Abstract
We examined the inhibitory effects of loquat methanol extract on the adhesion, migration, invasion and matrix metalloproteinase (MMP) activities of MDA-MB-231 human breast cancer cell line. Cells were cultured with DMSO or with 10, 25, or 50 µg/ml of loquat methanol extract. Both leaf and seed extracts significantly inhibited growth of MDA-MB-231 cells in a dose-dependent manner, although leaf extract was more effective. Adhesion and migration were significantly inhibited by loquat extracts in a dose-dependent manner. Loquat extract also inhibited the invasion of breast cancer cells in a dose-dependent manner and leaf extract was more effective than seed extract. MMP-2 and MMP-9 activities were also inhibited by loquat extract. Our results indicate that methanol extracts of loquat inhibit the adhesion, migration and invasion of human breast cancer cells partially through the inhibition of MMP activity and leaf extract has more anti-metastatic effects in cell based assay than seed extract. Clinical application of loquat extract as a potent chemopreventive agent may be helpful in limiting breast cancer invasion and metastasis.
Keywords: Loquat (Eriobotrya japonica), adhesion, migration, invasion, MMP
Introduction
Metastasis is a series of events including the detachment of tumor cells from the primary site, adhesion, migration and invasion of tumor cells into the blood or lymphatic vessel, extravasations out of the vessel, and interactions with the target tissues. Motility and invasion into the new target tissue result in the formation of a secondary tumor (Song et al., 2006; Weber, 2008). These events occur repeatedly during tumor invasion, and inhibition of adhesiveness, motility and invasiveness is likely to contribute to prevention of cancer progression (Festuccia et al., 1998; Festuccia et al., 1999; Weber, 2008). These stages are determined by characteristics inherent to the tumor cells, such as hormone receptor status, production of proteolytic enzymes, expression of cell adhesion molecules and increase in motility (Kohn & Liotta, 1995; Shaikh et al., 2008), as well as by a number of growth factors, matrix molecules and cytokines in the metastatic microenvironment (Yoneda et al., 1994).
Matrix metalloproteinases (MMPs) promote tumor progression by degrading normal barriers (Johansson et al., 2000). MMP-2 and MMP-9 (gelatinase A and B) are related to tumor invasion and metastasis because of their capacities for tissue remodeling via the extracellular matrix, basement membrane degradation and induction of angiogenesis (Sun et al., 2009).
Loquat (Eriobotrya japonica Lindley) has been used as a medicinal plant in Japan and China. Astringent leaves of loquat have been used for a long time to treat chronic bronchitis, coughs, phlegm, high fever and gastroenteric disorders. Terpenoids isolated from loquat leaves have anti-tumor, antiviral and anti-inflammatory properties (Liang et al., 1990; Nozato et al., 1994; Shimizu et al., 1996; Tommasi et al., 1992). Euscaphic acid, one of triterpene acids isolated from the methanol extract of loquat, was reported to exhibit marked anti-tumor promoting activity in an in vivo two-stage carcinogenesis test of mouse tumor (Banno et al., 2005). Polyphenol compounds in loquat leaves are cytotoxic against human oral tumor cell lines (Ito et al., 2000). In contrast, loquat seeds have the inhibitory effect of oxidative-stress and hypoglycemic property (Kim et al., 2009). However, a little has been performed on the investigations of their pharmacological importance.
Thus, we investigated anti-metastatic effects of loquat leaf and seed extracts in this study and reported the inhibitory effects of loquat extracts on the adhesion, migration, invasion and MMP activity of MDA-MB-231 human breast cancer cells.
Materials and Methods
Preparation of the methanol extract
Loquat leaves were collected from the Herb Garden of Mokpo National University in Muan, Korea. Leaves were freeze-dried and powdered. Loquat seeds were collected from Wan-Do in Jeonnam, Korea and used for preparation of extract after peeling of hard shells. The methanol extracts were obtained by macerating leaf powder and seeds with 80% methanol for 2 days at room temperature, and this procedure was repeated twice. The respective extract was filtered under reduced pressure and freeze-dried. The yields obtained were 32% for leaves and 27% for seeds.
Cells and culture conditions
The MDA-MB-231 human breast cancer cells were obtained from the American Type Culture and Collection (Rockville, MD). All cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco, Gaithersburg), supplemented with penicillin 100units/ml, streptomycin 100 µg/ml and 10% fetal bovine serum, and incubated in a humidified incubator at 37℃ and 5% CO2. Cultures used in subsequent experiments were at < 20 passages.
