Abstract
Background
Data from retrospective studies have suggested that there may be an interaction between fluconazole and nevirapine, increasing nevirapine concentrations and potentially leading to hepatotoxicity.
Methods
This study was nested within a large double-blind placebo-controlled study designed to determine if primary prophylaxis with fluconazole (200 mg three times per week) could reduce cryptococcal disease [CRYPTOPRO (ISRCTN 76481529)] in HIV-infected adults in rural south-western Uganda. Detailed pharmacokinetic studies were performed on 49 participants (22 on placebo and 27 on fluconazole) who had been on fluconazole or placebo with nevirapine for ≥4 weeks.
Results
The geometric mean pre-dose concentrations of nevirapine were 3865 ng/mL [95% confidence interval (95% CI) 3452–4758 ng/mL] and 5141 ng/mL (95% CI 4760–6595 ng/mL) (P = 0.009) in the placebo and fluconazole arms, respectively. The change in the peak nevirapine concentration in plasma (Cmax) was also higher in the fluconazole arm compared with the placebo arm [median 6546 (95% CI 6040–7974) versus 5126 (95% CI 4739–5773) ng/mL, P = 0.012]. Fluconazole increased the nevirapine area under the curve (AUC) from 0 to 8 h by 29% [geometric mean AUC0–8 46 135 (95% CI 42 432–57 173) versus 35 871 (95% CI 32 808–41 372) ng·h/mL, P = 0.016]. In the larger cohort from which the participants were drawn, co-administration of fluconazole did not increase the risk of hepatotoxicity.
Conclusions
Fluconazole led to significant increases in nevirapine exposure, but was not associated with evidence of increased hepatotoxicity.
Keywords: Uganda, pharmacokinetics, drug interactions, antiretroviral therapy
Introduction
Fungal infections are a common cause of morbidity and mortality in HIV-infected individuals in sub-Saharan Africa. Fluconazole, an azole derivative, is effective against a range of fungal infections in this setting. It has recently been shown to prevent cryptococcal disease and mucosal Candida infections in severely immunosuppressed Ugandan adults.1 Fluconazole is a potent inhibitor of cytochrome P450 isoenzymes and increases the plasma concentrations of a number of co-administered drugs, potentially causing toxicity.2
Nevirapine, a non-nucleoside reverse transcriptase inhibitor, is a common component of antiretroviral therapy regimens in sub-Saharan African countries, including Uganda. Nevirapine undergoes extensive hepatic metabolism into inactive compounds via P450 isoenzymes CYP3A4 and CYP2B6.3 Nevirapine is associated with severe hepatotoxic reactions and, in some cases, hepatic failure and death.4 This usually occurs within the first 6 months after initiation and is associated with higher CD4 counts, particularly in women. Routine monitoring of liver function is not recommended in a sub-Saharan African setting.5
Data from retrospective studies have suggested that there may be an interaction between fluconazole and nevirapine, increasing nevirapine concentrations and potentially leading to an increase in severe hepatotoxicity.6 This could be particularly important in sub-Saharan Africa, where access to laboratory testing and monitoring of drug toxicity is very limited.
There are currently limited data on the interaction of fluconazole and nevirapine, and no formal controlled pharmacokinetic study has ever been performed in HIV-infected individuals. We examined the interaction between nevirapine and fluconazole or placebo in 49 HIV-infected Ugandans and relate this to clinical observations in the cohort from which these patients were drawn.
Methods
Participants
This study was nested within a large double-blind placebo-controlled study designed to determine if primary prophylaxis with fluconazole (200 mg three times per week) reduced cryptococcal disease [CRYPTOPRO (ISRCTN 76481529)] in rural south-western Uganda.1 Study participants were antiretroviral-naive adults, with laboratory confirmation of HIV infection, a CD4 count of <200 cells/mm3 and a negative cryptococcal antigen test. Exclusion criteria were pregnancy or lactation and aspartate transaminases or alanine transaminases more than three times the upper limit of normal (ULN). Other liver enzymes were not assayed. Testing for hepatitis B and hepatitis C viruses was not carried out unless clinically indicated. Liver function tests were performed on all participants every 8 weeks.
Ethics
Information sheets were translated into the local language and written consent was obtained by trained medical personal. This study received ethical approval from the institutional review boards of the Uganda Virus Research Institute and Uganda National Council for Science and Technology (G/C 127).
