Abstract
Rationale: There is increasing evidence of increased ventilatory instability in patients with obstructive sleep apnea (OSA), but previous investigations have not studied whether the hypocapnic apneic threshold is altered in this group.
Objectives: To compare the apneic threshold, CO2 reserve, and controller gain between subjects with and without OSA matched for age, sex, and body mass index.
Methods: Hypocapnia was induced via nasal mechanical ventilation for 3 minutes. Cessation of mechanical ventilation resulted in hypocapnic central hypopnea or apnea depending upon the magnitude of the hypocapnia. The apnea threshold (PetCO2–AT) was defined as the measured PetCO2 at which the apnea closest to the last hypopnea occurred. The CO2 reserve was defined as the change in PetCO2 between eupneic PetCO2 and PetCO2–AT. Controller gain was defined as the ratio of change in Ve between control and hypopnea or apnea to the ΔPetCO2.
Measurements and Main Results: Eleven pairs of subjects were studied. There was no difference in the PetCO2–AT between the two groups. However, the CO2 reserve was smaller in the subjects with OSA (2.2 ± 0.6 mm Hg) compared with the control subjects (4.5 ± 1.4 mm Hg; P < 0.001). The controller gain was increased in the subjects with OSA (3.7 ± 1.3 L/min/mm Hg) compared with the control subjects (1.6 ± 0.5 L/min/mm Hg; P < 0.001). Controller gain decreased and CO2 reserve increased in seven subjects restudied after using continuous positive airway pressure for 1 month.
Conclusions: Ventilatory instability is increased in subjects with OSA and is reversible with the use of continuous positive airway pressure.
Keywords: control of breathing, obstructive sleep apnea, controller gain, apneic threshold, complex sleep apnea, central sleep apnea
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Ventilatory instability, manifested by increased chemoresponsiveness is observed in subjects with obstructive sleep apnea and when combined with unfavorable upper airway mechanics, explains the propensity to sleep-disordered breathing in such individuals. However, the role of continuous positive airway pressure in affecting the parameters of central apnea in such patients has not been completely defined.
What This Study Adds to the Field
In subjects with obstructive sleep apnea, ventilatory instability is manifested by a lower CO2 reserve and increased controller gain, both of which are reversed by the use of continuous positive airway pressure.
There is increasing evidence of instability of the ventilatory control system in patients with obstructive sleep apnea (OSA) (1–5). One manifestation is the persistence of periodic breathing and repetitive central apnea after “curative” tracheotomy in patients with OSA (4, 6). More recent evidence includes the response to hypercapnia administered in the pseudorandom binary stimulation test (1) and the finding, using proportional-assist ventilation, that the patients with severe OSA have a higher magnitude of chemical control system instability than patients with milder OSA (3). In fact, there is evidence that loop gain and pharyngeal collapsibility interact as potential determinants of the apnea/hypopnea index (3, 7).
Several lines of evidence suggest a mechanistic interaction between obstructive and central sleep apnea. This is supported by studies demonstrating that oscillating ventilatory motor output during periodic breathing is associated with reciprocal changes in upper airway resistance (8, 9); complete upper airway obstruction occurs in individuals with unfavorable upper airway anatomy. Similarly, upper airway narrowing or occlusion occurs during central apnea (10). Likewise, several studies have revealed that reversal of obstructive apnea with nasal CPAP therapy may lead to the emergence of central apnea, often referred to as “complex sleep apnea” (11–13).
The association between ventilatory control instability and OSA may be due to factors such as age, sex, or obesity. Moreover, chemical control instability may be a cause or a consequence of obstructive apnea. The reported resolution of “complex sleep apnea” and the amelioration of ventilatory control abnormalities after positive pressure therapy suggest that OSA may lead to ventilatory control abnormalities. We hypothesized that patients with OSA demonstrate a higher degree of chemoreflex sensitivity to changes in Pco2 below eupnea resulting in decreased CO2 reserve and a closer proximity of the apneic threshold to eupneic PetCO2. Therefore, the purpose of the investigation was to determine whether patients with OSA are more susceptible to central apnea than subjects without OSA. Preliminary results of this analysis have been previously published in abstract form (14).
