of the mitogen-activated protein kinases (MAPKs), is involved in modulating a variety of cellular functions, including proliferation, differentiation, and cell cycle progression [27, 28]. It has been suggested that ERK is essential for the proliferation of KIT-expressing type A1–A4 spermatogonia stimulated by SCF [27]. We have recently demonstrated that GDNF signals via the MAPK1/3 (ERK1/2) pathway to promote the proliferation of mouse spermatogonial stem/progenitor cells [29]. In the current study, we found that the phosphorylation of MAPK1/3 was up-regulated in human GPR125-positive spermatogonia after being cultured for 2 wk compared to freshly isolated GPR125-positive spermatogonia. Our ability to systematically characterize, isolate, and culture human spermatogonia allows us to phenotypically identify putative SSCs and progenitors in human testes.
MATERIALS AND METHODS
Procurement of Testes from Organ Donors
Testes were obtained from organ donors to the Washington Regional Transplant Consortium (WRTC; Annandale, VA). Written approval from the next of kin was obtained by the WRTC for the use of the testes in a research protocol. Prior to organ retrieval, the donors were maintained on a heart-lung machine with artificial respiration. During organ retrieval in a hospital operating suite, the surgeon first dissects the abdominal organs away from the posterior abdominal wall in order to facilitate rapid organ removal after blood flow is stopped. In adults, 30 000 units of heparin are introduced intravenously prior to aortic “cross clamping” in the thorax and in the lower abdomen. Immediately upon clamping, the aorta is perfused with Viaspan (DuPont Pharma), a standard organ preservation solution. Viaspan enters all abdominal organs as well as the testes; the testicular arteries originate from the aorta in close proximity to the renal arteries. The testes are retrieved after a 10-min perfusion, packed in cold Viaspan, and sent to the Georgetown University Medical Center by courier within 1–2 h of removal from the donor. It is important to note that as soon as the blood supply to the testes was interrupted by clamping of the aorta, the Viaspan solution entered the organ. In our laboratory, the testes were immediately placed aseptically in Dulbecco modified Eagle medium (DMEM) containing 1× antibiotic (Gibco). The data generated for this research was obtained from five donors (16–58 yr old) with testis weights ranging from 13 to 22 g each. Immunohistochemistry, cell isolation by MACS using a GPR125 antibody, and cell culture of the GPR125-positive spermatogonia were performed with testicular tissues from all five donors, with three independent experiments from each donor. The results obtained were consistent among the five donors. The causes of death included stroke, auto accident, and myocardial infarction. Most of the testis tissue was frozen as ∼1-g pieces in 10% DMSO, 80% fetal bovine serum (FBS), and 10% DMEM in liquid nitrogen, and the integrity of the tissue was excellent as assayed by hematoxylin and eosin (H&E) staining (Fig. 1) and subsequent culture. Other testis tissue was used immediately for testis section preparation, spermatogonial isolation, and cell culture as described below.
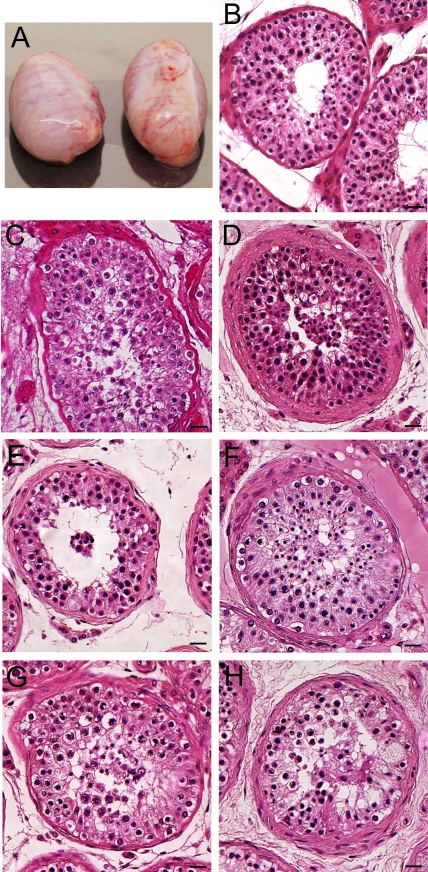
FIG. 1.
Morphology of the donor testes. A) The human testes from the 16-yr-old donor. Each testis weighed ∼14 g and was about 4.1 × 2 × 2.5 cm. B–H) H&E staining shows the morphology of the human testes from the five donors. Spermatogenesis appeared normal in all donors, although at times the fixation was not ideal in all regions of the testes. Bars = 20 μm (B–H).