Study design
Participants were selected from 1063 individuals taking nevirapine in CRYPTOPRO by an independent monitor unblinded to trial drug allocation. All participants had been stable on an antiretroviral regimen containing 200 mg of nevirapine taken twice per day with fluconazole or placebo for ≥4 weeks without evidence of hepatotoxicity. Power calculations were based on data from a previous study and the US Food and Drug Administration and the European Agency for the Evaluation of Medicinal Products cut-offs for bioequivalence (80%–125%). Assuming a median area under the curve from 0 to 12 h (AUC0–12) of 73 341 ng·h/mL (±14 818), a sample size of 22 in each group (total sample size 44) was required to detect a difference of 25% in nevirapine concentrations with 80% power and 95% confidence.7
Pharmacokinetic studies
Pharmacokinetic studies were performed on 49 participants: 22 (8 female) on placebo and 27 (12 female) on fluconazole. Heparinized blood (2 mL) was collected via a small-bore canula pre-nevirapine dosing and at 2, 4 and 8 h after taking antiretroviral therapy. The trial drug (fluconazole or placebo) was taken at the same time as antiretroviral therapy. Plasma was separated for measurement of nevirapine concentrations and frozen for transport to Liverpool, UK.
Nevirapine concentrations were assayed using validated liquid chromatography–tandem mass spectrometry (Waters Quattro Premier, Manchester, UK) with a lower limit of quantification of 450 ng/mL and inter- and intra-assay variabilities [coefficient of variation (%); using low, medium and high quality controls] of ∼11% and ∼10%, respectively.7 The laboratory participates in an external quality assurance programme (KKGT, The Netherlands).
Data analysis
Data were entered into a Microsoft® Access database. The primary outcome was a change in nevirapine AUC from 0 to 8 h (AUC0–8) and the secondary outcomes were the nevirapine pre-dose concentration, the change in peak concentration in plasma (Cmax), the concentration at 8 h post-dosing (C8) and ‘clearance’ (estimated as dose over 12 h/AUC0–8). All concentration data were log-transformed to improve approximation to normality. Non-compartmental analysis was applied to all the data (WinNonlin v. 5.2) and differences were tested using unpaired t-tests (Stats Direct).
Results
Participants
The median age was 37 years [interquartile range (IQR) 32–42 years], 20/49 (41%) were female and the median CD4 count was 52 cells/mm3 (IQR 22–104 cells/mm3). Patients had been taking fluconazole (27/49) or placebo (22/49) and a nevirapine-containing antiretroviral regimen for a median of 55 weeks (IQR 48–68 weeks). All were taking two nucleoside reverse transcriptase inhibitors [10 were on Combivir (lamivudine and zidovudine), 36 were on stavudine and lamivudine and 3 were on Truvada (tenofovir and emtricitabine)].
Pharmacokinetics
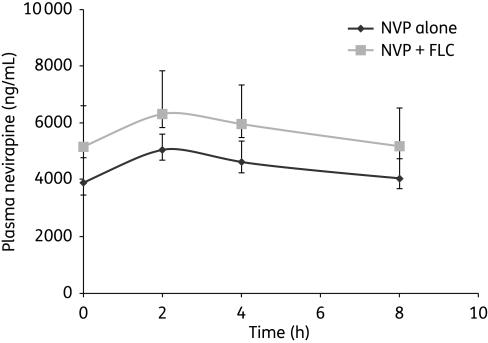
The geometric mean pre-dose concentrations of nevirapine were 3865 ng/mL [95% confidence interval (95% CI) 3452–4758 ng/mL] and 5141 ng/mL (95% CI 4760–6595 ng/mL) (P = 0.009) in the placebo and fluconazole arms, respectively. Cmax was also higher in the fluconazole arm compared with the placebo arm [median 6546 (95% CI 6040–7974) versus 5126 (95% CI 4739–5773) ng/mL, P = 0.012]. Fluconazole increased the nevirapine AUC from 0 to 8 h by 29% [geometric mean AUC0–8 46 135 (95% CI 42 432–57 173) versus 35 871 (95% CI 32 808–41 372) ng·h/mL, P = 0.016] (Figure 1) and the concentration at 8 h by 28% from 4023 to 5168 ng/mL (P = 0.02). Fluconazole also reduced the nevirapine ‘clearance’ from 5.58 (95% CI 5.15–6.34) to 4.34 (95% CI 3.90–5.54) L/h (P = 0.0172). Gender had no effect upon the nevirapine AUC0–8.