Cell proliferation assay
To determine the effect of leaf and seed methanol extracts on MDA-MB-231 cell proliferation, cells were seeded in 6-well culture plates at a concentration of 6×104/well. Complete medium with DMSO or with 10, 25 or 50 µg/ml of leaf or seed extract were replaced every other day, beginning the day after seeding. Cells cultured in complete medium were harvested with trypsin-EDTA on day 5 after treatment and counted by a model Z1 Coulter particle counter (Coulter Corp, Miami, Florida).
Cell adhesion assay
Plates (12-wells) were coated with 30 µg of Matrigel (Becton Dickinson, Bedford, MA). To block nonspecific binding sites, Matrigel coated culture plates were re-hydrated with serum free DMEM containing 0.1% BSA for 90 min at 37℃ and then washed with the same medium. MDA-MB-231 cells were trypsinized and resuspended in serum free DMEM containing 0.1% BSA. Cells were seeded at a concentration of 2×105/well in the presence of DMSO or of 10, 25 or 50 µg/ml of leaf or seed extract and incubated for 90 min at 37℃ and 5% CO2. At the end of 90 min, the medium was removed and the cells were washed gently two times with PBS to remove unattached cells. The attached cells were harvested by trypsinization, resuspended and counted. Each assay was performed in triplicate and repeated in three independent experiments. Values were expressed as the average of triplicate experiment.
Cell migration assay
Motility was assessed using a scratch wound assay (Goodman et al., 1985). Cells were seeded into 6-well cell culture plates at a concentration of 3×105cells and cultured in 10% FBS DMEM to near confluence. The confluent monolayer was carefully wounded using a sterile pipette tip and cellular debris was washed gently with PBS. The wounded monolayer was incubated in 10% FBS DMEM containing 20 µg/mL fibronectin and DMSO or 10, 25 or 50 µg/ml of leaf or seed extract for 24 hours. Migrating cells were examined under 10× by phase contrast microscope and photographed.
Invasion assay
The ability of cells to migrate across a Matrigel barrier (invasion) was determined by a modified Boyden chamber. Briefly, Boyden chambers were assembled using 8 µm Falcon transwell inserts (Becton Dikinson, Bedford, MA) as the upper chamber and 12-well plates as the lower chamber. Matrigel was applied to the insert and left overnight to dry in a laminar flow fume hood. The insert was re-hydrated with serum free DMEM for 90 min at 37℃ and 5% CO2. Following rehydration, Matrigel coated inserts were washed with serum free DMEM. Exponentially growing breast tumor cells were harvested by trypsin/EDTA and resuspended in 0.1% BSA DMEM. Cells were seeded at a concentration of 2×105 cells. DMSO or 10, 25 or 50 µg/ml of leaf or seed extract was carefully added to the upper chamber. Twenty micrograms of fibronectin was added as a chemoattractant to the lower chamber. The Boyden chamber was incubated for 24 hours at 37℃ and 5% CO2. At the end of incubation, the cells in the upper chamber were removed by wiping gently with a cotton swab. Cells that transversed the Matrigel and attached to the lower surface of the insert were fixed with 10% formalin and stained with 0.5% crystal violet. Inserts were examined under 20× or 40× phase contrast field microscopy and photographed. Values for invasion were expressed as the number of migrated cells per microscopic field. Four fields were counted. Each assay was performed in triplicate and repeated in three independent experiments. Values were expressed as the average of triplicate experiments.
Gelatin zymography
The activity of MMP-2 and MMP-9 in the conditioned media was assayed by gelatin zymography. Briefly, MDA-MB-231 cells were seeded at a concentration of 2×105/well in 6-well plates. After treatment with DMSO or 10, 25 or 50 µg/ml of leaf or seed extract for 24 hours, the supernatants were collected and subjected to gel electrophoresis on 10% running gels containing 0.1% gelatin. The gels were washed in 2.5% Triton X-100 for 30 minutes, followed by incubation for 20 hours at 37℃ in the incubation buffer containing 50 mM Tris-HCl (pH 7.5), 0.2 M NaCl, 1 mM CaCl2 and 0.2% NaN3. The gels were stained for 10 hours in 0.5% Coomassie brilliant blue, then destained with destaining solution (45% methanol and 10% acetic acid) and photographed.