Immunohistochemistry
To prepare sections, human testes were fixed in 4% paraformaldehyde for 3 h or in Bouin fixative overnight, dehydrated through a series of graded alcohols, and embedded in paraffin at 60°C overnight. For immunohistochemistry, 5-μm sections were dewaxed in xylene and rehydrated through a series of graded alcohols. Antigen retrieval was performed using the antigen retrieval Citra Plus solution (BioGenex Laboratories Inc.), and the endogenous peroxidase activity was quenched with 3% hydrogen peroxide. After permeabilization with Triton X-100 and blocking with 10% normal serum, the sections were incubated with primary antibodies (ABs), including rabbit polyclonal AB to GPR125 (Abcam Inc.) at a 1:100 dilution, mouse monoclonal AB to PLZF (Calbiochem) at a 1:100 dilution, mouse monoclonal AB to THY1 (LifeSpan Biosciences) at a 1:100 dilution, mouse monoclonal AB 57 B raised against MAGEA4 (melanoma antigen family A, 4; a gift from Professor Giulio C. Spagnoli, University Hospital of Basel, Switzerland) at a dilution of 1:200, rabbit polyclonal AB to KIT (Abcam Inc. and Santa Cruz Biotechnology, Inc.) at a 1:100 dilution, mouse monoclonal AB to PCNA (proliferating cell nuclear antigen; EMD Biosciences Inc.) at a 1:200 dilution, rabbit polyclonal AB to UCHL1 (AbD Serotec) at a 1:100 dilution, rabbit polyclonal AB to GFRA1 (Abcam Inc.) at a 1:100 dilution, mouse monoclonal AB to POU5F1 (Chemicon International) at a 1:100 dilution, phycoerythrin (PE)-conjugated mouse monoclonal AB to ITGA6 (BD Biosciences) with a dilution of 1:100, normal rabbit IgG (Santa Cruz Biotechnology, Inc.) at a 1:100 dilution, or normal mouse IgG (Santa Cruz Biotechnology, Inc.) at a 1:100 dilution, pursuant to the procedures described previously [4]. To determine the expression of GRR125, GFRA1, UCHL1, and THY1, the testis sections were incubated with secondary antibody to fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG or rhodamine-conjugated anti-rabbit or anti-mouse IgG at a 1:200 dilution for 30 min at 34°C and washed three times with PBS. All the secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. 4′, 6′-Diamidino-2-phenylindole (DAPI) was used to stain the nuclei of the cells in the testis, and the sections were observed for epifluorescence using an Olympus Fluoview 500 laser scanning microscope. To determine the expression of MAGEA4, KIT, POU5F1, PCNA, and ZBTB16 (PLZF), the sections were incubated with the biotinylated second antibody for 20 min. Next, streptavidin-peroxidase enzyme conjugate was added to the cells and reaction product was generated by addition of diaminobenzidine as the chromogen. After immunostaining, testis sections were counterstained with hematoxylin and examined under a light microscope. Replacement of primary antibody with PBS was used as a negative control.
Isolation and Culture of Human GPR125-Positive Spermatogonia
The seminiferous tubules were isolated from human testes as described above [30, 31]. The germ cells were separated using a second enzymatic digestion with 4 mg/ml collagenase IV, 2.5 mg/ml hyaluronidase (Sigma), 2 mg/ml trypsin (Sigma) and 1 μg/μl DNase I, and followed by differential plating to remove potential contamination by Sertoli and myoid cells [32]. For differential plating, germ cells and somatic cells were placed in a 15-cm-diameter tissue culture dish in DMEM/F12 medium supplemented with 10% FBS for 3 h at 34°C. Sertoli and myoid cells attached to the culture plates, and germ cells, which remained in suspension, were collected by centrifuging at 1000 rpm for 5 min. GPR125-positive spermatogonia were obtained by MACS using an antibody to GPR125 at a dilution of 1:40 (e.g., 25 μl of anti-GPR125 were added to 1 ml of cell suspension in DMEM containing 10% NU serum and 1 μg/μl DNase I), pursuant to the procedures described recently [33]. Cell isolation of GPR125-positive cells was repeated at least three times from different donor testes.
Human GPR125-positive spermatogonia were seeded in 24-well plates coated with 0.1% gelatin at a concentration of 5000 cells/well in 500 μl StemPro-34 SFM medium (Invitrogen) supplemented with 100 ng/ml human GDNF (R&D Systems), 300 ng/ml GFRA1-Fc fusion protein (GFRA1-Fc; R&D Systems), 10 ng/ml human NUDT6 (BD Biosciences), 10 ng/ml LIF (Chemicon International), 20 ng/ml EGF (BD Biosciences), 30 ng/ml TGFB (BD Biosciences), and 100 ng/ml Nodal (R&D Systems). The StemPro-34 SFM medium consisted of a basal liquid medium and frozen supplement (StemPro-Nutrient Supplement; Invitrogen) which was added at the time of use. All cultures were maintained at 34°C in a humidified 5% CO2 incubator.
Proliferation Assay of GPR125-Positive Spermatogonia
GPR125-positive spermatogonia were seeded at a density of 3000 cells in 96-well microtiter plates coated with 0.1% gelatin in 100 μl StemPro medium supplemented as above and cultured for 14 days. Proliferation assays were performed at 2-day intervals using the Non-Radioactive Cell Proliferation Assay according to the protocol of the manufacturer (Promega). The principal for the assay is that the conversion of the MTS tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] into aqueous and soluble formazan is accomplished by the dehydrogenase enzymes in metabolically active cells, and the quantity of formazan product as measured by the amount of absorbance at 490 nm is directly proportional to the number of living cells per well in culture. The C18–4 cells were established by stably transfecting type A spermatogonia from 6-day-old mice with the Large T antigen gene under the control of a ponasterone A-driven promoter [34], and they were used to generate the standard curve showing the relationship between the amount of absorbance at 490 nm and the number of living cells per well [35]. After 3 h of incubation at 34°C in a 5% CO2 incubator, optical densities were recorded by the Bio-Rad Ultra Microplate reader at 490 nm wavelength, and the data were presented as mean ± SEM from three experiments.
Immunocytochemistry
Immunocytochemistry was performed on freshly isolated GPR125-positive spermatogonia, GPR125-negative male germ cells, and GPR125-positive spermatogonia after culture for 2 wk according to the procedure described previously [4, 29]. Following extensive washes with PBS, the cells were incubated with primary antibody, including GPR125, GFRA1, THY1, PE-conjugated anti-human ITGA6, or normal rabbit IgG at a 1:100 dilution, for 1 h at 34°C. The species from which the primary antibodies were generated and the dilutions of primary antibodies are described above in Immunohistochemistry. After three washes in PBS, the cells were incubated with the secondary antibody, including FITC-conjugated donkey goat anti-rabbit or rhodamine-conjugated goat anti-rabbit IgG at a 1:200 dilution for 30 min at 34°C, and washed three times with PBS. DAPI was used to stain the nuclei, and the cells were observed for epifluorescence using an Olympus Fluoview 500 laser scanning microscope. All the secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. Replacement of primary antibody with PBS was used as a negative control.
