Abstract
OBJECTIVE
Progressive β-cell loss causes catabolism in cystic fibrosis. Existing diagnostic criteria for diabetes were based on microvascular complications rather than on cystic fibrosis–specific outcomes. We aimed to relate glycemic status in cystic fibrosis to weight and lung function changes.
RESEARCH DESIGN AND METHODS
We determined peak blood glucose (BGmax) during oral glucose tolerance tests (OGTTs) with samples every 30 min for 33 consecutive children (aged 10.2–18 years). Twenty-five also agreed to undergo continuous glucose monitoring (CGM) (Medtronic). Outcome measures were change in weight standard deviation score (wtSDS), percent forced expiratory volume in 1 s (%FEV1), and percent forced vital capacity (%FVC) in the year preceding the OGTT.
RESULTS
Declining wtSDS and %FVC were associated with higher BGmax (both P = 0.02) and with CGM time >7.8 mmol/l (P = 0.006 and P = 0.02, respectively) but not with BG120 min. A decline in %FEV1 was related to CGM time >7.8 mmol/l (P = 0.02). Using receiver operating characteristic (ROC) analysis to determine optimal glycemic cutoffs, CGM time above 7.8 mmol/l ≥4.5% detected declining wtSDS with 89% sensitivity and 86% specificity (area under the ROC curve 0.89, P = 0.003). BGmax ≥8.2 mmol/l gave 87% sensitivity and 70% specificity (0.76, P = 0.02). BG120 min did not detect declining wtSDS (0.59, P = 0.41). After exclusion of two patients with BG120 min ≥11.1 mmol/l, the decline in wtSDS was worse if BGmax was ≥8.2 mmol/l (−0.3 ± 0.4 vs. 0.0 ± 0.4 for BGmax <8.2 mmol/l, P = 0.04) or if CGM time above 7.8 mmol/l was ≥4.5% (−0.3 ± 0.4 vs. 0.1 ± 0.2 for time <4.5%, P = 0.01).
CONCLUSIONS
BGmax ≥8.2 mmol/l on an OGTT and CGM time above 7.8 mmol/l ≥4.5% are associated with declining wtSDS and lung function in the preceding 12 months.
Progressive β-cell loss causes catabolism and weight loss in cystic fibrosis (1,2). Weight is a prognostic indicator (3), and prevention of weight decline is a major clinical objective in children and adolescents with cystic fibrosis. Median life expectancy of patients with cystic fibrosis has risen progressively over recent decades but remains drastically shorter (∼36 years) than that of the general population (4). The presence of cystic fibrosis–related diabetes (CFRD) is associated with an increase in early mortality of up to sixfold (5). CFRD is usually diagnosed by the North American Cystic Fibrosis Foundation criteria (6) or World Health Organization (WHO) criteria for diabetes (7). These criteria were designed to identify patients at risk of microvascular complications in type 2 diabetes (8) and were not designed with cystic fibrosis–specific outcomes in mind. Microvascular complications occur in cystic fibrosis (9); however, catabolic decline in weight and deteriorating lung function may be more relevant outcomes. Poor weight gain is associated with worsening lung function (10,11), and both are associated with early mortality (12,13). Weight and lung function declines have been shown to precede the diagnosis of CFRD by standard criteria (2), but the earliest glycemic abnormality associated with clinical decline has not been determined. Glycemic status can be assessed in detail using an oral glucose tolerance test (OGTT) with 30-min samples and, more recently, continuous interstitial fluid glucose monitoring (CGM). We aimed to determine the relationship between glycemic status and the change in weight standard deviation score (wtSDS) and the change in lung function over the preceding year.
