Abstract
Zonadhesin is a rapidly evolving protein in the sperm acrosome that confers species specificity to sperm-zona pellucida adhesion. Though structural variation in zonadhesin likely contributes to its species-specific function, the protein has not previously been characterized in organisms capable of interbreeding. Here we compared properties of zonadhesin in several animals, including the horse (Equus caballus), donkey (E. asinus), and Grevy's zebra (E. grevyi) to determine if variation in zonadhesin correlates with ability of gametes to cross-fertilize. Zonadhesin localized to the apical acrosomes of spermatozoa from all three Equus species, similar to its localization in other animals. Likewise, in horse and donkey testis, zonadhesin was detected only in germ cells, first in the acrosomal granule of round spermatids and then in the developing acrosomes of elongating spermatids. Among non-Equus species, D3-domain polypeptides of mature, processed zonadhesin varied markedly in size and detergent solubility. However, zonadhesin D3-domain polypeptides in horse, donkey, and zebra spermatozoa exhibited identical electrophoretic mobility and detergent solubility. Equus zonadhesin D3-polypeptides (p110/p80 doublet) were most similar in size to porcine and bovine zonadhesin D3-polypeptides (p105). Sequence comparisons revealed that the horse zonadhesin precursor's domain content and arrangement are similar to those of zonadhesin from other large animals. Partial sequences of horse and donkey zonadhesin were much more similar to each other (>99% identity) than they were to orthologous sequences of human, pig, rabbit, and mouse zonadhesin (52%–72% identity). We conclude that conservation of zonadhesin D3-polypeptide properties correlates with ability of Equus species to interbreed.
Keywords: acrosome, equine, evolution, fertilization, sperm, spermatozoa, stallion, zona pellucida
Zonadhesin, a rapidly evolving sperm protein that confers species specificity to egg adhesion in mammals, varies among species; for three Equus species, there is a correlation between the zonadhesin structure and the ability to interbreed.
INTRODUCTION
Adhesion of spermatozoa to the mammalian egg's zona pellucida (ZP) represents the first direct, physical interaction of the male and female germ cells during fertilization, and it occurs by sequential or simultaneous binding of sperm proteins to glycoprotein components of the ZP [1, 2]. ZP adhesion molecule activity has been ascribed to several sperm proteins [3], including β-1,4-galactosyltransferase and SED-1 on the cell surface [4, 5] and zonadhesin, sp56/ZP3R, and proacrosin in the acrosome (Tardif et al., unpublished data) [6–11]. Among ZP adhesion molecules, zonadhesin is unique in its ability to bind in a species-specific manner directly to native, particulate ZP [6]. Zonadhesin localizes to the perimeter of the sperm acrosome near the outer acrosomal membrane [11, 12] and becomes exposed at the cell surface as a consequence of sperm capacitation (Tardif et al., unpublished data). In spermatozoa with vesiculated plasma and outer acrosomal membranes, zonadhesin is primarily detected between membrane vesicles at the leading edge of matrix emanating from the acrosome [11, 12]. Antibody binding to the surface-exposed D3p18 domain of mouse zonadhesin specifically inhibits fertilization and ZP adhesion in vitro (Tardif et al., unpublished data). Collectively, these results show that zonadhesin likely mediates ZP adhesion at the onset of acrosomal exocytosis.
Zonadhesin's function in ZP adhesion was established by characterizing the phenotype of zonadhesin-deficient mice produced by targeted disruption of Zan. Zonadhesin-null males are fertile, and their spermatozoa readily adhere to mouse ZP and fertilize mouse eggs in vitro (Tardif et al., unpublished data). Remarkably, though spermatozoa from wild-type and zonadhesin-null mice adhere equally well to the ZP of mouse oocytes, loss of zonadhesin dramatically increases adhesion of mouse spermatozoa to pig, cow, and rabbit ZP. This finding demonstrates that zonadhesin confers species specificity to ZP adhesion, and that a function of the sperm acrosome in species-specific gamete adhesion, previously shown only in marine invertebrates, is conserved in mammals. Furthermore, the observation that in the absence of zonadhesin other components of mouse spermatozoa mediate adhesion that is not species specific shows that ZP interaction is molecularly degenerate (mediated by multiple sperm proteins with overlapping function) and robust (insensitive to loss of individual adhesion molecules because of the compensatory activity of other proteins; Tardif et al., unpublished data).
The active form of zonadhesin in the sperm acrosome is produced by proteolysis of a large, mosaic precursor uniquely expressed in testicular germ cells [13]. The precursor comprises primarily three different domain types—MAM (meprin/A5 antigen/receptor tyrosine phosphatase mu), mucin, and VWD (von Willebrand type D)—present in other cell adhesion molecules [7, 14, 15]. The active form of pig zonadhesin is a heterodimer of Mr 45 000 (p45) and 105 000 (p105) polypeptides collectively spanning the VWD1–VWD4 domains of the precursor [11]. The zonadhesin precursor's amino acid sequence, predicted domain structure, and processing vary among species (Cheung et al., unpublished data; Tardif et al., unpublished data) [11, 14–16]. Indeed, like gamete recognition proteins in marine invertebrates, zonadhesin is evolving rapidly by positive selection [17, 18], which suggests divergence of its sequence is beneficial because it alters ZP binding specificity and thereby promotes development of species differences in ZP recognition. Though zonadhesin contributes to species specificity of ZP adhesion, no studies have yet correlated the protein's structural properties with species specificity of fertilization.