Western Blots
The freshly isolated GPR125-positive spermatogonia and GPR125-positive spermatogonia after culture for 2 wk were lysed respectively with RIPA lysis buffer (Santa Cruz Biotechnology, Inc.). Western blots using 20 μg of proteins were carried out according to the protocol we described previously [4]. The primary antibody was against phospho-MAPK1/3 (Thr 202/Tyr 204; Santa Cruz Biotechnology, Inc.) at a dilution of 1:500, and the secondary antibody was horseradish peroxidase-conjugated anti-goat IgG at a 1:2000–1:5000 dilution. The membranes were stripped with Restore Western Blot Stripping Buffer (Pierce Biotechnology, Inc.) and reprobed with an antibody against MAPK1 (ERK2; Santa Cruz Biotechnology, Inc.). Western blotting band intensities were captured by the Fujifilm LAS-1000 imager (Fujifilm) and quantified using ImageJ software (NIH).
Statistical Analysis
The values were presented as mean ± SEM from three independent experiments, and statistically significant differences (P < 0.05) among freshly isolated GPR125-positive spermatogonia and cultured GPR125-positive spermatogonia were determined by one-way ANOVA and Tukey posttests using SPSS statistical software.
RESULTS
Morphology of the Testes
Figure 1 shows representative images of seminiferous tubules from the five donors that we employed for the present study. Spermatogenesis generally appeared normal in all donors, with many tubules in each donor showing all the germ cells. There was little variability among the five donors, although in certain regions of the testes the fixation did not appear optimal.
Phenotypic Characteristics of Human Spermatogonia and Other Germ Cells
We first explored the phenotypic characteristics of human spermatogonia and other germ cells using consensus markers for SSCs and progenitors in other species, including GPR125, ZBTB16, THY1, POU5F1, UCHL1, ITGA6, and GFRA1, as well as KIT (a marker for differentiating spermatogonia) and PCNA (a marker for proliferating cells).
GPR125 Is Expressed in a Subpopulation of Human Spermatogonia but Not in Sertoli Cells or More Differentiated Germ Cells
Immunohistochemistry using adult human testes revealed that GPR125 was localized to the plasma membrane and possibly in the cytoplasm of a subpopulation of human spermatogonia lying adjacent to the basement membrane of the seminiferous tubules, but not in Sertoli cells or differentiated germ cells (Fig. 2, A–D). Higher-magnification images confirmed that GPR125 was expressed only in human spermatogonia within the seminiferous tubules (Fig. 2, E–G). Notably, very few spermatogonia (one or two spermatogonia) were found on average to be positive for GPR125 in each seminiferous tubule cross section. No staining was observed in the negative controls using normal rabbit IgG (Supplemental Fig. S1A available at www.biolreprod.org) or PBS (Supplemental Fig. S1C) but without primary antibody, confirming the specific staining of GPR125 expression in human testis.
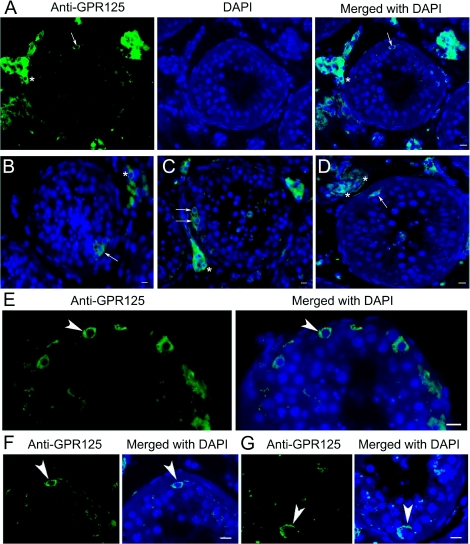
FIG. 2.
Immunohistochemistry shows GPR125 expression in human testes. A–G) GPR125 was localized at plasma membrane of a subset of human spermatogonia (arrows in A–D; arrowheads in E–G) adjacent to the basement membrane of the seminiferous epithelium. Representative pictures show that very few spermatogonia (arrows and arrowheads) were positive for GPR125 in each seminiferous tubule cross section. GPR125 staining was also observed in Leydig cells (asterisks). Bars = 10 μm.
MAGEA4 Is Expressed in Human Spermatogonia and Probably in Preleptotene Spermatocytes
Immunohistochemistry, using adult human testes, showed that MAGEA4 was expressed in all the spermatogonia and probably in preleptotene spermatocytes, but not in more differentiated germ cells or somatic cells, including Sertoli cells and Leydig cells (Fig. 3, A and B). Replacement of primary antibody with PBS served as a negative control, and no staining was observed (Fig. 3D).
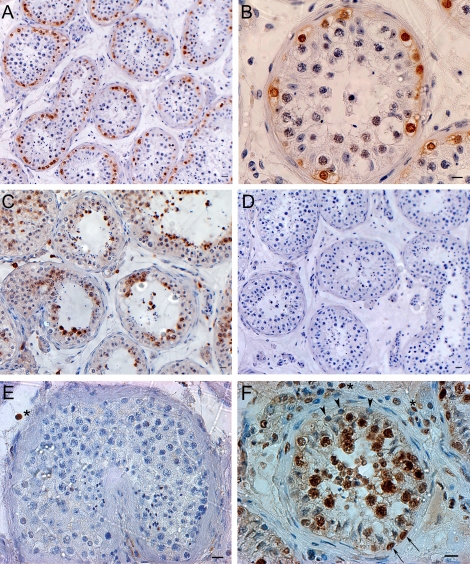
FIG. 3.