Figure 1.
Pharmacokinetic curve of nevirapine (NVP) with and without fluconazole (FLC). Bars represent 95% confidence intervals.
Hepatotoxicity and rash
In the larger cohort from which the participants in this pharmacokinetic study were drawn, co-administration of fluconazole did not increase the risk of hepatotoxicity. One thousand and sixty-three (81.9%) received an antiretroviral therapy regimen that contained nevirapine. In those on a nevirapine-containing antiretroviral regimen, 27/522 (5.2%) and 34/541 (6.3%) taking fluconazole and placebo, respectively, stopped the trial drug due to elevated transaminases (>5× ULN). None of the 49 participants had elevated transaminases (>5× ULN) in the first 6–12 weeks of taking nevirapine and the trial drug (fluconazole or placebo). In the main study, 14/1063 (1.3%) had liver function tests >5× ULN in the first 6–12 weeks of nevirapine and the trial drug. Of the 14, 5 were taking fluconazole and 9 were on placebo (Fisher's exact test, P = 0.42). In trial participants taking nevirapine, 11/522 (2.1%) on fluconazole and 20/541 (3.7%) on placebo had a rash. There was only one case of Stevens–Johnson syndrome in a patient on fluconazole.
Discussion
Fluconazole is increasingly used as a treatment for cryptococcal disease or candidiasis in sub-Saharan Africa and recent findings of its efficacy as prophylaxis against cryptococcal disease may lead to more widespread use. In this setting, when laboratory monitoring is limited, establishing its safety is crucial, particularly when toxicity may be potentially higher due to the high burden and often additive effects of infection, malignancy or malnutrition.
There are very limited clinical data on the pharmacokinetic interaction of azoles and nevirapine. A healthy volunteer study suggested that co-administration of 200 mg of itraconazole daily and 200 mg of nevirapine daily did not increase nevirapine concentrations.8 In contrast, a retrospective Thai study suggested that co-administration of fluconazole (200 or 400 mg daily) increased mean trough nevirapine concentrations from 6.5 ± 3.0 mg/L in those not taking fluconazole to 9.8 ± 4.3 and 12.2 ± 6.8 mg/L in those taking it (200 and 400 mg, respectively).9 In our prospective study with controls, we have demonstrated an almost 30% increase in total exposure (AUC) to nevirapine when taking fluconazole.
Hepatic toxicity is a serious and potentially life-threatening complication among HIV-infected individuals who receive nevirapine as part of their antiretroviral regimen. Asymptomatic hepatitis occurs in 8%–28% of individuals and clinical hepatitis occurs in ∼1%–5%.4,10 The rate of severe biochemical hepatotoxicity (defined as transaminases >5× ULN) in our main cohort was 5.2% and 6.3% in the fluconazole and placebo arms, respectively. Despite the increased exposure of nevirapine in patients receiving fluconazole, we could find no evidence of a concentration–toxicity relationship. This may be partially explained by data suggesting that dose-dependent hepatotoxicity does not occur until nevirapine concentrations are >6000–8000 ng/mL.11 In this study, the median Cmax for those taking fluconazole and nevirapine was 6546 ng/mL. The lack of increased risk of hepatotoxicity with co-administration is also in keeping with Thai data that did not detect increased toxicity, despite increases in trough nevirapine concentrations.9
There are caveats to the interpretation of this study. The dose of fluconazole used for prophylaxis (200 mg three times per week) was relatively low and there is a potential for higher treatment doses of 400–800 mg per day to cause even greater elevations of nevirapine concentrations. The absence of increased rates of hepatotoxicity in the main study overall does need to be interpreted in the context that study participants were at low risk for nevirapine hepatotoxicity with normal transaminases at baseline, had relatively low CD4 cell counts, had been on nevirapine for a median of 55 weeks and the hepatitis B and C status of participants was uncertain.4 Nevertheless, this study clearly demonstrates that despite significant increases in exposure when nevirapine was co-administered with 200 mg of fluconazole three times a week, this was not associated with evidence of hepatotoxicity and gives reassurance for the use of these two agents together in settings when monitoring is not available.