Depending in part on the experimental conditions and pairwise combinations tested, mammalian spermatozoa adhere preferentially to homologous ZP in vitro [19–21]. Nevertheless, some closely related mammals readily interbreed, indicating that mammalian fertilization is not strictly species specific. Indeed, over the ages mankind has successfully bred countless viable Equus hybrids, most notably mules (E. asinus × E. caballus), which continue to be commercially important animals because of their unique physical characteristics and temperament. Mules are produced by breeding a jack to a mare, whereas the reverse combination (breeding a stallion to a jenny) produces a hinny; hence jack and stallion spermatozoa are both able to fertilize eggs of the opposite species. Hybrids have also been produced by interbreeding horses and asses with zebras [22]. Consequently, Equus species are ideal for assessing the relationship between zonadhesin structure and ability of spermatozoa to fertilize eggs of other species. Here we tested the hypothesis that the ability of Equus species to interbreed correlates with similarity in the properties of zonadhesin.
MATERIALS AND METHODS
Semen Collection and Sperm Preparation
Semen from stallions housed at Texas Tech Ranch Horse Center (n = 4; all fertile, ages 5–18 yr) and from a large standard donkey (fertile, age 16 yr; Snook, TX) was collected using the Missouri artificial vagina (NASCO, Fort Atkinson, WI). Semen from stallions at Colorado State University (n = 18; all fertile, ages 4–21 yr) was collected using the Colorado artificial vagina (Lane Manufacturing, Inc., Denver, CO). Semen collected by manual stimulation [23] from a Grevy's zebra (fertile, age 13 yr) was generously provided by Roanoke Artificial Insemination Laboratory (Roanoke, VA). Sperm concentration was determined immediately after semen collection using an Equine Densitometer (Model 534A; Animal Reproduction Systems, Chino, CA), and motility was evaluated by light microscopy. After removing the gel fraction from the ejaculate, semen was extended according to equine reproductive industry standards (25–50 × 106 cells per milliliter) with EZ Mixin-CST stallion extender (Animal Reproduction Systems). Extended semen was transferred to an Equitainer (Hamilton Research, South Hamilton, MA), and within 24 h spermatozoa were recovered by centrifugation (10 min, 750 × g, 23°C) and washed once with PBS (10 mM NaPO4 [pH 7.4], 150 mM NaCl) by centrifugation, then resuspended at 25–50 × 106 cells per milliliter in PBS. All procedures involving use of animals were reviewed and approved by the Texas Tech University or Colorado State University Institutional Animal Care and Use Committees and were performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Immunofluorescence
Air-dried spermatozoa smeared on glass slides were permeabilized for 30 min with 100% methanol (23°C). Slides were then washed once with Tris-suffered saline (10 mM Tris-HCl [pH 7.4], 150 mM NaCl) containing 0.1% Tween-20 (TBST) and soaked for 45 min in 10% heat-inactivated goat serum (HIGS) diluted in TBST (23°C). Primary antibody (rabbit polyclonal antiserum to pig zonadhesin holoprotein [11] diluted 1:2000 in TBST containing 10% HIGS) was added overnight at 4°C, and zonadhesin was visualized on sperm cells with anti-rabbit IgG conjugated to Alexia 594 (Molecular Probes, Carlsbad, CA). For zonadhesin localization in testis, tissues from 15-, 24-, or 36-mo-old stallions were recovered and placed on ice for up to 2 h prior to further processing. Pieces 1 cm3 were cut from each testis and fixed in 35 ml of 4% paraformaldehyde solution overnight at 4°C, and immunofluorescence on paraffin sections was performed with antigen retrieval as previously described [24].
Western Blotting
Washed spermatozoa were either extracted with SDS-PAGE 1× sample buffer (106 cells per microliter) containing 2% SDS and 25 mM dithiothreitol or sequentially extracted with 1% Triton X-100/PBS and 1% SDS as described by Bi et al. [11]. Sperm proteins were separated by SDS-PAGE (4%–10% polyacrylamide linear gradient) and Western blotting performed as previously described [11]. For antigen detection, affinity-purified rabbit antibody to pig zonadhesin D3 was diluted 1:50 000, affinity-purified rabbit antibody to mouse zonadhesin D3 was diluted 1:25 000, and rabbit antisera to pig zonadhesin holoprotein was diluted 1:10 000.
Equus Zonadhesin cDNA Cloning
Horse and donkey zonadhesin cDNA fragments spanning the D3-D4 domain boundary were generated by RT-PCR using primers (sense: AGGAACAGCAGCTTCTGCCC; antisense: CTTCAQCCTCCCAGCTGTTGCCA) with well-conserved sequences (0–1 mismatch) among pig, human, mouse, and rabbit zonadhesin cDNAs. PCR products (0.9 kb) were cloned in pGEM-Teasy [11], and both strands of multiple clones were sequenced to at least two-fold coverage to derive the composite sequences for the two Equus species.
Horse ZAN Bioinformatics
The horse zonadhesin locus (ZAN) was identified (see Results) in partially annotated genomic sequence of Twilight, a thoroughbred mare (Horse Genome Project; http://www.uky.edu/Ag/Horsemap/). Zonadhesin coding regions were identified in a 33.85-kb segment (nts 8728094–8761942 of a 42.6-Mb contig, GenBank Accession NC_009156.2) of horse chromosome 13 by Dotplot comparison (60% match threshold, 30-residue window; MegAlign program; Lasergene 8 software, DNASTAR Inc., Madison, WI) of amino acid sequences translated from the three sense strand reading frames to amino acid sequences of pig zonadhesin and human zonadhesin Variant 6 (deduced by translating the major open reading frames [ORFs] of the 7.8-kb pig cDNA, GenBank Accession U40024, and 8.4-kb human cDNA, GenBank Accession AF332980, respectively). Exon boundaries flanking the putative coding regions were then deduced in cerebro by locating intron splice donor/acceptor dinucleotide pairs (GT/AG) that, when introns were spliced, would preserve the major zonadhesin ORF. Two predicted cDNA sequence contigs were then produced by joining 32 exon sequences identified upstream and nine exon sequences identified downstream of an estimated >1-kb gap in NC_009156. A predicted horse zonadhesin composite cDNA sequence was then generated by assembling (SeqMan program; Lasergene 8) the two conceptually spliced genomic contigs with the sequence of the 895-bp horse zonadhesin RT-PCR product (see previous) spanning the missing exons in the NC_009156 sequence gap.