RESEARCH DESIGN AND METHODS
In a prospective protocol, 33 consecutive children with cystic fibrosis (median age 13.1 years, range 10.2–18 years) underwent an OGTT when clinically stable with respect to lung disease, as part of an annual screening program for all patients with cystic fibrosis aged ≥10 years. All were under the care of a pediatric respiratory physician at the Sydney Children's Hospital cystic fibrosis clinic. For the OGTT, patients fasted for at least 8 h and then consumed 1.75 g/kg of carbonated dextrose solution (maximum 75 g). No patient refused the OGTT. Venous or fingerprick samples were collected at 0, 30, 60, 90, and 120 min for measurement of glucose and insulin. Glucose levels were determined by the hospital laboratory, using a standard glucose oxidase method (Beckman Coulter, Fullerton, CA). Insulin levels were determined with a standard chemiluminescence immunoassay (Immulite, limit of detection 2 mU/l; Siemens Healthcare Diagnostics, Deerfield, IL). β-Cell function and insulin sensitivity were estimated using homeostasis model assessment (HOMA2) (14).
Twenty-five patients (76%) also agreed to CGM (Medtronic). Patients refusing CGM were not different from those undergoing CGM according to the clinical characteristics listed in Table 1. Local anesthetic cream and play therapy were used to minimize the distress of intravenous cannulation and insertion of the CGM device. Mean ± SD duration of CGM was 60.2 ± 14.6 h. Patients entered capillary blood glucose values into the CGM device at 60 min after CGM insertion and subsequently before breakfast and dinner each day. These premeal calibration times were selected to avoid moments of rapid changes in interstitial and blood glucose levels (J. Mastrototaro, Medtronic, personal communication). Percentage of time >7.8 mmol/l and area >7.8 mmol/l (millimoles per liter per day) were determined using MiniMed Solutions software (MMT-7310 version 3.0C [3.0.128]; Medtronic). The 7.8 mmol/l threshold corresponds to the WHO cutoff for impaired glucose tolerance (IGT) at 120 min on an OGTT.
Table 1.
Characteristics of patients with cystic fibrosis at the time of OGTT and change over the preceding 12 months in weight, height, and lung function
| Age (years) | 13.7 ± 2.8 |
| Sex (male/female) | 16 (49)/17 (51) |
| Exocrine enzyme replacement | 30 (91) |
| Genotype | |
| Homozygous F508del | 20 (61) |
| Heterozygous F508del | 8 (24) |
| Other | 3 (9) |
| Unknown | 2 (6) |
| Age of diagnosis of cystic fibrosis (years) | 0.47 ± 2.0 |
| wtSDS | −0.78 ± 1.1 |
| Height SDS | −0.41 ± 0.92 |
| Change in wtSDS* | −0.16 ± 0.38 |
| Change in height SDS* | 0.03 ± 0.24 |
| %FEV1 | 85 ± 20 |
| %FVC | 94 ± 17 |
| Change in %FEV1* | −5 ± 8 |
| Change in %FVC* | −3 ± 8 |
Data are n (%) or means ± 1 SD. n = 33.
*Over the preceding year.
Patients were instructed to continue their high-energy, high-fat, cystic fibrosis–specific diet and routine activities. No patients were treated with glucocorticoids at the time of the OGTT or during CGM.
Height, weight, and lung function were measured by respiratory laboratory staff at each visit, using a stadiometer, electronic weight scales, and a SensorMedics Vmax system (Cardinal Health, Dublin, OH). Percent predicted forced expiratory volume in 1 s (%FEV1) and percent predicted forced vital capacity (%FVC) were calculated using the method reported by Knudson et al. (15).
wtSDS was calculated using Centers for Disease Control and Prevention 2000 growth data with Growth Analyser Software (version 3.5, www.growthanalyser.org). For each patient, the rate of change in %FEV1, %FVC, and wtSDS over the 12 months before the OGTT was determined from the slope obtained by linear regression analysis. The number of data points during the 12-month period was 10 ± 8.
The study was approved by the local ethics committee. Statistical analysis was performed with SPSS software (version 17).