Funding
This work was funded by the Medical Research Council, UK (grant number G0100035). Further project support came from the National Institute of Health Research (NIHR–Department of Health), UK and the Northwest Development Agency (NWDA), UK. The funding sources had no involvement in the design, data collection, analysis, writing or decision to submit for publication.
Transparency declarations
None to declare.
Author contributions
K. W. drafted this article. K. W., R. P.-R., V. W., A.-B. G., S. K. and D. G. L. participated in the study conception, design and data collection. V. W. and S. K. participated in the data analysis. R. P.-R., V. W., A.-B. G., S. K. and D. G. L. participated in reviewing and critical revision of the manuscript. All authors saw and approved the final version of the manuscript.
Acknowledgements
We would like to thank the trial participants, staff of The AIDS Support Organisation (TASO) Masaka, Kitovu Mobile AIDS Organisation (especially Dr Carla Symmons), Uganda Cares Masaka and Ministry of Health teams. We are grateful to Professor Heiner Grosskurth for critical review and revision of the manuscript, to Dr Jonathan Levin for advice on statistical analysis and Christine Watera, Sebastian Owilla, Jessica Nakiyingi-Miiro and Jim Todd for help with randomization.
References
- 1.Parkes-Ratanshi R, Kamali A, Wakeham K, et al. Successful primary prevention of cryptococcal disease using fluconazole prophylaxis in HIV-infected Ugandan adults. Abstracts of the Sixteenth Conference on Retroviruses and Opportunistic Infections; 2009; Montreal. Abstract P-32. Foundation for Retrovirology and Human Health, Alexandria, VA, USA. [Google Scholar]
- 2.Bruggemann RJ, Alffenaar JW, Blijlevens NM, et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48:1441–58. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 3.Wen B, Chen Y, Fitch WL. Metabolic activation of nevirapine in human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab Dispos. 2009;37:1557–62. doi: 10.1124/dmd.108.024851. [DOI] [PubMed] [Google Scholar]
- 4.Manosuthi W, Sungkanuparph S, Tansuphaswadikul S, et al. Incidence and risk factors of nevirapine-associated severe hepatitis among HIV-infected patients with CD4 cell counts less than 250 cells/microL. J Med Assoc Thai. 2008;91:159–65. [PubMed] [Google Scholar]
- 5.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach, 2006 Revision. http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. (18 November 2009, date last accessed) [PubMed] [Google Scholar]
- 6.Geel J, Pitt J, Orrell CJ, et al. The effect of fluconazole on nevirapine. Abstracts of the Fifteenth International Conference on AIDS; 2004; Bangkok, Thailand. Abstract WeOrB1239. [Google Scholar]
- 7.Kikaire B, Khoo S, Walker AS, et al. Nevirapine clearance from plasma in African adults stopping therapy: a pharmacokinetic substudy. AIDS. 2007;21:733–7. doi: 10.1097/QAD.0b013e3280121801. [DOI] [PubMed] [Google Scholar]
- 8.Jaruratanasirikul S, Sriwiriyajan S. Pharmacokinetic study of the interaction between itraconazole and nevirapine. Eur J Clin Pharmacol. 2007;63:451–6. doi: 10.1007/s00228-007-0280-x. [DOI] [PubMed] [Google Scholar]
- 9.Manosuthi W, Athichathanabadi C, Uttayamakul S, et al. Plasma nevirapine levels, adverse events and efficacy of antiretroviral therapy among HIV-infected patients concurrently receiving nevirapine-based antiretroviral therapy and fluconazole. BMC Infect Dis. 2007;7:14. doi: 10.1186/1471-2334-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phanuphak N, Apornpong T, Teeratakulpisarn S, et al. Nevirapine-associated toxicity in HIV-infected Thai men and women, including pregnant women. HIV Med. 2007;8:357–66. doi: 10.1111/j.1468-1293.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 11.Almond LM, Boffito M, Hoggard PG, et al. The relationship between nevirapine plasma concentrations and abnormal liver function tests. AIDS Res Hum Retroviruses. 2004;20:716–22. doi: 10.1089/0889222041524670. [DOI] [PubMed] [Google Scholar]