Immunohistochemistry reveals the expression of MAGEA4, KIT, POU5F1, and PCNA in human testes. A, B) MAGEA4 was expressed in all spermatogonia (Adark, Apale, and type B spermatogonia) and possibly in preleptotene spermatocytes. C) KIT was expressed in late-stage spermatocytes and round spermatids but not in other germ cells or Sertoli cells. D) Replacement of primary antibody with PBS served as negative control and no staining was observed. E) POU5F1 was undetected in human germ cells but was found occasionally in the nucleus of interstitial cells (asterisk). F) PCNA was expressed in some proliferating spermatogonia (arrows), possibly the renewing Apale, along the basement membrane of the seminiferous tubules and proliferating spermatocytes including pachytene spermatocytes, but not in nonproliferating spermatogonia (possibly the reserve Adark) and preleptotene spermatocytes (arrowheads). Some proliferating interstitial cells (asterisks) were also positive for PCNA. Bars = 20 μm (A, C–F), 10 μm (B).
KIT Is Expressed in Late Stage Spermatocytes and Round Spermatids but Not in Spermatogonia, Whereas POU5F1 Is Not Detected in Human Germ Cells; PCNA Is Expressed in Proliferating Spermatogonia and Spermatocytes but Not in Nonproliferating Spermatogonia or Preleptotene Spermatocytes
We next determined the expression of KIT, POU5F1, and PCNA in adult human testes. Immunohistochemistry revealed that KIT was expressed in late spermatocytes and round spermatids, but not in spermatogonia or early spermatocytes (Fig. 3C). Interestingly, POU5F1 was not detected in human germ cells or Sertoli cells, whereas it was expressed in a few interstitial cells (Fig. 3E). PCNA was found to be expressed in proliferating spermatogonia and proliferating spermatocytes including pachytene spermatocytes, but it was not detected in nonproliferating spermatogonia or preleptotene spermatocytes (Fig. 3F). PCNA staining was also found in proliferating interstitial cells (Fig. 3F).
UCHL1 and GFRA1 Are Present in Human Spermatogonia Whereas ITGA6 Is Expressed in Spermatogonia and Sertoli Cells; THY1 Is Expressed in a Subpopulation of Spermatogonia Whereas ZBTB16 Is Expressed in Spermatogonia
We further probed the expression of UCHL1, ITGA6, THY1, ZBTB16, and GFRA1 in adult human testes. Immunohistochemistry revealed that UCHL1 was localized to the cytoplasm of human spermatogonia along the basement membrane of seminiferous tubules, but not in Sertoli cells or differentiated germ cells (Fig. 4A). ITGA6 was localized to the plasma membrane of spermatogonia and Sertoli cells within seminiferous tubules (Fig. 4B). ITGA6 staining was also observed in Leydig cells (Fig. 4B). Furthermore, we found that THY1 was localized to the plasma membrane of very few spermatogonia (Fig. 4C), whereas ZBTB16 was expressed in the nuclei of spermatogonia (Fig. 4D). GFRA1 was found to be present in the plasma membrane of spermatogonia but not in Sertoli cells or more differentiated germ cells (Fig. 4E). Normal rabbit IgG (Supplemental Fig. S1A) was used as a negative control for anti-UCHL1 and anti-GFRA1; normal mouse IgG (Supplemental Fig. S1B) was used as a negative control for anti-ZBTB16 and anti-THY1. Replacement of primary antibody with PBS also served as a negative control (Fig. 4F).
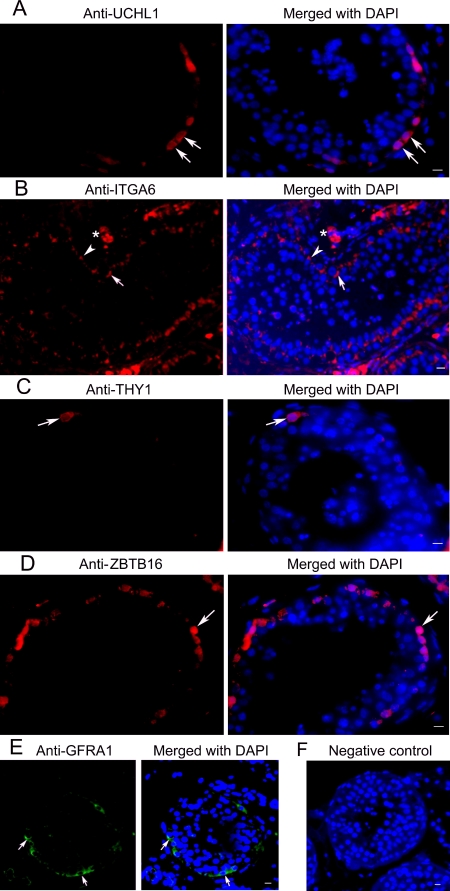
FIG. 4.
Immunohistochemistry shows expression of UCHL1, ITGA6, THY1, ZBTB16, and GFRA1 in human testes. A) Expression of UCHL1 was observed in the cytoplasm of the spermatogonia (arrows). B) Expression of ITGA6 was seen in the plasma membrane of spermatogonia (arrows). Often rows of spermatogonia along the basement membrane were stained (arrows). ITGA6 staining was also observed in Sertoli cells (arrowheads) and Leydig cells (asterisks). C) THY1 was expressed in a subpopulation of spermatogonia (arrows). D) ZBTB16 was localized to human spermatogonia (arrows) lying at the basement membrane. E) Expression of GFRA1 was found in the plasma membrane of the spermatogonia (arrows). F) Replacement of primary antibody with PBS served as negative control and no staining was observed. Bars = 10 μm.