RESULTS
According to the blood glucose values at 0 and 120 min (BG120 min) on the OGTT, 18 patients (55%) had normal glucose tolerance (NGT) by WHO criteria, 13 (39%) had IGT (BG120 min 7.8–11 mmol/l), and 2 (6%) were diabetic (CFRD) (BG120 min ≥11.1 mmol/l). None had fasting hyperglycemia (≥7 mmol/l).
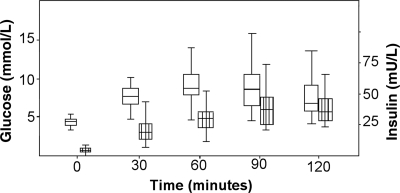
Figure 1 shows the time course of blood glucose and insulin levels during the OGTT. The peak blood glucose (BGmax) occurred at 30 min in 6 patients (18%), at 60 min in 15 patients (45%), at 90 min in 11 patients (33%), and at 120 min in only 1 patient (3%). In contrast with peak blood glucose, peak insulin was delayed. The time of peak insulin occurred at 120 min in 14 of 27 (42%), at 90 min in 8 of 27 (24%), at 60 min in 5 of 27 (15%), and at 0 or 30 min in no patients (insulin values were incomplete or not performed in the remaining 6 of 33 patients). Time to insulin peak was 58 ± 19 min for those with NGT vs. 75 ± 16 min for those with IGT (P = 0.03). Median fasting insulin was only 4 mU/l (range 0–21 mU/l). Using HOMA2, mean β-cell function for the whole group was only 29.5% (95% CI 24.6–34.5), and median insulin sensitivity was 90.1% (interquartile range 30–417%).
Figure 1.
Glucose and insulin levels during the OGTT. Boxes show the median and interquartile range. Whiskers show the 5th and 95th percentiles.
Table 1 outlines the clinical characteristics of the 33 patients at the time of the OGTT. Mean wtSDS for the 33 patients was −0.78 (95% CI −1.2 to −0.4). A decline in wtSDS over the preceding year occurred in 23 of 33 patients (70%).
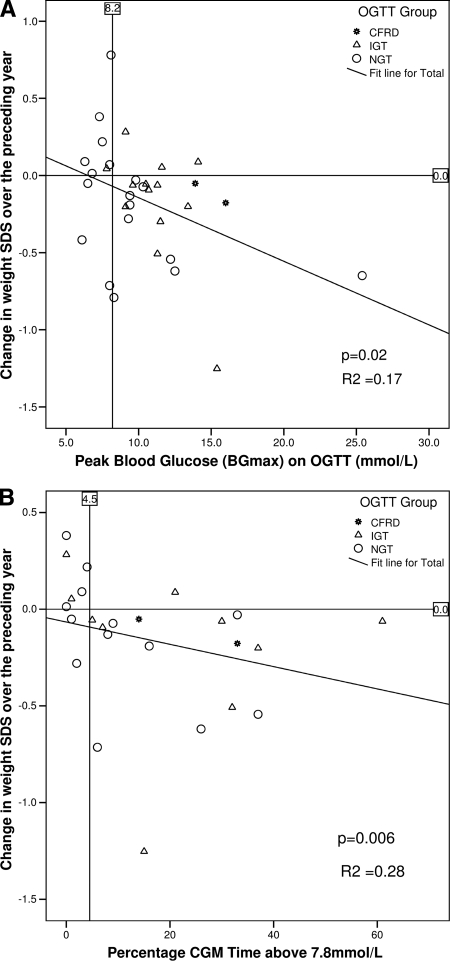
A decline in wtSDS over the preceding year was associated with higher BGmax (R2 = 0.17, P = 0.02) (Fig. 2) and longer percentage of CGM time above 7.8 mmol/l (R2 = 0.28, P = 0.006) (Fig. 2), but not with BG120 min (R2 = 0.000025, P = 0.98). A decline in %FEV1 was associated with a longer percentage of CGM time above 7.8 mmol/l (R2 = 0.16, P = 0.05), but not with BGmax (R2 = 0.12, P = 0.06) or BG120 min (R2 = 0.0036, P = 0.75). A decline in %FVC was associated with higher BGmax (R2 = 0.16, P = 0.02) and percentage of CGM time above 7.8 mmol/l (R2 = 0.20, P = 0.02) but not with BG120 min (R2 = 0.032, P = 0.30).