RESULTS
Species Diversity of Zonadhesin Polypeptides
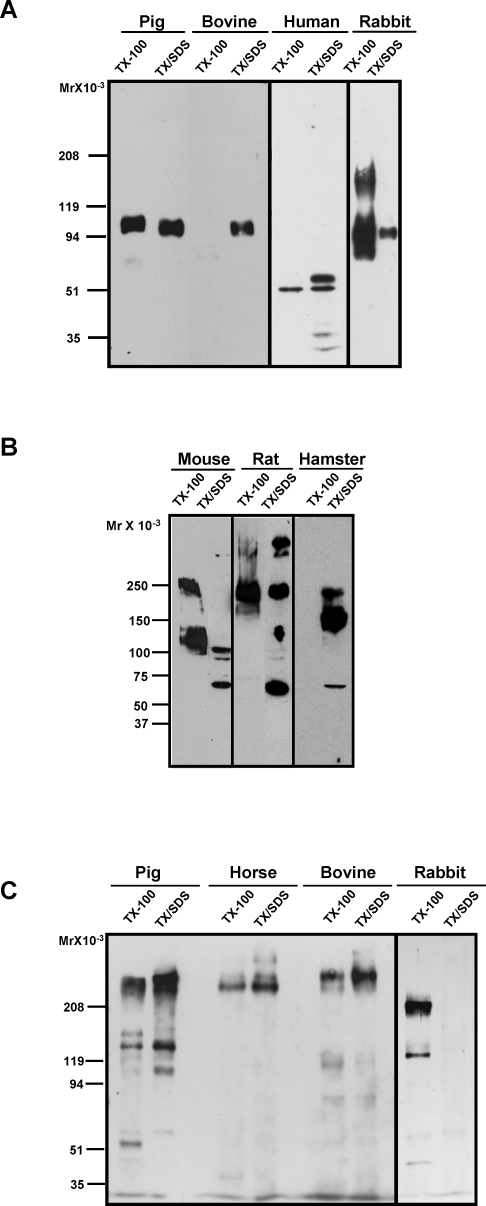
Despite similar protein domain content and order of zonadhesin precursors, we found significant species variation in the biochemical properties of zonadhesin's processed forms in mature spermatozoa (Fig. 1). Affinity-purified antibodies to the VWD3 domain of pig zonadhesin detected diverse D3-polypeptides (protein disulfides reduced) on Western blots of pig, bovine, human, and rabbit sperm proteins (Fig. 1A). Both the size and detergent extractability of the D3-polypeptides varied among these species. As in our previous studies [11, 13], anti-D3 readily detected the Mr 105 000 polypeptide (p105) spanning D2–D4 in mature, active pig zonadhesin. Nonionic detergent (Triton X-100) extracted approximately half of p105 from pig spermatozoa. In bovine spermatozoa, no comparable D3-polypeptide was extractable with Triton X-100, but subsequent re-extraction with a denaturing, ionic detergent (SDS) solubilized a polypeptide similar in size to pig p105. In the sequential detergent extracts of human spermatozoa, anti-D3 detected multiple smaller polypeptides, including predominant Mr 50 000 (p50) and 60 000 (p60) proteins. Extraction with Triton X-100 solubilized less than half of the p50 and none of the p60 from human cells. In contrast, extraction of rabbit spermatozoa with Triton X-100 solubilized nearly all anti-D3 immunoreactive proteins, which included Mr 75 000, 95 000, and 150 000 polypeptides.
FIG. 1.
Interspecies diversity of zonadhesin D3-domain polypeptides in spermatozoa from animals representing five taxonomic orders. Proteins extracted from spermatozoa with Triton X-100 (TX-100) or with SDS after prior extraction with Triton X-100 (TX/SDS) were separated by SDS-PAGE, and zonadhesin was detected on Western blots with affinity-purified antibodies to the D3 domain or with antisera to the zonadhesin holoprotein. A) D3-polypeptides (protein disulfides reduced) of pig (Sus scrofa; order: Artiodactyla), bovine (Bos taurus; order: Artiodactyla), human (Homo sapiens; order: Primates), and rabbit (Oryctolagus cuniculus; order: Lagomorpha) spermatozoa detected with antibody to pig D3 domain. Note the species differences in polypeptide size, detergent solubility, or both. B) D3 polypeptides (protein disulfides reduced) of mouse, rat, and hamster spermatozoa (Mus musculus, Rattus norvegicus, and Cricetinae mesocricetus, respectively; order: Rodentia) detected with antibody to mouse D3 domain. Note the species differences in both polypeptide size and detergent solubility. C) Zonadhesin (protein disulfides not reduced) of pig, horse (Equus caballus; order: Perissodactyla), bovine, and rabbit spermatozoa detected with holoprotein antisera. Note the relatively similar size of the largest (multimeric) holoprotein form among the three ungulates.
Zonadhesin D3-polypeptides (disulfides reduced) were similarly diverse in spermatozoa from three rodents (mouse, rat, and hamster). Affinity-purified antibodies to the mouse zonadhesin D3 domain detected Mr 125 000 and 250 000 mouse zonadhesin polypeptides extracted with Triton X-100 (Fig. 1B). Similar to rabbit zonadhesin, most anti-D3 immunoreactivity was extracted with nonionic detergent. Extraction with Triton X-100 also solubilized a dominant Mr 250 000 D3-polypeptide from rat spermatozoa, but a heterogeneous set of five rat D3-polypeptides was not solubilized. In contrast, zonadhesin D3-polypeptides were not extracted from hamster spermatozoa with nonionic detergent; instead, a dominant Mr 180 000 D3-polypeptide was subsequently solubilized with SDS.