METHODS
The Human Investigation Committee of the Wayne State University School Medicine and the Dingell Veterans Affairs Medical Center approved the experimental protocol. Participants gave written informed consent to participate. We studied healthy nonsnoring individuals free of sleep apnea and individuals with recently diagnosed OSA based upon polysomnography. All subjects with OSA were naive to nasal continuous positive airway pressure (CPAP) therapy. We excluded subjects with severe daytime sleepiness (ESS >15), significant comorbidity, and operators of commercial vehicles. Subjects with OSA who completed the experimental night were offered CPAP and invited to return 6 wk later for a repeat study. Objective compliance with CPAP was downloaded from the CPAP unit at the time of the second study.
Measurements
Sleep stage was scored according to standard methods (15). Airflow was measured by a heated pneumotachometer connected to a tight-fitting nasal mask. Vt was obtained by integrating the pneumotachograph flow signal. PetCO2 was measured with a gas analyzer. Supraglottic pressure was measured with a solid-state catheter (Millar, Houston, TX), positioned in the hypopharynx just below the base of the tongue.
Mechanical Ventilation Protocol
We used a nasal mechanical ventilator to induce brief hyperventilation as previously described (16, 17). The expiratory pressure was kept constant throughout the study. For control subjects, the ventilator was set at an expiratory positive airway pressure (EPAP) of 2.0 cm H2O. For subjects with OSA, we set the EPAP at the opening pressure that eliminated apneic and hypopneic episodes. We did not attempt to eliminate flow limitation in either group because this would have required repeated changes in the EPAP level, with potential confounding effects on lung volumes and PetCO2. During periods of hyperventilation, the ventilator was set in spontaneous timed mode with a back-up rate of 4 to 8 breaths per minute. We increased the inspiratory pressure in increments of 1.0 cm H2O from the baseline EPAP for each successive trial. Mechanical ventilation was continued for 3 minutes and was terminated during expiration to the baseline EPAP. Each trial was repeated twice, with trials separated by a minimum of 3 minutes. The ensuing hypocapnia resulted in a hypopnea or central apnea. If expiratory time was at least 5 seconds, it was defined as a central apnea.
Data Analysis
Only trials with stable sleep-state (Stages N2/N3, absence of arousal or ascent to Stage N1) were analyzed. For each trial, the control period was represented by the average of five breaths immediately preceding the onset of mechanical ventilation. The hyperventilation data were the calculated average of the last five mechanically ventilated breaths before the ventilator was turned back to the baseline EPAP. The change in PetCO2 (ΔPetCO2) was calculated as the difference between the control period and the last five mechanical ventilation breaths. Ve was given a value of 0 during central apnea. The apneic threshold (PetCO2–AT) was defined as the measured PetCO2 at which the apnea closest to the last hypopnea occurred. The CO2 reserve (ΔPetCO2–AT) was defined as the change in PetCO2 between eupneic PetCO2 and PetCO2–AT.
The propensity to central apnea during NREM sleep is determined by an interaction between the response of the brain and chemoreceptors to changing PetCO2, representing the controller, and the effectiveness of the lung/respiratory system in lowering PetCO2 in response to hyperventilation (the plant) (18). The chemoreflex sensitivity to reduced PetCO2 was calculated for each trial as representative of the gain of the controller, defined as the ratio of change in Ve between control and apnea to the ΔPetCO2–AT (18, 19). The effectiveness of the plant in translating ventilatory changes into changes in PaCO2 represents the plant gain. The calculation of plant gain is described in the online supplement.
Statistical Analysis
For analysis #1, 11 subjects with OSA and 11 control subjects were paired for sex, age, and BMI (see Table E1 in the online supplement). Paired t tests were used to compare the NREM PetCO2, PetCO2–AT, ΔPetCO2–AT, and controller gain between the two groups.
For analysis #2, seven subjects with OSA were restudied after the use of CPAP (Table E2). Five control subjects and three subjects with OSA not on CPAP were also restudied (Table E3). Paired t tests were used to compare the NREM PetCO2, PetCO2–AT, ΔPetCO2–AT, and controller gain before and after CPAP use for the subjects with OSA and between studies for the control subjects.