Figure 2.
Decline in wtSDS over the preceding year by BGmax on an OGTT (A) and by percentage of CGM time above 7.8 mmol/l (B). The vertical lines at 8.2 mmol/l and 4.5% CGM time above 7.8 mmol/l represent optimal cutoffs determined by the ROC analysis in Fig. 3.
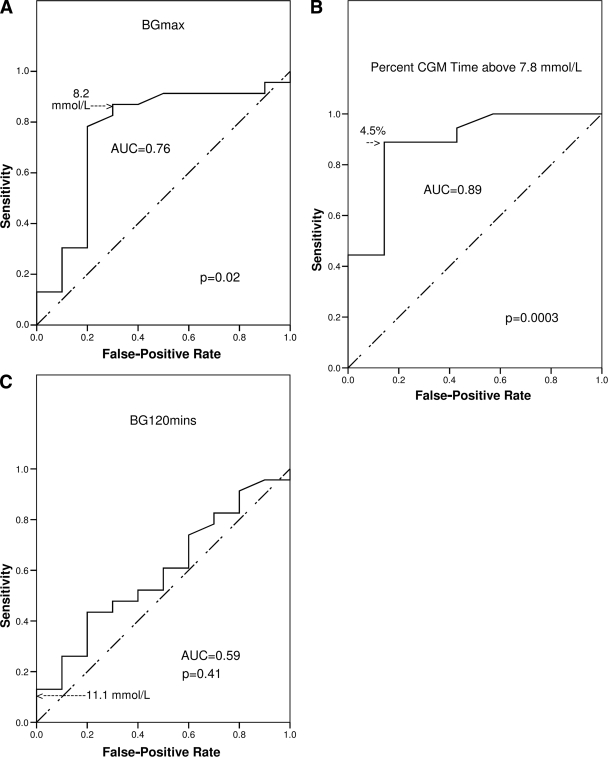
We used receiver operating characteristic (ROC) analysis (16) to determine optimal glycemic cutoffs for detecting cystic fibrosis–specific outcomes. Figure 3 shows the results of the ROC analyses for declining wtSDS:
CGM time above 7.8 mmol/l ≥4.5% detected declining wtSDS with 89% sensitivity and 86% specificity (area under the ROC curve [AUC] 0.89, P = 0.003).
CGM time above 7.8 mmol/l was ≥4.5% in 17 of 25 patients (68%).
BGmax ≥8.2 mmol/l gave 87% sensitivity and 70% specificity (AUC 0.76, P = 0.02).
BGmax was ≥8.2 mmol/l in 23 of 33 patients (69.7%).
In contrast with BGmax, BG120 min did not detect declining wtSDS (AUC 0.59 [95% CI 0.38–0.80] P = 0.41) and BG120 min ≥11.1 mmol/l had only 10% sensitivity.
Figure 3.
Determination of optimal glycemic cut points for detecting decline in wtSDS over the preceding 12 months by ROC analysis. A: Plot of sensitivity vs. false-positive rate (1 − specificity) for all possible cut points in BGmax by OGTT. The point closest to the top left-hand corner (8.2 mmol/l) maximizes sensitivity and specificity and is the optimal cut point. B: Percentage of CGM time above 7.8 mmol/l with the optimal cut point of 4.5%. C: Same data for BG120 min on OGTT, which did not detect declining wtSDS. The cut point of 11.1 mmol/l used in the WHO diagnostic criteria is marked.