Species differences in zonadhesin holoprotein (multiple polypeptides joined by intermolecular disulfides) in the sequential detergent extracts were also detected on Western blots probed with antisera to zonadhesin purified from pig spermatozoa. The zonadhesin holoprotein antisera detected Mr 150 000 and 300 000 proteins (p45/105 and a dimer of p45/105, respectively [13]) in both Triton X-100 and subsequent SDS extracts of pig spermatozoa (protein disulfides not reduced; Fig. 1C). In horse spermatozoa, the holoprotein antisera recognized only a higher Mr protein similar in size to the Mr ∼300 000 pig multimer. A similar multimer also predominated in bovine spermatozoa. In contrast, the holoprotein antisera detected primarily an Mr 200 000 protein in a Triton X-100 extract of rabbit spermatozoa. Also unlike pig, horse, and bovine zonadhesin, nearly all zonadhesin holoprotein in rabbit spermatozoa was extracted with nonionic detergent.
Expression of Zonadhesin in Stallion Testis
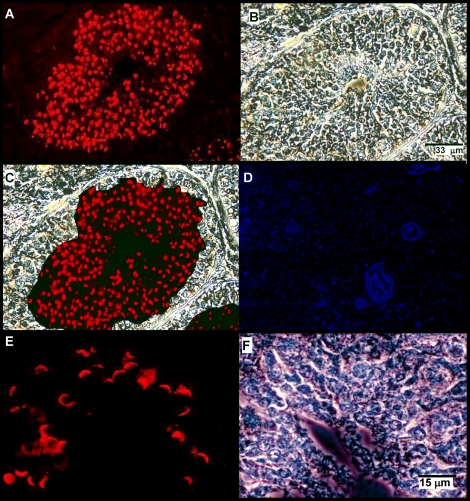
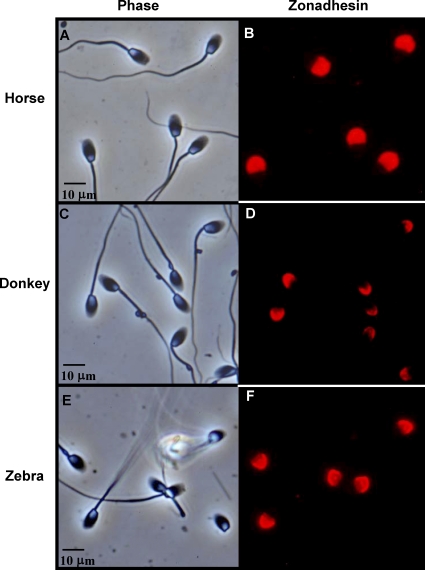
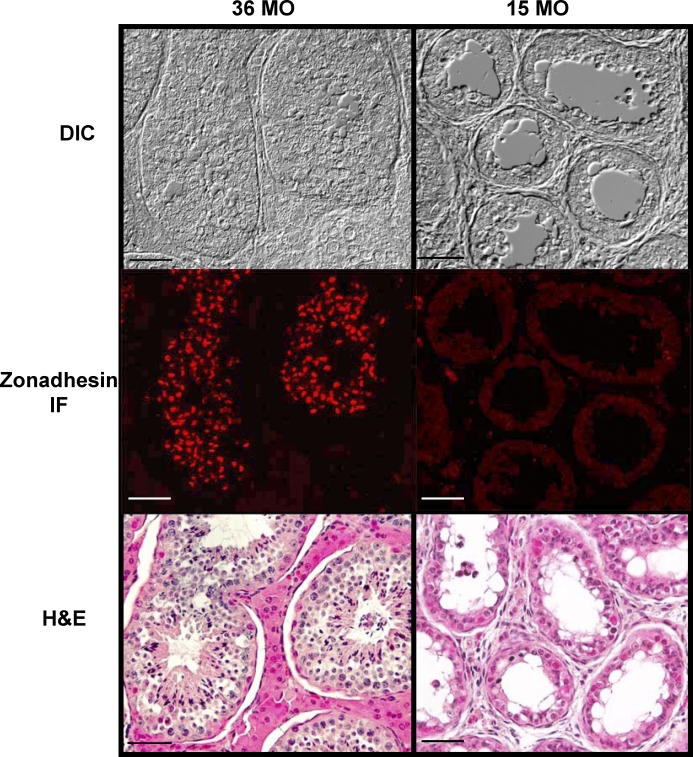
The species diversity in properties of zonadhesin D3-polypeptides suggested that expression and processing of the zonadhesin precursor varies among species. Therefore, we localized zonadhesin in horse testis and spermatozoa by immunofluorescence (Figs. 2–4) both to establish the protein's expression pattern for the first time in a representative Equus species and to compare that pattern to previous findings in pig and hamster zonadhesin [11, 12]. In mature horse testes, strong zonadhesin expression was detected in developing germ cells within the seminiferous tubules (Fig. 2). Zonadhesin was present in all stages of spermatids but not in diploid spermatogonia or spermatocytes (Fig. 2, A and C). In round and elongating spermatids zonadhesin localized in a polarized manner corresponding to its first appearance and persistence in the developing acrosomal granule (Fig. 2E). The zonadhesin localization pattern in mature donkey testis (data not shown) was identical to that observed in horse testis. Restriction of zonadhesin expression to haploid germ cells was confirmed by localization on sections of testis from an immature stallion (less than 15 mo; Fig. 3). No zonadhesin immunoreactivity was evident in immature testis, in contrast to strong zonadhesin expression in all tubules of sexually mature testis. Zonadhesin was also detected in the apical acrosomes of 100% of horse, donkey, and zebra spermatozoa (Fig. 4).
FIG. 2.