Isolation and Phenotypic Features of Human GPR125-Positive Spermatogonia
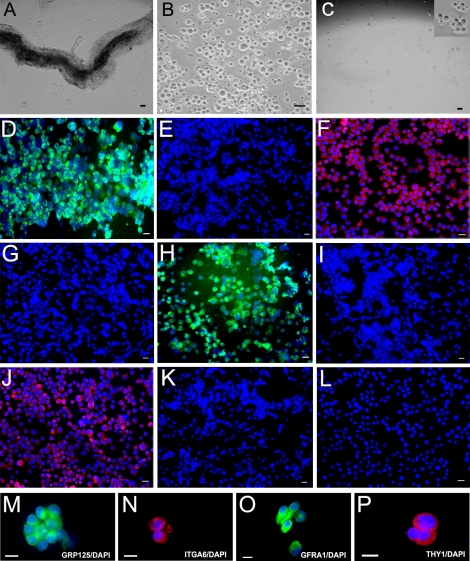
Because we observed that GPR125 was localized to the plasma membrane of a subset of human spermatogonia but not in Sertoli cells or differentiated germ cells (Fig. 2, A–G), we conducted MACS, using an antibody to GPR125, to select GPR125-positive spermatogonia from human testes. We isolated seminiferous tubules using mechanical dissociation and a one-step enzymatic digestion (Fig. 5A). Germ cells were further separated using a second enzymatic digestion followed by differential plating to remove potential contamination by Sertoli and myoid cells. We removed clumps of elongating spermatids with a 40-μm mesh filter and we obtained ∼6 × 106 germ cells from each 1 g of human testis tissue (Fig. 5B). We then obtained ∼3 × 104 human GPR125-positive spermatogonia after MACS (Fig. 5C).
FIG. 5.
Isolation and characterization of the GPR125-positive spermatogonia from human testis (freshly isolated). A) Seminiferous tubules were isolated from human testes using mechanical dissociation and a one-step enzymatic digestion. B) Human germ cells were obtained using a second enzymatic digestion and different plating. C) GPR125-positive spermatogonia were obtained using MACS with an antibody to GPR125. The inset in C is a high-magnification view showing the GPR125-positive spermatogonia. Images in A, B, and C are phase contrast. After MACS, immunocytochemical analysis showed the expression of GPR125 (D), ITGA6 (F), GFRA1 (H), and THY1 (J), in the isolated GPR125-positive spermatogonia. M–P) High magnification showed that freshly isolated GPR125- positive spermatogonia expressed GPR125 (M), ITGA6 (N), GFRA1 (O), and THY1 (P). No staining was observed in GPR125-negative male germ cells using antibody to GPR125 (E), ITGA6 (G), GFRA1 (I), or THY1 (K). L) Replacement of primary antibody with PBS in isolated GPR125-positive spermatogonia served as a negative control. Bars = 10 μm (A, C, D, F, H, J, L–P), 20 μm (B, E, G, I, and K).
We next determined the phenotypic features of the freshly isolated human GPR125-positive spermatogonia. Immunocytochemistry revealed that GPR125 was present in the plasma membrane of more than 95% of the isolated human GPR125-positive spermatogonia (Fig. 5D). Moreover, we found that freshly isolated human GPR125-positive spermatogonia coexpressed ITGA6 (Fig. 5F), GFRA1 (Fig. 5H), and THY1 (Fig. 5J). Higher magnification confirmed that the isolated GPR125-positive spermatogonia expressed GPR125 (Fig. 5M), ITGA6 (Fig. 5N), GFRA1 (Fig. 5O), and THY1 (Fig. 5P). No staining was observed in the negative controls using PBS but not primary antibody (Fig. 5L). To confirm the purity of GPR125-positive cells by MACS, we checked the GPR125-negative male germ cells. Immunocytochemistry revealed that GPR125-negative male germ cells didn't express GPR125 (Fig. 5E), ITGA6 (Fig. 5G), GFRA1 (Fig. 5I), or THY1 (Fig. 5K).
Culture and Phenotypic Characteristics of Human GPR125-Positive Spermatogonia
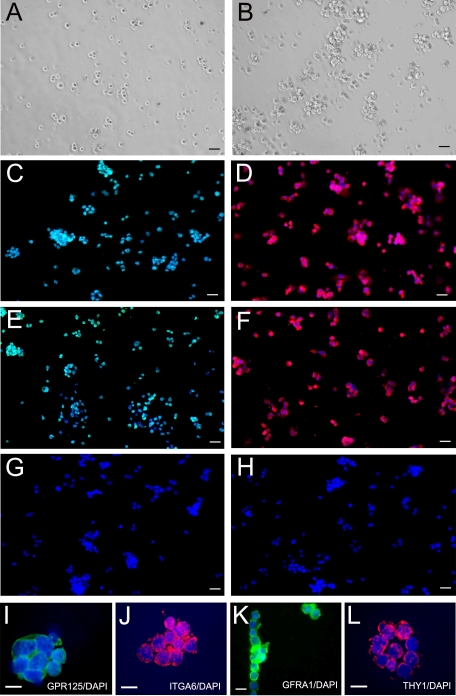
We then cultured the GPR125-positive spermatogonia in StemPro medium supplemented with a number of growth factors (Fig. 6, A and B). A proliferation assay showed that human GPR125-positive spermatogonia started to proliferate at Day 2 after plating, and the doubling time of the cells was around 5.3 days (Fig. 7A). One week after plating, these cells grew relatively faster and cell number increased by more than 5-fold (500%) after they were cultured for 14 days (Figs. 6B and 7A).
FIG. 6.
Culture for 14 days and characterization of human GPR125-positive spermatogonia. A) GPR125-positive spermatogonia were cultured in StemPro medium supplemented with GDNF, soluble GFRA1, NUDT6 LIF, EGF, Nodal, and TGFB (cells shown at day 1). B) GPR125-positive spermatogonia were cultured in StemPro medium supplemented with growth factors as mentioned above (cells shown at day 14). Panels A and B are phase contrast images. After culture for 14 days, immunocytochemical analysis showed the expression of GPR125 (C), ITGA6 (D), GFRA1 (E), and THY1 (F) in the cultured GPR125-positive spermatogonia. G) Replacement of primary antibody with PBS served as a negative control. H) Replacement of primary antibody with normal rabbit IgG served as another negative control. I–L) High magnification shows that cultured GPR125-positive spermatogonia expressed GPR125 (I), ITGA6 (J), GFRA1 (K), and THY1 (L). Bars = 20 μm (A–H), 10 μm (I–L).