Percentage of CGM time above 7.8 mmol/l was not significantly better at detecting declining wtSDS than BGmax because the 95% CIs around the AUCs in the ROC analyses overlapped (0.5–1 and 0.56–0.96, respectively). A longer percentage of CGM time above 7.8 mmol/l correlated with higher BGmax on the OGTT (P = 0.006 and R2 = 0.28).
By using ROC analysis to examine lung function, CGM time above 7.8 mmol/l ≥4.5% detected a fall in %FVC of ≥3% over the preceding 12 months (a clinically significant decline) (17) with 79% sensitivity and 46% specificity (AUC 0.79, P = 0.01), but BGmax did not (AUC 0.69, P = 0.07). Neither percentage of CGM time above 7.8 mmol/l nor OGTT BGmax detected a fall in %FEV1 over the preceding year of ≥3% (respectively, AUC 0.65, P = 0.20 and AUC 0.58, P = 0.44). BG120 min was unable to detect a fall ≥3% in either %FVC (AUC 0.69, P = 0.06) or %FEV1 (AUC 0.55, P = 0.64). ROC analyses for declining weight and lung function using percentage of CGM time above 8.2 mmol/l and CGM area above 8.2 mmol/l and CGM area above 7.8 mmol/l did not improve the ROC AUC (data not shown).
Fasting insulin (but not β-cell function or insulin sensitivity as measured by HOMA2) was higher in patients with BGmax<8.2 mmol/l (6.5 mU/l, range 3–9 mU/l) than in those with BGmax ≥8.2 mmol/l (3 mU/l, range 0–21 mU/l, P = 0.02). WHO groups of NGT or IGT had indistinguishable fasting insulin, β-cell function, and insulin sensitivity (data not shown).
After the two patients with CFRD by WHO criteria were excluded (because they had BG120 min ≥11.1 mmol/l and would currently be eligible for insulin treatment), we compared groups defined with cut points from the ROC analysis (Table 2). The decline in wtSDS was significantly worse in patients with BGmax ≥8.2 mmol/l (−0.3 ± 0.4) than in those with BGmax <8.2 mmol/l (0.0 ± 0.4, P = 0.04). The decline in wtSDS was also worse in those with CGM time above 7.8 mmol/l ≥4.5% (−0.3 ± 0.4 vs. 0.1 ± 0.2 in those <4.5%, P = 0.01). There was, however, no significant difference in wtSDS comparing NGT (BG120 min <7.8 mmol/l) and IGT (BG120 min 7.8–11 mmol/l) groups defined by WHO criteria (−0.2 ± −0.4 vs. −0.2 ± 0.4, P = 0.94).
Table 2.
Change in wtSDS and lung function over the preceding year by glycemic groups
| Change in* | Cystic fibrosis–specific OGTT cutoff |
WHO categories based on BG120 |
Cystic fibrosis–specific CGM-based cutoff |
||||||
|---|---|---|---|---|---|---|---|---|---|
| BGmax ≥8.2 mmol/l | BGmax <8.2 mmol/l | P | IGT: 7.8–11 mmol/l | NGT: <7.8 mmol/l | P | CGM >7.8 mmol/l ≥4.5% | CGM >7.8 mmol/l <4.5% | P | |
| n (%) | 21/31 (68)† | 10/31 (32) | 13/31 (42)† | 18/31 (58) | 15/23 (65)† | 8/23 (35) | |||
| wtSDS | −0.3 ± 0.4 | 0.0 ± 0.4 | 0.04 | −0.2 ± 0.4 | −0.2 ± 0.4 | 0.94 | −0.3 ± 0.4 | 0.1 ± 0.2 | 0.01 |
| %FEV1 | −6 ± 7 | −4 ± 11 | 0.48 | −6 ± 7 | −5 ± 9 | 0.83 | −6 ± 7 | −2 ± 11 | 0.31 |
| %FVC | −4 ± 7 | 0.5 ± 10 | 0.21 | −5 ± 6 | −1 ± 9 | 0.21 | −4 ± 7 | 1 ± 10 | 0.13 |
Data are means ± SD unless otherwise indicated. Change in weight and lung function in the preceding 12 months in 31 patients with cystic fibrosis, excluding the 2 patients with BG120 min ≥11.1 mmol/l (diabetic by WHO criteria) are shown. Groups are compared according to three different glycemic cutoffs.