Zonadhesin distribution and expression in mature stallion testis. Zonadhesin was localized by immunofluorescence on sections of testis from a 3-yr-old stallion using antisera to pig zonadhesin holoprotein. A) Zonadhesin immunoreactivity in a seminiferous tubule (epifluorescence). Note the strong labeling in the region of developing spermatids. B) Phase contrast view of the tubule shown in A. C) Panels A and B merged. D) Epifluorescence view of a seminiferous tubule stained with fluorescent secondary antibody only (no zonadhesin antiserum). E) Higher magnification view of zonadhesin immunoreactivity in elongating spermatids near the lumen of a tubule (epifluorescence). F) Phase contrast view of the field shown in E. Bars indicate relative magnification of the images.
FIG. 4.
Zonadhesin localization in spermatozoa from three Equus species. Zonadhesin was detected by immunofluorescence on methanol-fixed spermatozoa using antisera to pig zonadhesin holoprotein. Phase contrast (A) and paired epifluorescence (B) views of zonadhesin immunoreactivity in horse (E. caballus) spermatozoa. C, D) Paired phase contrast and epifluorescence views of zonadhesin immunoreactivity in donkey (E. asinus) spermatozoa. E, F) Same as A–D except zonadhesin in zebra (E. grevyi) spermatozoa. Bars indicate relative magnification of the images.
FIG. 3.
Zonadhesin distribution and localization in immature stallion testis. Zonadhesin was localized by immunofluorescence as for Figure 2. Shown are differential interference contrast (DIC), epifluorescence (zonadhesin IF), and light microscopic (H&E) images of seminiferous tubules in testis sections from a sexually mature (36 mo old) and immature (15 mo old) stallion. The DIC and zonadhesin IF images from each animal are of the same microscopic field. The H&E images depict similar fields of the same tissue sections after post-staining with hematoxylin and eosin. In the 15-mo-old testis, note the absence of spermatids, indicating that spermiogenesis has not begun, and the absence of zonadhesin immunoreactivity. The vacuolar appearance of the tubules in the immature testis is characteristic of testicular development in the horse. Note also in the 36-mo-old testis the presence of developing spermatids and detection of zonadhesin in all tubules. Bar = 50 μm.
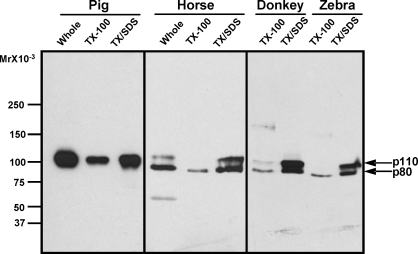
Zonadhesin Polypeptides in Equus Species
In contrast to the variation in detergent solubility and sizes of zonadhesin D3-polypeptides observed in comparisons between pig, human, rabbit, bovine, mouse, rat, and hamster spermatozoa, among Equus species these zonadhesin properties were largely identical (Fig. 5). In sperm extracts from three Equus species (horse, donkey, and Grevy's zebra), affinity-purified antibody to the pig D3 domain cross-reacted predominantly with Mr 110 000 and Mr 80 000 polypeptides. Detergent extractability of these peptides was also similar among Equus species; a small fraction of the Mr 80 000 D3-polypeptide was extracted with Triton X-100, whereas little or no Mr 110 000 polypeptide was extracted, and both polypeptides were readily solubilized upon subsequent re-extraction with SDS. The pattern of zonadhesin D3-polypeptide content was the same in extracts of spermatozoa from all 22 fertile stallions examined (not shown). The sizes of Equus zonadhesin D3-polypeptides differed from those of all other species tested, but were most similar in size to the Mr 105 000 polypeptide in pig and bovine spermatozoa.
FIG. 5.
Conservation of zonadhesin D3-polypeptide properties in spermatozoa from three Equus species. Zonadhesin D-polypeptides (protein disulfides reduced) extracted either immediately with 2% SDS/25 mM DTT (Whole) or sequentially with Triton X-100 and SDS were detected by Western blotting as in Figure 1. Detection of the pig D3-polypeptide (p105) is included for comparison. Note the identical sizes and detergent solubilities of the p80 and p110 D3-polypeptides in spermatozoa of all three Equus species.
Equus Zonadhesin Sequence Analysis
To test whether zonadhesin D3-polypeptide variation correlates with divergence of zonadhesin sequences, we determined the domain organization of the horse zonadhesin precursor (Fig. 6) and compared partial amino acid sequences among six species, including horse and donkey (Fig. 7). No horse zonadhesin cDNA or annotated genomic sequence was retrievable by querying NCBI data. Therefore, we located the horse EPO gene, which in both the human and mouse genomes is immediately upstream of ZAN exon 1 [15]. By scanning horse Chromosome 13 sequence adjacent to EPO for similarity to ZAN, we found a 33.85-kb locus (LOC100147025; Accession NC_009156.2) that included 45 putative exons identified by automated annotation using GNOMON. Though the locus included a >1-kb sequence gap and was designated as a pseudogene by the Horse Genome Project, its conserved synteny with flanking EPO and EPHB4 genes and similar exon content to the 48-exon human ZAN gene suggested it was horse ZAN. Pairwise comparisons of the horse genomic locus to pig and human zonadhesin sequences, together with in cerebro determination of intron/exon boundaries, revealed that the automated annotation had correctly predicted 35 horse ZAN exons, 28 upstream and 7 downstream of the sequence gap. Boundaries of six GNOMON-predicted exons were corrected by sequence comparison, but four predicted exons could not be confirmed.
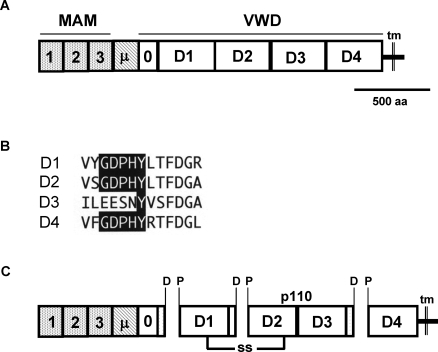
FIG. 6.