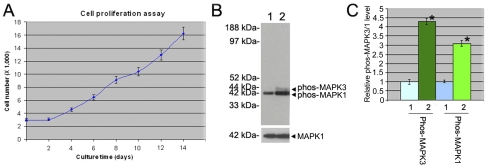
FIG. 7.
Cell number increased and MAPK1/3 phosphorylation was activated in human GPR125-positive spermatogonia after culture for 14 days. A) Proliferation assay showed a cell growth curve of human GPR125-positive spermatogonia. B) Western blots revealed that the phosphorylation of MAPK1/3 (upper panel) was activated in human GPR125-positive spermatogonia after culture for 14 days with a number of growth factors compared to the freshly isolated GPR125-positive spermatogonia. MAPK1 was used as a loading control for total proteins (lower panel). C) Relative levels of MAPK1/3 phosphorylation in the cultured GPR125-positive spermatogonia compared to the freshly isolated GPR125-positive spermatogonia (designated as 1) after normalization to the signal obtained with MAPK1. Statistically significant differences (P < 0.05) between the cultured and fresh GPR125-positive spermatogonia are indicated by asterisks. B and C) 1, freshly isolated GPR125-positive spermatogonia; 2, the cultured GPR125-positive spermatogonia.
To test whether the cultured GPR125-positive spermatogonia retained phenotypic characteristics of fresh GPR125-positive spermatogonia, we carried out an immunocytochemical analysis. As shown in Figure 6, the GPR125-positive spermatogonia after 14 days of culture still expressed GPR125 (Fig. 6C), ITGA6 (Fig. 6D), GFRA1 (Fig. 6E), and THY1 (Fig. 6F). Higher-magnification images confirmed that the cultured GPR125-positive spermatogonia expressed GPR125 (Fig. 6I), ITGA6 (Fig. 6J), GFRA1 (Fig. 6K), and THY1 (Fig. 6L). Replacement of primary antibody with PBS (Fig. 6G) and with normal rabbit IgG (Fig. 6H) served as negative controls and no staining was observed.
Human GPR125-Positive Spermatogonia Proliferate via the Activation of MAPK1/3 Pathway
To gain an insight into the molecular mechanisms that control the proliferation of human GPR125-positive spermatogonia, we further asked whether the MAPK1/3 signaling pathway was activated in human GPR125-positive spermatogonia after culture for 2 wk. Western blots clearly revealed that MAPK3 and MAPK1 phosphorylation was increased in the cultured GPR125-positive spermatogonia compared to freshly isolated GPR125-positive spermatogonia (Fig. 7B). Densitometric analyses showed a 4.3-fold and 3.1-fold increase of MAPK3 and MAPK1 phosphorylation, respectively (Fig. 7C).
DISCUSSION
In the current study, we sought to probe phenotypic characteristics of human spermatogonia using markers for SSCs and progenitors normally found in rodent, pig, and nonhuman primate testis. GPR125 is thought to be a marker for mouse SSCs and their progeny, and it is present in all types of mouse spermatogonia, including type A, intermediate, and type B spermatogonia and possibly even in preleptotene spermatocytes [13, 36]. We localized GPR125 at the plasma membrane of a subpopulation of human spermatogonia adjacent to the basement membrane of the seminiferous tubules. Unlike the wide expression in mouse [13], it is noteworthy that the frequency of GPR125-positive cells in human testes was only one or two spermatogonia per seminiferous tubule cross-section. Clermont reported that there are approximately seven Adark and Apale spermatogonia, in a 1:1 ratio, around each human seminiferous tubule cross-section [17]. Thus, our results suggest that human GPR125-positive spermatogonia are a subset of the Adark and/or the Apale spermatogonia, and GPR125-positive spermatogonia may be the SSCs in the human testis. To our knowledge, the GPR125 staining we are describing in human spermatogonia is the first report demonstrating specific staining of very, very few cells around the periphery of human seminiferous tubules, further suggesting that these may indeed be stem cells. To prove this conclusively, it will be necessary to transplant this subpopulation into sterile nude mice and determine whether seeding of the seminiferous tubules occurs, although the efficiency of the xenotransplantation assay of primate germ cells to immunodeficient mouse testes needs to be explored further [23, 37].
Human spermatogonia and progenitors share some phenotypes with rodent, pig, and monkey spermatogonia and SSCs. This can be illustrated by our observations that human spermatogonia and their progenitors expressed GPR125, THY1, GFRA1, ITGA6, and ZBTB16, markers for rodent SSCs and progenitors [1–4, 9–12]. Recently, THY1 and GFRA1 were found to be markers for monkey putative SSCs [22], indicating that these two molecules are conserved in nonhuman primates and human. Similar to rodents and rhesus monkeys [22], surface markers such as THY1 and GFRA1 may be used to select human spermatogonia. One problem is that ITGA6 is also expressed in human Sertoli cells. Thus, it is necessary to remove Sertoli cells before MACS or FACS if ITGA6 will be used to select spermatogonia from the mixture of male germ cells and somatic cells within human seminiferous tubules. In adult monkey, the expression of ZBTB16 appears to be confined to the Adark and/or Apale spermatogonia [23], suggesting that ZBTB16 may be conserved in mice, primates, and human undifferentiated spermatogonia. We also found that human spermatogonia and their progenitors expressed UCHL1, a marker for pig spermatogonia [24]. Moreover, we observed that MAGEA4 [26] was present in all human spermatogonia and possibly in preleptotene spermatocytes but not in more differentiated germ cells or somatic cells, suggesting that MAGEA4 may be involved in the early but not the late stages of differentiation of human spermatogenesis. Because of the widespread presence of MAGEA4, it cannot be considered a marker for the SSC.