*Over the preceding year.
†Excluding 2 patients (BG120 min >11.1 mmol/l).
Groups defined by glycemic thresholds (OGTT BGmax ≥8.2 or <8.2 mmol/l, CGM time above 7.8 mmol/l ≥4.5 or <4.5%) and markers of catabolic decline (change in wtSDS ≥0 or <0 or change in %FEV1 or %FVC ≥3 or <3) were indistinguishable by sex, genotype category (F508del homozygous, F508del heterozygous, other, or unknown), or the presence or absence of exocrine pancreatic sufficiency (data not shown).
CONCLUSIONS
We found that glucose abnormalities are related to the cystic fibrosis–specific outcomes of decline in lung function and weight over the preceding year. Current WHO glycemic categories of NGT and IGT (based on BG120 min during OGTT) could not distinguish patients with declining wtSDS from those with stable or increasing scores. In contrast, we found that peak blood glucose ≥8.2 mmol/l on 30-min sampling in the OGTT and CGM time above 7.8 mmol/l ≥4.5% could do so reliably.
In a retrospective analysis of 38 diabetic children and adults with cystic fibrosis and nondiabetic cystic fibrosis control subjects, Lanng et al. (2) reported a gradual decline in weight, BMI, and lung function up to 4 years preceding the diagnosis of CFRD. Lanng et al. diagnosed CFRD by 1985 WHO criteria (based on BG120 min during an OGTT, but with higher thresholds than in current use). Our findings suggest that BG120 min elevation is a late event, whereas peak blood glucose ≥8.2 mmol/l on 30-min sampling in the OGTT and CGM time above 7.8 mmol/l ≥4.5% are early glucose abnormalities that are associated with decline in lung function and weight over the preceding year. The patients of Lanng et al. had lung function decline of greater magnitude than those in our patients, probably reflecting improved outcomes over recent decades.
The central problem leading to CFRD is insulin deficiency (1,18). Relative insulin resistance due to stress hormones may occur during exacerbations of lung disease, leading to temporary worsening of glycemic status (18,19). We avoided this confounding effect by ensuring that the OGTT and CGM were performed when the patients were clinically stable. Furthermore, fasting insulin levels and HOMA2 calculations of β-cell function and insulin sensitivity in our patients demonstrated insulin deficiency (rather than insulin resistance) as the underlying problem. This result confirms the findings of Mohan et al. (20), who found abnormalities of insulin secretion and not insulin resistance among 60 adult patients with cystic fibrosis. We found that glucose abnormalities (on the OGTT and CGM) and markers of clinical decline (fall in wtSDS or lung function over the preceding year) were common and were not limited to those with the more severe genotype with homozygous F508del or to those with exocrine insufficiency.
Screening practices vary, and many centers have yet to adopt the recommendation (21,22) to perform yearly OGTTs for all patients aged >10 years. Moran et al. (23) do perform annual OGTTs and have reported improvements in mortality, nutritional status, and lung function in patients with CFRD, attributing these improvements to early diagnosis and aggressive treatment.
We found that peak blood glucose is missed in the vast majority of patients by 0- and 120-min samples (32 of 33, 97%), indicating the need for sampling every 30 min during the OGTT. This conclusion is supported by the findings of Dobson et al. (24), who showed that nondiabetic patients with cystic fibrosis had substantial hyperglycemia at 30, 60, and 90 min, but identical 0 and 120 min blood glucose values compared with those of healthy aged-matched control subjects. Hyperglycemia promotes bacterial growth, and it is possible that this may contribute to the clinical decline seen even in those with relatively preserved β-cell function. Palerm (25) recently reported blood glucose values in normal subjects after meals and found that they rarely exceeded 150 mg/dl (8.3 mmol/l). This result is very similar to our cystic fibrosis–specific cutoff of 8.2 mmol/l for BGmax on an OGTT.