RESULTS
Analysis #1: Comparison of Chemoresponsiveness between OSA and Control Subjects
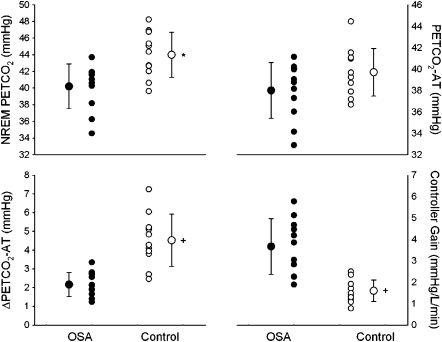
Eleven pairs of subjects with OSA and control subjects were included in the detailed analysis. Subject demographics can be found in Table E1, and the results are presented in Figure 1. Subjects with OSA had a lower NREM PetCO2 (OSA 40.2 ± 2.7 mm Hg vs. control, 44.0 ± 2.7 mm Hg; P = 0.013). There was no difference in PetCO2–AT between the two groups (OSA 38.0 ± 2.6 mm Hg vs. control, 39.5 ± 2.4 mm Hg; P = ns). Plant gain was not different between the two groups (OSA, 1.8 ± 1.3 mm Hg/L/min vs. control, 1.9 ± 0.9 mm Hg/L/min; P = ns). However, the CO2 reserve was smaller in subjects with OSA (2.2 ± 0.6 mm Hg) compared with the control subjects (4.5 ± 1.4 mm Hg; P < 0.001). Chemoreflex sensitivity was elevated in the subjects with OSA (3.7 ± 1.3 L/min/mm Hg) compared with the control subjects (1.6 ± 0.5 L/min/mm Hg; P < 0.001). The Ve required to achieve apnea, as a percentage of the control Ve, was higher in the control group (172.0 ± 36.6%) than in the OSA group (127.6 ± 27.8%; P = 0.031). Vt, as a percentage of the control Vt, was marginally significantly higher in the control group (18.3 ± 65.5%) than in the OSA group (140.3 ± 33.5%; P = 0.051).
Figure 1.
Individual and group mean data comparing NREM PetCO2, apnea threshold (PetCO2–AT), ΔPetCO2, and controller gain between subjects with obstructive sleep apnea (OSA) (closed circles) and control subjects (open circles). There were significant differences in the NREM PetCO2 (*P = 0.013), ΔPetCO2–AT, and controller gain (+P < 0.001).
Analysis #2: Comparison of Chemoresponsiveness before and after Treatment with CPAP
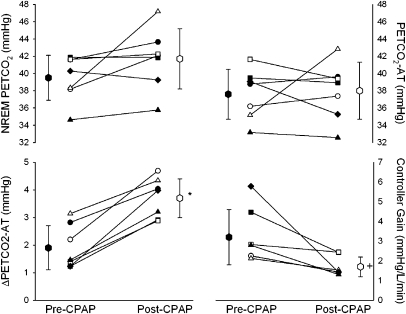
Seven subjects with OSA were studied before and after the use of CPAP for an average of 28.7 ± 9.5 days (60.2 ± 23.8% of potential nights) with an average use of 3.8 ± 2.1 hours on nights used. Subject demographics are presented in Table E2, and the results before and after CPAP are presented in Figure 2. Chemoreflex sensitivity decreased significantly with the use of nasal CPAP (pre-CPAP, 3.2 ± 1.4 L/min/mm Hg vs. post-CPAP, 1.7 ± 0.5 L/min/mm Hg; P = 0.016), whereas the ΔPetCO2–AT was significantly increased with CPAP use (pre-CPAP, 1.9 ± 0.8 mm Hg vs. post-CPAP, 3.7 ± 0.7 mm Hg; P < 0.001). There was no difference in NREM PetCO2 (pre-CPAP, 39.5 ± 2.6 mm Hg vs. post-CPAP, 41.7 ± 3.5 mm Hg; P = ns), PetCO2–AT (pre-CPAP, 37.6 ± 2.9 mm Hg vs. post-CPAP, 38.0 ± 3.3 mm Hg; P = ns), and plant gain (pre-CPAP, 2.1 ± 1.0 L/min/mm Hg vs. post-CPAP, 2.3 ± 1.3 L/min/mm Hg; P = ns) before and after CPAP use.