Domain structure and predicted processing of the horse zonadhesin precursor. The precursor's structural characteristics were inferred from its amino acid sequence deduced by conceptual translation of a composite cDNA. A) Predicted domain structure of the horse zonadhesin precursor. MAM, meprin/A5/receptor tyrosine phosphatase mu domain; μ, mucin domain; VWD, von Willebrand D domains; tm, transmembrane segment. B) Paralogous sequences in the horse zonadhesin precursor's VWD1, VWD2, VWD3, and VWD4 domains corresponding to sites of proteolytic processing in the pig protein. Note the conservation of the GDPHY sequence motif (shaded) in D1, D2, and D4. C) Predicted polypeptides produced by proteolytic cleavage of Asp-Pro bonds of GDPHY motifs during the functional maturation of horse zonadhesin. Note that a p110 polypeptide spans the VWD3 domain.
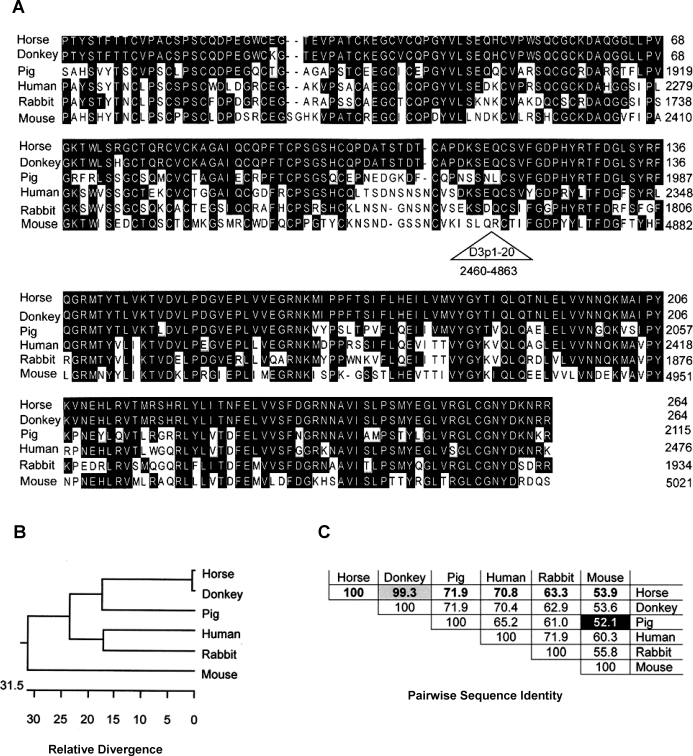
FIG. 7.
Comparison of partial zonadhesin sequences from six species representing four taxonomic orders. Orthologous segments (264 amino acids) spanning the VWD3-VWD4 domain boundary of horse, donkey, pig, human, rabbit, and mouse zonadhesin were aligned using MegAlign (Lasergene 8). A) Aligned sequences. Amino acids comprising the expansion of partial D3 domains in the mouse sequence (residues 2460–4863) were deleted (triangle) to attain proper alignment with the D3 and D4 domain sequences of the other species. Pig, human, rabbit, and mouse sequences were from Accession NP_999548, AAK28052, P57999, and AAK28824, respectively. B) Dendrogram depicting similarity of the sequences. Branch lengths between any two sequences indicate their relative similarity. C) Percent identities of pairwise comparisons. Note that the horse and donkey sequences are much more similar (only one amino acid difference) than any other pair.
Joining 32 confirmed or corrected exon sequences upstream of the sequence gap in NC_009156.2 produced a predicted cDNA contig encoding MAM, mucin, and VWD domains of horse zonadhesin. Likewise, joining nine confirmed or corrected exon sequences downstream of the gap produced a contig encoding VWD, transmembrane, and C-terminal tail regions of the horse protein. A full-length predicted coding sequence, assembled from the two conceptually spliced contigs and the sequence of a 985-bp RT-PCR product spanning the genomic sequence gap, specified a horse zonadhesin precursor composed of three MAM domains, a mucin domain, five tandem full or partial VWD domains, a transmembrane segment, and a C-terminal tail (Fig. 6A). The horse zonadhesin precursor lacked the expansion of 20 partial VWD domains present in the mouse protein, and instead exhibited a domain structure comparable to zonadhesin proteins of other large animals. However, the mucin domain of the horse precursor comprised fewer repeats of the PTE(R/K)PTV consensus sequence (25 repeats) than are present in the pig (53 repeats), mouse (80 repeats), and human (66 repeats) proteins, and more repeats than are present in the rabbit protein (20 repeats). The sequence of the horse precursor's tandem MAM domains overall was 70%–74% identical to corresponding sequences of the pig and human proteins. Likewise, the sequence of the horse precursor's tandem VWD domains overall was 69%–77% identical to corresponding sequences of the pig and human proteins.
The horse zonadhesin precursor's VWD1, D2, and D4 domains included consensus GDPHY sequence motifs (Fig. 6B) that in pig zonadhesin are sites of proteolysis in the protein's functional maturation. The motif was not conserved in the corresponding region of the horse D3 domain. Conceptual processing by hydrolysis of the Asp-Pro bonds embedded in the GDPHY motifs predicted a mature horse zonadhesin protein that includes the p110 polypeptide detected by the anti-D3 antibody (Fig. 6C).