On the other hand, we also found that some rodent markers for SSCs and progenitors are not applicable to humans. For example, our observation that POU5F1, a marker for mouse spermatogonial stem and progenitor cells [2, 6–8], is not detected in adult normal human spermatogonia and other male germ cells, which is consistent with the observation by Looijenga et al. [38] showing that POU5F1 is present in human tumor germ cells but not in normal germ cells. Likewise, KIT is recognized as a hallmark for mouse differentiating spermatogonia, including type A1–A4 spermatogonia [4, 5] and it is also present in monkey spermatogonia [22]. We found that KIT was not detected in any types of human spermatogonia but was present in later germ cells including spermatocytes and round spermatids. It is still possible that other KIT antibodies may recognize an epitope on human spermatogonia, but we have tested antibodies from two companies (Abcam Inc. and Santa Cruz Biotechnology, Inc.) and found that these two antibodies did not stain human spermatogonia. PCNA, a cofactor of DNA polymerase delta found during the activation of DNA replication, has been used previously as an index of the proliferative activity in human testicular tissues [39, 40]. We found that PCNA, a marker for proliferating germ cells from mouse spermatogonia to pachytene spermatocytes [41], was expressed in human proliferating spermatogonia and spermatocytes. Because PCNA expression is absent in the cells at G0 phase (a period in the cell cycle in which cells exist in a quiescent state) [40], we speculate that PCNA-negative spermatogonia may be the nondividing reserve stem cells in human testis. Thus, our data indicate that there are clear differences in the phenotypic characteristics between human spermatogonia and spermatogonia from other species, such as mouse or monkey. More research is required to clarify fully the spermatogonial marker expression differences among the species.
Our observations show that GPR125 is expressed in only one or two spermatogonia per human seminiferous tubule cross section, but is absent in other types of germ cells and Sertoli cells; thus, GPR125 can be used to effectively isolate and purify human spermatogonia, possibly a subpopulation of the Adark and/or the Apale spermatogonia. Compared to ITGA6, another surface marker for human spermatogonia and their progenitors [42], GPR125 has a unique advantage because it is not present in human Sertoli cells; thus, by using MACS, the purity of the isolated spermatogonia is increased because of lack of Sertoli cell contamination. Indeed, we obtained a purity of more than 95% of human spermatogonia using MACS as assayed by immunocytochemistry. These freshly isolated GPR125-positive spermatogonia coexpress ITGA6, THY1, and GFRA1, markers for mouse SSCs and progenitors, suggesting that human GPR125-positive spermatogonia are phenotypically putative human SSCs. One important characteristic of stem cells, including SSCs and progenitors, is proliferation. Thus, we sought to determine whether human GPR125-positive spermatogonia could proliferate in vitro. A proliferation assay showed that over a 2-week culture period, human GPR125-positive spermatogonia proliferated, indicating that human GPR125-positive spermatogonia possess the proliferative features of human SSCs and progenitors. The stem cell potential of human GPR125-positive spermatogonia needs to be confirmed by the human-to-nude mouse xenotransplantation assay. However, xenotransplantation of nonhuman primate germ cells to immunodeficient mouse testes does not result in complete spermatogenesis, and the efficiency of the xenotransplantation assay is not yet known [23].
Nothing is known about the molecular mechanisms regulating the proliferation of human SSCs and progenitors. We have recently demonstrated that the RAS/MAPK pathway is involved in the proliferation of mouse SSCs and progenitors [29]. In the present study, we revealed for the first time that the phosphorylation of both MAPK1 and MAPK3 activation was increased after 2 wk of culture of human GPR125-positive spermatogonia, compared to freshly isolated GPR125-positive spermatogonia, suggesting that the MAPK1/3 signaling pathway is involved in the proliferation of putative human SSCs and progenitors.
In view of the recent findings that human SSCs and/or their progenitors can revert spontaneously (without the addition of exogenous genes) back to pluripotent embryonic stem (ES)-like cells (non-canonical induced Pluripotent Stem [iPS] cells) [42–44], the next important step in this research is to identify with certainty the cell type in the testis that is capable of the reprogramming to the ES-like cells; presumably it is the human SSC, but this remains to be determined. Secondly, the identification of the regulatory molecules and signaling mechanisms that allow for this reprogramming must be investigated for a potential application of these SSC/progenitor cells in regenerative medicine.
In conclusion, we have demonstrated that human spermatogonia share some phenotypes with rodent, monkey, and pig SSCs and progenitors because they express GPR125, ITGA6, ZBTB16, UCHL1, GFRA1, and THY1. On the other hand, human spermatogonia do not express some phenotypic markers of rodent spermatogonia and SSCs, because POU5F1 is not detected in adult human male germ cells and KIT is apparently not present in human spermatogonia. We have isolated with high purity human GPR125-positive spermatogonia that coexpress ITGA6, THY1, and GFRA1, markers for rodent SSCs and progenitors. Human GPR125-positive spermatogonia proliferate in vitro via the MAPK1/3 signaling pathway and remain in an undifferentiated state. We believe that human GPR125-positive spermatogonia are phenotypically putative human SSCs. This study provides an analysis of the phenotypic characteristics of human spermatogonia, including SSCs and their progenitors; demonstrates that it is possible to culture human spermatogonia; and offers a novel insight into molecular mechanisms controlling the proliferation of the putative human SSC.
Acknowledgments
We thank Professor Giulio C. Spagnoli, University Hospital of Basel, Switzerland, for providing a monoclonal antibody 57 B raised against MAGEA4.
Footnotes
1Supported by NIH grant HD033728.