Figure 2.
Individual and group mean data comparing NREM PetCO2, PetCO2–apnea threshold (AT), ΔPetCO2–AT, and controller gain gain before (mean pre–continuous positive airway pressure [CPAP] value, closed hexagon) and after (mean post-CPAP value, open hexagon) use of CPAP. There were significant differences in the ΔPetCO2–AT (*P < 0.001) and controller gain (+P < 0.016).
Eight subjects were restudied after a median of 64 ± 54 days from the original study. Three of the subjects had OSA, and the repeat studies were without having used CPAP. Because the results appeared similar between the three subjects with OSA and the five control subjects, the results were combined. There was no change in chemoreflex sensitivity (first study, 2.6 ± 1.4 mm Hg/L/min vs. second study, 2.8 ± 1.6 mm Hg/L/min; P = 0.51), NREM PetCO2 (first study, 38.9 ± 3.5 mm Hg vs. second study, 40.0 ± 2.9 mm Hg; P = 0.32), PetCO2–AT (first study, 35.8 ± 3.0 mm Hg vs. second study, 37.1 ± 2.9 mm Hg; P = 0.23), or ΔPetCO2–AT (first study, 3.1 ± 1.5 mm Hg vs. second study, 2.9 ± 1.3 mm Hg; P = 0.61) between the two studies.
DISCUSSION
The major findings of our study are (1) CO2 chemoreflex sensitivity below eupnea was elevated in patients with OSA as compared with normal control subjects, (2) the CO2 reserve was smaller in patients with sleep apnea, and (3) treatment with nasal CPAP resulted in decreased chemoreflex sensitivity and increased CO2 reserve.
Methodological Considerations
Several considerations may influence the interpretation of our findings. First, we used nasal CPAP in patients with sleep apnea to stabilize the upper airway. We selected a CPAP level that eliminates apnea/hypopnea but maintains a moderate degree of inspiratory flow limitation to avoid overdistending the upper airway and to mitigate changes in lung volume or the development of hypocapnia secondary to the CPAP rather than the mechanical ventilation. This allowed us to minimize an additional confounder and allowed to us to prevent the development of CPAP emergency central apnea. The degree of flow limitation was similar between the two groups. Other investigators have used a similar approach to assess ventilatory control in patients with sleep apnea (6, 20). Second, our analysis included only trials with stable sleep state to ensure that sleep state changes did not influence the apneic threshold. In addition, we instituted partial sleep deprivation one night before the study to maximize the likelihood of stable sleep during the experiment. It is unlikely that mild sleep deprivation has affected our measures of the apneic threshold. For example, a recent study that rigorously controlled for a variety of factors showed that severe sleep deprivation does not affect the ventilatory response to CO2 (21). Third, it is unlikely that mechanical ventilation caused volume-related ventilatory decline because Vt rarely exceeded 200% of control and because subjects with OSA developed central apnea at a Vt below 150% of control Vt. Most of our study participants were men because we were unable to identify sufficient number of women with OSA who met the inclusion and exclusion criteria. Using the present model, we have previously shown that men, as compared with women, are more susceptible to the development of central apnea (16, 17). However, we do not believe this would influence our results because we matched subjects by sex. Finally, we studied only healthy subjects free of significant obesity, comorbidities, and medications, all of which influence the hypocapnic apneic threshold and/or CO2 reserve (17, 22, 23). Our findings do not address the potential interactive effects of obesity and low oxygen stores on breathing stability during sleep.