Amino acid sequences encoded by the horse and donkey RT-PCR products spanning the VWD3-VWD4 domain boundary were much more similar to each other than they were to orthologous regions of pig, human, rabbit, and mouse zonadhesin (Fig. 7, A–C). Horse and donkey zonadhesin were >99% identical to each other in this region, whereas all other pairwise comparisons among the six species ranged from 52%–72% identity (Fig. 7C). The sequence similarity among horse, human, and pig zonadhesin in this region (65%–72% identity) was comparable to the similarity observed in the entire VWD domain comparison (69%–77% identity) among these species.
DISCUSSION
The results of this study show that mature, processed zonadhesin in spermatozoa varies markedly among species representing five different taxonomic orders of mammals (Rodentia, Lagomorpha, Artiodactyla, Perissodactyla, and Primates) but is similar in Equus species (order: Perissodactyla) capable of interbreeding. We compared properties of zonadhesin polypeptides containing the VWD3 domain because this domain is present in the p105 component of the pig zonadhesin form with highest ZP binding activity, so species differences in these polypeptides are likely to reflect species differences in the protein's function in fertilization. Despite the species variation in the protein's properties, however, the pattern of zonadhesin precursor expression in horse testis is the same as previously shown in pig and hamster [11, 12], and the protein's presence in the developing acrosomal granule of horse spermatids and localization to the apical acrosomes of mature spermatozoa from horse, donkey, and zebra are also the same as in all other species examined. This conservation of zonadhesin's cellular expression, subcellular trafficking, and ultimate destination in mature spermatozoa implies conservation among species of the protein's function in ZP recognition (Tardif et al., unpublished data) [11]. Though it is formally possible that Equus zonadhesins are inactive and simply don't contribute to ZP adhesion in these species, the sequence similarity among them strongly implies conservation of their binding activity. Furthermore, antibody inhibition experiments (Tardif et al., unpublished data) suggest that the presence of a very large, inactive zonadhesin protein would hinder the functions of other acrosomal adhesion molecules (proacrosin/acrosin and sp56/ZP3R) and thereby block ZP adhesion entirely. The correlation between zonadhesin's biochemical properties and the ability of species to interbreed is consistent with our demonstration that zonadhesin confers species specificity to sperm-ZP adhesion (Tardif et al., unpublished data). These findings provide additional support for the idea that the rapid evolution of zonadhesin [17, 18] produces species differences in the protein that underlie its species-specific ZP binding activity and function in egg recognition.
The posttranslational processing of zonadhesin has been studied most extensively in the pig. The pig zonadhesin precursor is rapidly proteolyzed to generate the MAM/mucin, p45, and p105 polypeptides present in mature spermatozoa, as no high-Mr polypeptide corresponding to the nascent precursor is detectable in testis [11]. The MAM/mucin polypeptide is heavily O-glycosylated, consistent with the presence of a predicted mucin domain, and p45 and p105 are both N-glycosylated [11]. In addition, the p45/105 heterodimer forms covalent multimers by interchain disulfide bonding similar to the multimerization of von Willebrand factor [13]. Each of these posttranslational processing events is a potential source of species variation in zonadhesin's biochemical properties that could be detected as species differences in polypeptide size or detergent extractability. Though we did not determine which of these possible sources of species variation contributed to the observed species differences in zonadhesin polypeptides, the similarity in D3-polypeptide sizes and detergent extractability of horse, donkey, and zebra zonadhesin does indicate that the sequences and domain content of the zonadhesin precursor are highly similar among these species, and that the posttranslational processing of the precursors is highly similar or identical as well. This conclusion is further supported by our observation that partial sequences of horse and donkey zonadhesin's VWD3-VWD4 domains are much more similar to each other than they are to corresponding regions in three non-Equus species.
Proteolysis of the pig zonadhesin precursor entails specific hydrolysis of Asp-Pro bonds embedded within a sequence motif (GDPHY) conserved in the protein's VWD1, VWD2, and VWD4 domains [11]. No known proteases exhibit substrate specificity corresponding to this hydrolytic event, so it is unclear whether it occurs nonenzymatically [25] or by action of a specific, uncharacterized protease. Regardless, conceptual hydrolysis at conserved GDPHY sites in deduced precursor sequences from mouse, human, and rabbit yields predicted polypeptides that correspond to the actual polypeptide sizes determined by Western blotting (Uppuluri et al., unpublished data; Tardif et al., unpublished data) [14–16]. Conservation or mutation of the GDPHY motif in zonadhesin's VWD domains therefore appears to determine the sites where proteolysis occurs during spermiogenesis to generate the polypeptides of the mature, active protein in spermatozoa. Consequently, a relatively subtle species difference in amino acid sequence can produce a dramatic species difference in zonadhesin polypeptide size. The conservation of the GDPHY motif in the VWD1, VWD2, and VWD4 domains of horse zonadhesin suggests its processing is similar to that of the pig precursor and likely explains the similarity in the sizes of D3-domain polypeptides in the mature horse and pig proteins.
The successful interbreeding of equine species illustrates not only that mammalian fertilization isn't strictly species-specific, but also that mating barriers between some closely related species either don't exist or can be overcome. All pairwise hybrids among horses, donkeys, and zebras have been produced [22], but these species each have different numbers of chromosomes, and hybrid offspring are nearly always sterile. Mules conform to Haldane's rule [26]; fertile females occur rarely, but males (the heterogametic sex) are invariably sterile. If mating barriers limit the chances that mammalian gametes from different species will ever interact, and hybrid sterility assures closely related species that do interbreed will remain separate even when cross-species fertilization occurs, what is the adaptive advantage of species specificity conferred by rapid evolution of zonadhesin? Although formation of sterile hybrids does not compromise the genetic diversity afforded by separate species, females carrying a sterile fetus are not contributing to the propagation of their species. Thus species specificity of egg recognition can work together with mating barriers to limit cross-species fertilization and thereby confer reproductive advantage to a species. Neither of these potential barriers is currently intact among Equus species. Nevertheless, our results suggest that the ongoing molecular evolution of zonadhesin will impart sufficient species specificity to fertilization in equids that in the distant future it will become impossible to produce hybrids such as mules by mating or simple artificial insemination techniques.