Statistical analysis
Statistics were analyzed using Sigma Stat software. Results were expressed as the mean ± S.D. of three independent experiments. Comparisons were based on one-way ANOVA followed by Duncan's multiple range test. A p-value < 0.05 was considered statistically significant.
Results
Effect of loquat on the proliferation of breast cancer cells
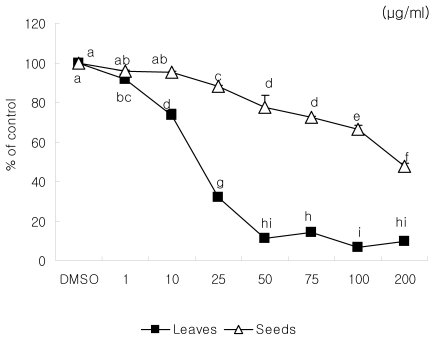
Loquat leaf extract inhibited the proliferation of MDA-MB-231 cells at all concentrations and was more effective than seed extract (Fig. 1). Seed extract significantly inhibited proliferation at the concentrations of 25 and 50 µg/ml, but not at 10 µg/ml. At the concentration above 100 µg/ml of loquat leaf or seed extract, the number of cells on the day 5 after treatment was decreased to less than 6×104/well, the number of cells seeded at the beginning of proliferation assay. However, extracts at the concentrations of 10, 25, and 50 µg/ml inhibited the cell growth and no cytotoxicity was observed. Thus, we used extracts at the concentrations of 10, 25, and 50 µg/ml for further investigation of anti-metastatic effect of loquat.
Fig. 1.
Effect of Eriobotrya japonica Lindley extracts on MDA-MB231 cell proliferation. Cells were seeded in 6-well culture plates at a concentration of 6×104/well. Complete medium with DMSO or with 10, 25 or 50 µg/ml of leaf or seed extract were replaced every the other day. Cells cultured in complete medium were harvested on day 5 after treatment and were counted. Data were expressed as mean ± SD. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple test (P < 0.05).
Effect of loquat on the adhesion of breast cancer cells
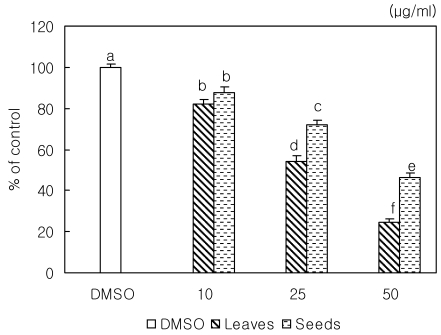
Loquat inhibited the adhesion of cancer cells. Leaf extracts down-regulated the binding of the cells to Matrigel and significantly inhibited adhesion of MDA-MB-231 cells in a dose dependant manner (Fig. 2). At a concentration of 50 µg/ml, adhesion was reduced by 56% compared to the control. Seeds had a significant inhibitory effect on adhesion only at a concentration of 50 µg/ml.
Fig. 2.
Effect of Eriobotrya japonica Lindley extracts on MDA-MB231 cell adhesion. Cells were added to matrigel coated plates and incubated for 90 min in present of DMSO or 10, 25 or 50 µg/ml of leaf or seed extract. Attached cells were counted. Data were expressed as mean ± SD. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple test (P < 0.05).
Effect of loquat on the migration of breast cancer cells
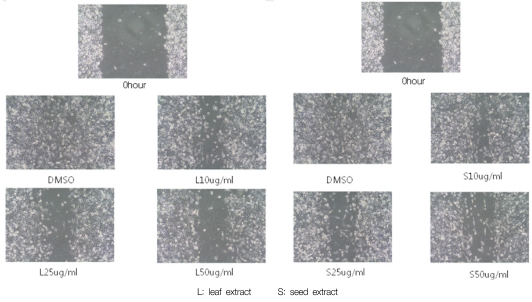
The effect of loquat on MDA-MB-231 cell migration was analyzed using the scratch wound assay. In the absence of loquat extract, MDA-MB-231 cells migrated along the edges of the wound and covered the wound. Significant inhibition of cell flattening and spreading was observed in the presence of loquat leaf and seed extracts (Fig. 3).
Fig. 3.
Effect of Eriobotrya japonica Lindley extracts on MDA-MB231 cell migration. Cells were seeded into 6 well cell culture plates and cultured in 10% FBS DMEM to near confluence. Confluent monolayer was carefully wounded and washed cellular debris gently with PBS. The wounded monolayer was incubated in 10% FBS DMEM containing 20 µg/ml fibronectin and DMSO or, 10, 25 or 50 µg/ml of leaf or seed extract for 24 hours.