Susceptibility to Hypocapnic Central Apnea and Ventilatory Control
We noted that the chemoreflex sensitivity was elevated, resulting in a closer proximity of the hypocapnic apneic threshold to the eupneic PetCO2 (narrowed CO2 reserve). Our findings corroborate previous studies demonstrating abnormal chemical ventilatory control in patients with OSA compared with normal subjects regardless of the metric used to assess ventilatory stability during wakefulness or sleep. For example, the ventilatory recruitment threshold is elevated in patients with sleep apnea compared with matched control subjects during wakefulness (24). Likewise, obese patients with OSA demonstrate a higher response to repeated exposure to a single breath of CO2 during wakefulness (1) or to acoustic arousal (20), suggesting that the chemoreflex control system is under dampened in patients with sleep apnea and may promote further instability.
Our finding that chemoreflex sensitivity is elevated in patients with OSA compared with normal participants corroborates previous work demonstrating higher loop gain in patients with sleep apnea (3, 6). Loop gain is an engineering concept used as a framework to express the overall ventilatory change for a given initial perturbation. Using proportional assist ventilation to measure “loop gain” in patients with sleep apnea, Younes and colleagues found higher loop gain in patients with severe disease compared with mild disease (3). Likewise, Wellman and colleagues found a strong correlation between loop gain and apnea/hypopnea index in a subset of patients with OSA (6). The present study suggests that increased controller gain may be the mechanism of increased loop gain reported in patients with sleep apnea.
Several important implications can be noted from the observed difference in the apneic threshold between normal participants and patients with OSA. First, the reduction of “CO2 reserve” in patients with sleep apnea compared with normal control participants was due to increased chemosensitivity to hypocapnia with the ensuing narrowing of the CO2 reserve. Second, lower baseline NREM PetCO2 in patients with OSA suggests decreased plant gain and hence decreased susceptibility to developing hypocapnia for a given ventilatory perturbation. There is no standardized measurement of plant gain, but we believe our measurement allows for a reasonable estimate. However, we used changing PetCO2 as the independent variable, and we did not investigate the determinants of plant gain, including alveolar volume or changes in blood flow, both of which are important factors in plant gain. However, using the present measurement, there was no consistent difference in the plant gain between the two groups. Third, controller gain and CO2 reserve normalized after CPAP therapy, suggesting that ventilatory control instability may be a consequence rather than a cause of OSA.
In summary, patients with sleep apnea demonstrate a reversible increase in controller gain in response to hypocapnia and a reversible narrowing of the CO2 reserve, both of which contribute to a higher propensity to develop central apnea.
Mechanisms of Increased Controller Gain in Patients with OSA
Peripheral chemoreflex sensitivity is potentiated in young patients with OSA with no other clinical conditions (25, 26). Peng and colleagues demonstrated that chronic intermittent hypoxia in rats exposed to intermittent hypoxia for 10 days augments the carotid body sensory response to hypoxia followed by prolonged activation of the carotid body sensory discharge for 1 hour after the last hypoxia exposure. This is consistent with the development of sensory long-term facilitation. Reexposure to normoxia for 10 days reverses the effects of intermittent hypoxia and suggests that chronic intermittent hypoxia may lead to the generation of reactive oxygen species in the carotid body during the reoxygenation phase (27). Enhanced ventilatory chemoreflex sensitivity may also occur at the level of the integration of the afferent output at the pontine respiratory centers and the subsequent translation of chemoreceptor afferent information at the CNS to appropriate ventilatory changes. Other putative mechanisms include alterations in gene expression, neurotransmitters, or sympathetic output. Our findings do not permit us to determine a specific mechanism.
Our findings demonstrate that the increased propensity to central apnea in patients with OSA is reversible with CPAP therapy, suggesting that the ventilatory control abnormality is a consequence rather than a cause of OSA. This may be due to repetitive central nervous system insult from recurrent apneas, chronic intermittent hypoxia, and sleep fragmentation. Our findings corroborate previous studies that have shown reversible abnormalities in ventilatory control or brain perfusion in patients with sleep apnea. Using magnetic resonance spectroscopy, Kamba and colleagues revealed metabolic changes in brain tissue of patients with sleep apnea (28). Likewise, studies have demonstrated that treatment with nasal CPAP resulted in changes in the ventilatory response to CO2 and O2 (29, 30) and reversal of impaired load compensation after 2 to 4 weeks of nasal CPAP therapy (31). Finally, in a recent study, Loewen and colleagues found that the dynamic ventilatory responses to combined hypoxia and hypercapnia decrease with 5 months of CPAP use in a group of subjects with severe OSA, indicating that OSA is associated with reversible changes in peripheral chemoresponsiveness (32).