REFERENCES
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J.Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod 2006; 74: 314–321. [DOI] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dym M.Isolation of male germ-line stem cells; influence of GDNF. Dev Biol 2005; 279: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000; 287: 1489–1493. [DOI] [PubMed] [Google Scholar]
- He Z, Jiang J, Hofmann MC, Dym M.Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biol Reprod 2007; 77: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T, Nishikawa S.Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development 1991; 113: 689–699. [DOI] [PubMed] [Google Scholar]
- Ohmura M, Yoshida S, Ide Y, Nagamatsu G, Suda T, Ohbo K.Spatial analysis of germ stem cell development in Oct-4/EGFP transgenic mice. Arch Histol Cytol 2004; 67: 285–296. [DOI] [PubMed] [Google Scholar]
- Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, Kuwana T, Kehler J, Abe K, Scholer HR, Suda T.Identification and characterization of stem cells in prepubertal spermatogenesis in mice small star, filled. Dev Biol 2003; 258: 209–225. [DOI] [PubMed] [Google Scholar]
- Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH.Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells 2008; 26: 2928–2937. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE.Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 2004; 36: 647–652. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP.Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 2004; 36: 653–659. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL.Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A 2003; 100: 6487–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Avarbock MR, Brinster RL.beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 1999; 96: 5504–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW, Yancopoulos GD, Murphy A, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature 2007; 449: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL.Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 2004; 101: 16489–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T.Long-term culture of mouse male germline stem cells under serum- or feeder-free conditions. Biol Reprod 2005; 72: 985–991. [DOI] [PubMed] [Google Scholar]
- Clermont Y.Spermatogenesis in man. A study of the spermatogonial population. Fertil Steril 1966; 17: 705–721. [PubMed] [Google Scholar]
- Clermont Y.Renewal of spermatogonia in man. Am J Anat 1966; 118: 509–524. [DOI] [PubMed] [Google Scholar]
- Clermont Y.Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 1972; 52: 198–236. [DOI] [PubMed] [Google Scholar]
- Clermont Y.The cycle of the seminiferous epithelium in man. Am J Anat 1963; 112: 35–51. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Schlatt S.A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction 2006; 132: 673–680. [DOI] [PubMed] [Google Scholar]
- Dym M, Kokkinaki M, He Z.Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res C Embryo Today 2009; 87: 27–34. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE.Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod 2009; 24: 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, Schatten GP, Orwig KE.Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells 2007; 25: 2330–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Megee S, Rathi R, Dobrinski I.Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev 2006; 73: 1531–1540. [DOI] [PubMed] [Google Scholar]
- Luo J, Megee S, Dobrinski I.Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J Cell Physiol 2009; 220: 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry F, Satie AP, Rioux-Leclercq N, Rajpert-De Meyts E, Spagnoli GC, Chomez P, De Backer O, Jegou B, Samson M.MAGE-A4, a germ cell specific marker, is expressed differentially in testicular tumors. Cancer 2001; 92: 2778–2785. [DOI] [PubMed] [Google Scholar]
- Dolci S, Pellegrini M, Di Agostino S, Geremia R, Rossi P.Signaling through extracellular signal-regulated kinase is required for spermatogonial proliferative response to stem cell factor. J Biol Chem 2001; 276: 40225–40233. [DOI] [PubMed] [Google Scholar]
- Yoon S, Seger R.The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 2006; 24: 21–44. [DOI] [PubMed] [Google Scholar]
- He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M.Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells 2008; 26: 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellvé AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M.Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol 1977; 74: 68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym M, Jia MC, Dirami G, Price JM, Rabin SJ, Mocchetti I, Ravindranath N.Expression of c-kit receptor and its autophosphorylation in immature rat type A spermatogonia. Biol Reprod 1995; 52: 8–19. [DOI] [PubMed] [Google Scholar]
- Dirami G, Ravindranath N, Pursel VG, Dym M.Effects of stem cell factor and granulocyte macrophage-colony stimulating factor on survival of porcine type A spermatogonia cultured in KSOM. Biol Reprod 1999; 61: 225–230. [DOI] [PubMed] [Google Scholar]
- Kokkinaki M, Lee TL, He Z, Jiang J, Golestaneh N, Hofmann MC, Chan WY, Dym M.The molecular signature of spermatogonial stem cells in the 6-day-old mouse testis. Biol Reprod 2009; 80: 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dettin L, Johnson E, Dym M.Immortalization of mouse germ line stem cells. Stem Cells 2005; 23: 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Jiang J, Kokkinaki M, Dym M.Nodal signaling via an autocrine pathway promotes proliferation of mouse spermatogonial stem/progenitor cells through Smad2/3 and Oct-4 activation. Stem Cells 2009; 27: 2580–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seandel M, Falciatori I, Shmelkov SV, Kim J, James D, Rafii S.Niche players: spermatogonial progenitors marked by GPR125. Cell Cycle 2008; 7: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmcke J, Wistuba J, Schlatt S.Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update 2006; 12: 275–282. [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res 2003; 63: 2244–2250. [PubMed] [Google Scholar]
- Ruwanpura SM, McLachlan RI, Matthiesson KL, Meachem SJ.Gonadotrophins regulate germ cell survival, not proliferation, in normal adult men. Hum Reprod 2008; 23: 403–411. [DOI] [PubMed] [Google Scholar]
- Steger K, Aleithe I, Behre H, Bergmann M.The proliferation of spermatogonia in normal and pathological human seminiferous epithelium: an immunohistochemical study using monoclonal antibodies against Ki-67 protein and proliferating cell nuclear antigen. Mol Hum Reprod 1998; 4: 227–233. [DOI] [PubMed] [Google Scholar]
- Salmand PA, Jungas T, Fernandez M, Conter A, Christians ES.Mouse heat-shock factor 1 (HSF1) is involved in testicular response to genotoxic stress induced by doxorubicin. Biol Reprod 2008; 79: 1092–1101. [DOI] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, et al. Generation of pluripotent stem cells from adult human testis. Nature 2008; 456: 344–349. [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Gallicano GI, Kokkinaki M, Pant D, Jiang J, Destefano D, Fernandez-Bueno C, Rome JD, Haddad BR, Dym M.Pluripotent stem cells derived from adult human testes. Stem Cells Dev 2009; 18: 1115–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA.Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells 2009; 27: 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]