Effect of loquat on the invasion of breast cancer cells
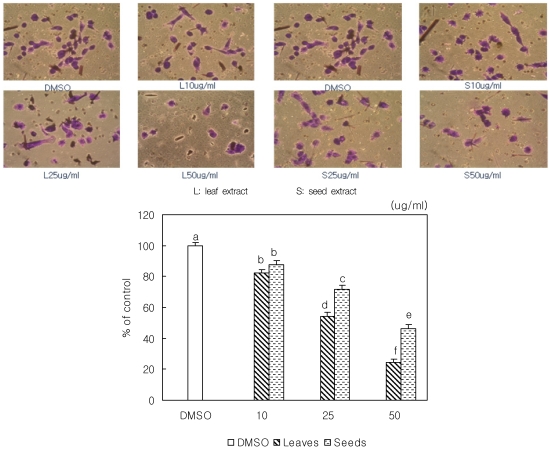
We analyzed the invasion of MDA-MB-231 using a Boyden chamber model, and both leaf and seed extract dose dependently suppressed the invasion of MDA-MB-231cells (Fig. 4). Loquat leaf extract inhibited the invasion of breast cancer cells more effectively than seed extract.
Fig. 4.
Effect of Eriobotrya japonica Lindley extracts on MDA-MB231 cell invasion (P < 0.05). Boyden chamber was used for the invasion assay. Cells were treated with DMSO or 10, 25 or 50 µg/ml of leaf or seed extract for 24 hours during assay. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Ducan's multiple test (P < 0.05).
Effect of loquat on MMP-2 and MMP-9 enzyme activities
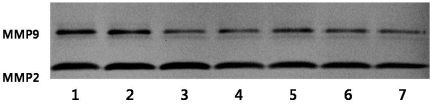
We analyzed the effect of loquat on the activities of gelatinolytic MMP-2 and MMP-9 in MDA-MB-231 breast cancer cells to determine whether the anti-invasive activity of loquat was correlated with the inhibition of MMP activity (Fig. 5). Loquat leaf extract had an inhibitory effect on the MMP-9 activity in a dose dependent manner, and MMP-2 activity only at the concentration of 50 µg/ml. Seed extract showed an inhibitory effect on the MMP-9 activity.
Fig. 5.
Effects of Eriobotrya japonica Lindley on the activities of MMP2 and MMP9 of MDA-MB231 cells. 1: DMSO 2: seed10 µg/ml 3: seed 25 µg/ml 4: seed 50 µg/ml 5: leaves 10 µg/ml 6: leaves 25 µg/ml 7: leaves 50 µg/ml
Discussion
Breast cancer invasion is a multiple step process characterized by altered cellular adhesion, increased motility and invasion of the extracellular matrix. We treated cells with loquat leaf or seed extract for 5 days to determine the effect on the proliferation of invasive estrogen receptor-negative MDA-MB-231 breast cancer cells. Loquat leaf extract inhibited the proliferation of MDA-MB-231 cells more effectively than seed extract (Fig. 1). Polyphenols of loquat were reported to have selective cytotoxicity against cancer cell lines relative to normal cells (Ito et al., 2000).
In the present study, we used methanol extract which was reported to have in vivo anti-tumor promoting activity (Banno et al., 2005) and used extracts at the concentrations of 10, 25, and 50 µg/ml, at which no cytotoxicity was observed.
The initial invasive action of metastatic cells requires interaction between tumor cells and the extracellular matrix (ECM) (Hazan et al., 2000; Zheng et al., 1999). We observed an inhibitory effect of loquat on cancer cell attachment ability. The inhibitory effect of loquat may be due to suppression of adhesion protein expression and/or the activity of adhesion molecules. Results in current study indicate that, in addition to the mechanism involving increased adhesion protein expression, the activity of adhesion molecules can increase the enhanced adhesion ability in human colon cancer (Shaikh et al., 2008).