Implications for Sleep Apnea
Our findings are relevant to the pathogenesis of “complex sleep apnea” syndrome. This is a condition characterized by the emergence or persistence of central apnea upon alleviation of upper airway obstruction with nasal CPAP (11, 12). Some authors suggest that cardiac dysfunction and/or dysfunctional ventilatory control are etiologic factors. However, several studies have shown amelioration or resolution of CPAP-related central apnea after 1 to 3 months of CPAP therapy in the majority of patients, suggestive of a reversible ventilatory control abnormality (33–35). Our findings are consistent with the notion that CPAP-emergent central apnea may be caused by a reversible increase in chemoreflex sensitivity to hypocapnia.
Increased susceptibility to central apnea in patients with OSA demonstrates the pathophysiologic link between central and obstructive apnea. Central apnea is associated with pharyngeal narrowing or occlusion, depending on the properties of the upper airway (9, 10). Conversely, recurrent obstructive apnea and associated intermittent hypoxia may increase the susceptibility to develop central apnea and promote further respiratory instability. Nasal CPAP therapy may be beneficial in restoring upper airway patency and in normalizing ventilatory control abnormalities.
In summary, we have shown that patients with OSA demonstrate increased ventilatory response to hypocapnia, below eupneic CO2 levels. Increased susceptibility to the development of hypocapnic central apnea, as measured by the hypocapnic apneic threshold, may contribute to the pathogenesis of OSA.
Supplementary Material
Supported by VA Merit Award, National Institutes of Health K24 and by NIH R01-HL053443.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200810-1658OC on September 17, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hudgel DW, Gordon EA, Thanakitcharu S, Bruce EN. Instability of ventilatory control in patients with obstructive sleep apnea. Am J Respir Crit Care Med 1998;158:1142–1149. [DOI] [PubMed] [Google Scholar]
- 2.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol 2007;103:1929–1941. [DOI] [PubMed] [Google Scholar]
- 3.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2001;163:1181–1190. [DOI] [PubMed] [Google Scholar]
- 4.Onal E, Lopata M. Periodic breathing and the pathogenesis of occlusive sleep apneas. Am Rev Respir Dis 1982;126:676–680. [DOI] [PubMed] [Google Scholar]
- 5.Sin DD, Jones RL, Man GC. Hypercapnic ventilatory response in patients with and without obstructive sleep apnea: do age, gender, obesity, and daytime PaCO2 matter? Chest 2000;117:454–459. [DOI] [PubMed] [Google Scholar]
- 6.Badr MS, Grossman JE, Weber SA. Treatment of refractory sleep apnea with supplemental carbon dioxide. Am J Respir Crit Care Med 1994;150:561–564. [DOI] [PubMed] [Google Scholar]
- 7.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 2004;170:1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudgel DW, Chapman KR, Faulks C, Hendricks C. Changes in inspiratory muscle electrical activity and upper airway resistance during periodic breathing induced by hypoxia during sleep. Am Rev Respir Dis 1987;135:899–906. [DOI] [PubMed] [Google Scholar]
- 9.Badr MS, Kawak A, Skatrud JB, Morrell MJ, Zahn BR, Babcock MA. Effect of induced hypocapnic hypopnea on upper airway patency in humans during NREM sleep. Respir Physiol 1997;110:33–45. [DOI] [PubMed] [Google Scholar]
- 10.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 1995;78:1806–1815. [DOI] [PubMed] [Google Scholar]
- 11.Gilmartin GS, Daly RW, Thomas RJ. Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med 2005;11:485–493. [DOI] [PubMed] [Google Scholar]
- 12.Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 2006;29:1203–1209. [DOI] [PubMed] [Google Scholar]
- 13.Thomas RJ. Effect of added dead space to positive airway pressure for treatment of complex sleep-disordered breathing. Sleep Med 2005;6:177–178. [DOI] [PubMed] [Google Scholar]
- 14.Salloum A, Mateika JH, Rowley JA, Badr MS. The effect of 6 weeks of CPAP treatment on hypocapnic apneic threshold in OSA patients [abstract]. Am J Respir Crit Care Med 2007;175:A70. [Google Scholar]
- 15.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. 2007. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine; 2007.