Footnotes
1Supported by a postdoctoral fellowship from the Lalor Foundation (S.T.), a grant from the Texas Equine Research Account (H.B.), and grants from the National Institutes of Health (HD-35166) and the Texas Department of Agriculture (D.M.H.).
REFERENCES
- Yanagimachi R.The physiology of reproduction. Knobil E, Neill JD.Mammalian Fertilization New York:Raven Press;1994: 189–317. [Google Scholar]
- Bleil JD, Wassarman PM.Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol 1983; 95: 317–324. [DOI] [PubMed] [Google Scholar]
- Bi M, Wassler MJ, Hardy DM.Sperm adhesion to the extracellular matrix of the egg. Hardy DM.Fertilization San Diego:Academic Press;2002: 153–180. [Google Scholar]
- Miller DJ, Gong X, Shur BD.Sperm require beta-N-acetylglucosaminidase to penetrate through the egg zona pellucida. Development 1993; 118: 1279–1289. [DOI] [PubMed] [Google Scholar]
- Ensslin MA, Shur BD.Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell 2003; 114: 405–417. [DOI] [PubMed] [Google Scholar]
- Hardy DM, Garbers DL.Species-specific binding of sperm proteins to the extracellular matrix (zona pellucida) of the egg. J Biol Chem 1994; 269: 19000–19004. [PubMed] [Google Scholar]
- Hardy DM, Garbers DL.A sperm membrane protein that binds in a species-specific manner to the egg extracellular matrix is homologous to von Willebrand factor. J Biol Chem 1995; 270: 26025–26028. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM.Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell 1980; 20: 873–882. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Zhuang T, Ord TS, Hui L, Moss SB, Gerton GL.Recombinant mouse sperm ZP3-binding protein (ZP3R/sp56) forms a high order oligomer that binds eggs and inhibits mouse fertilization in vitro. J Biol Chem 2008; 283: 12438–12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Azuma S, Kashiwabara S, Toyoda Y.Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem 1994; 269: 31845–31849. [PubMed] [Google Scholar]
- Bi M, Hickox JR, Winfrey VP, Olson GE, Hardy DM.Processing, localization and binding activity of zonadhesin suggest a function in sperm adhesion to the zona pellucida during exocytosis of the acrosome. Biochem J 2003; 375: 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP, Bi M, Hardy DM, NagDas SK.Zonadhesin assembly into the hamster sperm acrosomal matrix occurs by distinct targeting strategies during spermiogenesis and maturation in the epididymis. Biol Reprod 2004; 71: 1128–1134. [DOI] [PubMed] [Google Scholar]
- Hickox JR, Bi M, Hardy DM.Heterogeneous processing and zona pellucida binding activity of pig zonadhesin. J Biol Chem 2001; 276: 41502–41509. [DOI] [PubMed] [Google Scholar]
- Gao Z, Garbers DL.Species diversity in the structure of zonadhesin, a sperm-specific membrane protein containing multiple cell adhesion molecule-like domains. J Biol Chem 1998; 273: 3415–3421. [DOI] [PubMed] [Google Scholar]
- Wilson MD, Riemer C, Martindale DW, Schnupf P, Boright AP, Cheung TL, Hardy DM, Schwartz S, Scherer SW, Tsui LC, Miller W, Koop BF.Comparative analysis of the gene-dense ACHE/TFR2 region on human chromosome 7q22 with the orthologous region on mouse chromosome 5. Nucleic Acids Res 2001; 29: 1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea IA, Sivashanmugam P, O'Rand MG.Zonadhesin: characterization, localization, and zona pellucida binding. Biol Reprod 2001; 65: 1691–1700. [DOI] [PubMed] [Google Scholar]
- Herlyn H, Zischler H.Identification of a positively evolving putative binding region with increased variability in posttranslational motifs in zonadhesin MAM domain 2. Mol Phylogenet Evol 2005; 37: 62–72. [DOI] [PubMed] [Google Scholar]
- Gasper J, Swanson WJ.Molecular population genetics of the gene encoding the human fertilization protein zonadhesin reveals rapid adaptive evolution. Am J Hum Genet 2006; 79: 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford JM.Sperm/egg interaction: the specificity of human spermatozoa. Anat Rec 1977; 188: 477–487. [DOI] [PubMed] [Google Scholar]
- Hanada A, Chang MC.Penetration of zone-free eggs by spermatozoa of different species. Biol Reprod 1972; 6: 300–309. [DOI] [PubMed] [Google Scholar]
- Peterson RN, Russell L, Bundman D, Freund M.Sperm-egg interaction: evidence for boar sperm plasma membrane receptors for porcine zona pellucida. Science 1980; 207: 73–74. [DOI] [PubMed] [Google Scholar]
- Gray AP. Mammalian hybrids. Slough, England:: Commonwealth Agricultural Bureas;; 1972. [Google Scholar]
- Crump JP, Jr, Crump JW.Manual semen collection from a Grevy's zebra stallion (Equus grevyi), onset of sperm production, semen characteristics, and cryopreservation of semen, with a comparison to the sperm production from a Grant's Zebra stallion (Equus burchelli boehmi). Theriogenology 1994; 41: 1011–1021. [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL.Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 1991; 39: 741–748. [DOI] [PubMed] [Google Scholar]
- Lidell ME, Johansson ME, Hansson GC.An autocatalytic cleavage in the C terminus of the human MUC2 mucin occurs at the low pH of the late secretory pathway. J Biol Chem 2003; 278: 13944–13951. [DOI] [PubMed] [Google Scholar]
- Haldane JBS.Sex ratio and unisexual sterility in hybrid animals. J Genet 1922; 12: 101–109. [Google Scholar]