Cell migration is crucial for cancer cell invasion and metastasis (Huang et al., 2008; Kawasaki et al., 2008). We analyzed the effect of loquat on MDA-MB-231 cell migration using the scratch wound assay and observed an inhibitory effect on cancer cell migration. Loquat leaf and seed extracts suppressed MDA-MB-231 cell migration compared to the control (Fig. 3). Antiproliferative properties can contribute to inhibitory effect on the migration (Azios & Dharmawardhane, 2005). We observed inhibitory effects of loquat on the proliferation of cancer cells (Fig. 1). However, in the present study, seed extracts were less effective on the inhibition of cell proliferation than leaf extracts, whereas the inhibitory effects of seed and leaf extracts on cell migration were similar. Azios and Dharmawardhane (2005) reported that resveratrol, a phytoestrogen present in grape skin and red wine, inhibited breast cancer cell migration by its antiproliferative and proapoptotic properties. Therefore, other factors which can affect cell migration need to be investigated to explain the discordance between the effect of loquat on cell proliferation and migration.
A critical event in breast cancer invasion and metastasis is the invasion of cancer cells through the extracellular matrix (Meng et al., 2000). In our Boyden chamber model, both leaf and seed extracts dramatically suppressed the invasion of MDA-MB-231cells (Fig. 4). Loquat leaf extract inhibited the invasion of breast cancer cells more effectively than seed extract. To successfully penetrate the Boyden chamber, cells must successfully adhere, degrade and transverse the Matrigel coated insert (Cos et al., 1998). Therefore, in the present study, the inhibitory effect of invasion of loquat could be explained by its inhibition of adhesion and migration.
Matrix metalloproteinases (MMPs) are crucial for degrading extracellular matrix components and promoting tumor cellular invasion in vitro and in vivo (Johansson et al., 2000). To determine whether the anti-invasive activity of loquat was correlated with the inhibition of gelatinolytic MMP activity, we analyzed the effect of loquat on the activities of gelatinolytic MMP-2 and MMP-9 in MDA-MB-231 breast cancer cells (Fig. 5). Both leaf and seed extracts more effectively inhibited MMP-9 activity than MMP-2 activities. MMP-2 and MMP-9 (gelatinase A and B) are related to tumor invasion and metastasis by their capacities for tissue remodeling via extracellular matrix basement membrane degradation and angiogenesis induction. Therefore, inhibitory effects of loquat on the activities of MMPs may exert to the inhibition of invasion and MMP9 is more important than MMP 2 for the inhibition of invasion. Loquat leaf extract was more effective on the inhibition of activities of MMPs than seed extract and this inhibitory effect was correlated with inhibition of invasion in the MDA-MB-231 cells.
In conclusion, we demonstrated that loquat extracts were effectively able to suppress invasiveness of breast cancer cells and inhibitory effects on the adhesion, migration and MMP activities are the possible mechanisms for the inhibition of invasiveness. Loquat leaf extract was more effective than seed extract on the inhibition of proliferation, adhesion, invasion, and MMP activities in the MDA-MB-231 cells. Our results provide a theoretical foundation for the possibility of loquat as a potential agent for the treatment of cancer metastasis.
Footnotes
This research was financially supported by the ministry of Education, Science Technology (MEST) and Korea Institute for Advancement of Technology (KIAT) through the Human Resource Training Project for Regional Innovation.
References
- 1.Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface acting structures and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia. 2005;7:128–140. doi: 10.1593/neo.04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banno N, Akihisa T, Tokuda H, Yasukawa K, Taguchi Y, Akazawa H, Ukiya M, Kinura Y, Suxuki T, Nishino H. Anti-inflammatory and antitumor-promotin effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol Pharm Bull. 2005;25:1995–1999. doi: 10.1248/bpb.28.1995. [DOI] [PubMed] [Google Scholar]
- 3.Cos S, Fernández R, Güézmes A, Sánchez-Barceló EJ. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998;58:4383–4390. [PubMed] [Google Scholar]