- 16.Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol 2003;94:101–107. [DOI] [PubMed] [Google Scholar]
- 17.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep 2006;29:95–103. [DOI] [PubMed] [Google Scholar]
- 18.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol 2005;90:13–24. [DOI] [PubMed] [Google Scholar]
- 19.Dempsey JA, Smith CA, Przybylowski T, Chenuel B, Xie A, Nakayama H, Skatrud JB. The ventilatory responsiveness to CO(2) below eupnoea as a determinant of ventilatory stability in sleep. J Physiol 2004;560:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asyali MH, Berry RB, Khoo MC. Assessment of closed-loop ventilatory stability in obstructive sleep apnea. IEEE Trans Biomed Eng 2002;49:206–216. [DOI] [PubMed] [Google Scholar]
- 21.Spengler CM, Shea SA. Sleep deprivation per se does not decrease the hypercapnic ventilatory response in humans. Am J Respir Crit Care Med 2000;161:1124–1128. [DOI] [PubMed] [Google Scholar]
- 22.Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med 2002;165:1245–1250. [DOI] [PubMed] [Google Scholar]
- 23.Xie A, Skatrud JB, Barczi SR, Reichmuth K, Morgan BJ, Mont S, Dempsey JA. Influence of cerebral blood flow on breathing stability. J Appl Physiol 2009;106:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mateika JH, Ellythy M. Chemoreflex control of ventilation is altered during wakefulness in humans with OSA. Respir Physiol Neurobiol 2003;138:45–57. [DOI] [PubMed] [Google Scholar]
- 25.Narkiewicz K, Kato M, Pesek CA, Somers VK. Human obesity is characterized by a selective potentiation of central chemoreflex sensitivity. Hypertension 1999;33:1153–1158. [DOI] [PubMed] [Google Scholar]
- 26.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 1999;99:1183–1189. [DOI] [PubMed] [Google Scholar]
- 27.Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol 2004;96:1236–1242. [DOI] [PubMed] [Google Scholar]
- 28.Kamba M, Inoue Y, Higami S, Suto Y, Ogawa T, Chen W. Cerebral metabolic impairment in patients with obstructive sleep apnoea: an independent association of obstructive sleep apnoea with white matter change. J Neurol Neurosurg Psychiatry 2001;71:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbraecken, J., M. Willemen, W. De Cock, W. Wittesaele, K. Govaert, van de Heyning P, De Backer W. Influence of longterm CPAP therapy on CO(2) drive in patients with obstructive sleep apnea. Respir Physiol 2000;123:121–130. [DOI] [PubMed] [Google Scholar]
- 30.Tun Y, Hida W, Okabe S, Kikuchi Y, Kurosawa H, Tabata M, Shirato K. Effects of nasal continuous positive airway pressure on awake ventilatory responses to hypoxia and hypercapnia in patients with obstructive sleep apnea. Tohoku J Exp Med 2000;190:157–168. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg HE, Scharf SM. Depressed ventilatory load compensation in sleep apnea: reversal by nasal CPAP. Am Rev Respir Dis 1993;148:1610–1615. [DOI] [PubMed] [Google Scholar]
- 32.Loewen A, Ostrowski M, Laprairie J, Atkar R, Gnitecki J, Hanly P, Younes M. Determinants of ventilatory instability in obstructive sleep apnea (OSA): inherent or acquired? Sleep 2009;32:1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med 2009;5:205–211. [PMC free article] [PubMed] [Google Scholar]
- 34.Kuzniar TJ, Morgenthaler TI. Treatment of complex sleep apnea syndrome. Curr Treat Options Neurol 2008;10:336–341. [DOI] [PubMed] [Google Scholar]
- 35.Dernaika T, Tawk M, Nazir S, Younis W, Kinasewitz GT. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night sleep studies. Chest 2007;132:81–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.