- 4.Festuccia C, Bologna M, Gravina GL, Guerra F, Angelucci A, Villanova I, Millimaggi D, Teti A. Osteoblast conditioned media contain TGF-β and modulate the migration of prostate tumor cells and their interactions with extracellular matrix component. Int J Cancer. 1999;81:395–403. doi: 10.1002/(sici)1097-0215(19990505)81:3<395::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Festuccia C, Guerra F, D'Acenzo S, Guinciuglio D, Albini A, Bologna M. In vitro regulation of pericellular proteolysis in prostatic tumor cells treated with bombesin. Int J Cancer. 1998;75:418–431. doi: 10.1002/(sici)1097-0215(19980130)75:3<418::aid-ijc16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Goodman SL, Vollmer HP, Birchmeier W. Control of cell locomotion: pertubation with an antibody directed against specific glycoproteins. Cell. 1985;41:1029–1038. doi: 10.1016/s0092-8674(85)80083-0. [DOI] [PubMed] [Google Scholar]
- 7.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaeonson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Kim MS, Ratovitski E. Midkine promote tetraspanin-integrin interaction and inuceds FAK-Stat1 alpha pathway contributing to migration/invasivemess of human head and neck squamous cell carcinoma cells. Biochem Biophys Res Commun. 2008;377:474–478. doi: 10.1016/j.bbrc.2008.09.138. [DOI] [PubMed] [Google Scholar]
- 9.Ito H, Kobayashi E, Takamatsu Y, Li S, Hatano T, Sakagami H, Kusama K, Satoh K, Sugita D, Shimura S, Itoh Y, Yoshida T. Polyphenols from Eribotrya japonica and their cytotoxicity against human oral cancer cell lines. Chem Pharm Bull. 2000;48:687–693. doi: 10.1248/cpb.48.687. [DOI] [PubMed] [Google Scholar]
- 10.Johansson N, Ahonen M, Kajari VM. Matrix metalliproteinases in tumor invasion. Cell Mol Life Sci. 2000;57:5–15. doi: 10.1007/s000180050495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki G, Yanamoto S, Yoshitomi I, Yamada S, Mizuno A. Overexpression of metastasis-associated MTA1 in oral squamous cell carcinomas:correlation with metastasis and invasion. Int J Oral Maxillofac Surg. 2008;37:1039–1046. doi: 10.1016/j.ijom.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Kim E, Kim MS, Rhyu DY, Min OJ, Baek HY, Kim YJ, Kim HA. Hypoglycemic effect of Eryobotrya japonica (E. japonica) in db/db mice. The Korean Journal of Food and Nutrition. 2009;22:159–165. [Google Scholar]
- 13.Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856–1862. [PubMed] [Google Scholar]
- 14.Liang ZZ, Aquino R, Feo VD, Shimone FD, Pizza C. Polyhaydroxylated triterpene from Eribotrya japonica. Planta Med. 1990;56:330–332. doi: 10.1055/s-2006-960973. [DOI] [PubMed] [Google Scholar]
- 15.Meng Q, Goldberf ID, Rosen EM, Fan S. Inhibitory effects of indole-3-carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res Treat. 2000;63:147–152. doi: 10.1023/a:1006495824158. [DOI] [PubMed] [Google Scholar]
- 16.Nozato N, Matsumoto K, Uemitsu N. Triterpene from the leaves of Eribotrya japonica. Nat Med (Tokyo) 1994;48:336. [Google Scholar]
- 17.Shaikh FM, Seales EC, Clem WC, Hennessy KM, Zhuo Y, Bellis SL. Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms. Exp Cell Res. 2008;314:2941–2950. doi: 10.1016/j.yexcr.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu M, Uemitsu N, Shirota M, Matsumoto K, Tezuka U. A new triterpene ester from Eribotrya japonica. Chem Pharm Bull. 1996;44:2181–2182. [Google Scholar]
- 19.Song G, Ohashi T, Sakamoto N, Sato M. Adhesive force of human hepatoma HepG2 cells to endothelial cells and expression of E-selectin. Mol Cell Biomech. 2006;3:61–68. [PubMed] [Google Scholar]
- 20.Sun Y, Lu N, Ling Y, Gao Y, Chen Y, Wang L, Hu R, Qi Q, Liu W, Yang Y, You Q, Guo Q. Oroxylin A suppress invasion through down-regulating the expression of matrix metallic proteinase-2/9 in MDA-MB-435 human breast cancer cells. Eur J Pharmacol. 2009;603:22–28. doi: 10.1016/j.ejphar.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Tommasi ND, Shimone FD, Pizza C. Cinstituents of Eribotrya japonica : a study of their antiviral properties. J Nat Prod. 1992;55:1067–1073. doi: 10.1021/np50086a006. [DOI] [PubMed] [Google Scholar]
- 22.Weber GF. Molecular mechanism of metastasis. Cancer Lett. 2008;270:181–190. doi: 10.1016/j.canlet.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Yoneda T, Sasaki A, Mundy GR. Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat. 1994;32:73–84. doi: 10.1007/BF00666208. [DOI] [PubMed] [Google Scholar]
- 24.Zheng DQ, Woodard AS, Fronaro M, Tallini G, Languino LR. Prostatic Carcinoma Cell Migration Via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]