Lippe et al. (26) reported reduced and delayed insulin secretion in subjects with cystic fibrosis compared with that in healthy age-matched control subjects. We also found delayed peak insulin secretion in our subjects. This finding is consistent with a model of progressive β-cell loss leading to progressive insulin deficiency and worsening glycemic status. We propose that stages of progressive cystic fibrosis insulin deficiency (CFID) can be usefully defined by blood glucose on an OGTT, as CFID1 (BGmax ≥8.2 and <11.1 mmol/l), CFID2 (BGmax ≥11.1 mmol/l and BG120 min <11.1 mmol/l), CFID3 (BG120 min ≥11.1 mmol/l, corresponding to CFRD without fasting hyperglycemia) (6), and CFID4 (fasting blood glucose≥7, corresponding to CFRD with fasting hyperglycemia). Moran et al. (27) recently showed in a randomized controlled trial that insulin treatment in adults with CFRD without fasting hyperglycemia (corresponding to CFID3) resulted in improved BMI, although there was no significant change in lung function or number of hospitalizations.
We found that CGM is a useful tool for detecting early glycemic abnormalities, which performed as well as OGTT on ROC analysis and correlated with peak blood glucose on an OGTT. It has been used previously in cystic fibrosis (24,28) but is not currently licensed for diagnostic use. Furthermore, 24% of our patients refused to undergo CGM, whereas all agreed to have the OGTT. It is possible that the acceptability of CGM may improve as the devices become smaller.
Our findings suggest that CFRD with and without fasting hyperglycemia is a late event, which is preceded by more subtle glycemic abnormalities on CGM and 30-min sampled OGTT. These early glucose abnormalities are associated with a decline in lung function and weight over the preceding year. It is likely that progressive deficiency of insulin, a powerful anabolic hormone, may be responsible for this decline, suggesting that early insulin therapy may be beneficial in patients with cystic fibrosis before the onset of CFRD as currently defined.
Acknowledgments
This study was supported by grants from the Sydney Children's Hospital Foundation and the Regional Diabetes Support Scheme.
This study was also supported by a grant from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
We thank Barbara O'Donovan and Brigitte Mason (respiratory scientists), Rebecca McDonald (clinical nurse consultant), Rhonda Bell (clinic coordinator), Dr. Sandra Chuang (respiratory fellow), Professor Jenny Peat (statistician), and the patients of Sydney Children's Hospital and their families.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Dobson L, Sheldon CD, Hattersley AT. Understanding cystic-fibrosis-related diabetes: best thought of as insulin deficiency? J R Soc Med 2004; 97( Suppl. 44): 26– 35 [PMC free article] [PubMed] [Google Scholar]
- 2.Lanng S, Thorsteinsson B, Nerup J, Koch C. Influence of the development of diabetes mellitus on clinical status in patients with cystic fibrosis. Eur J Pediatr 1992; 151: 684– 687 [DOI] [PubMed] [Google Scholar]
- 3.Sharma R, Florea VG, Bolger AP, Doehner W, Florea ND, Coats AJ, Hodson ME, Anker SD, Henein MY. Wasting as an independent predictor of mortality in patients with cystic fibrosis. Thorax 2001; 56: 746– 750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellis G, Cazes MH, Parant A, Gaimard M, Travers C, Le Roux E, Ravilly S, Rault G. Cystic fibrosis mortality trends in France. J Cyst Fibros 2007; 6: 179– 186 [DOI] [PubMed] [Google Scholar]
- 5.Rodman HM, Doershuk CF, Roland JM. The interaction of 2 diseases: diabetes mellitus and cystic fibrosis. Medicine (Baltimore) 1986; 65: 389– 397 [DOI] [PubMed] [Google Scholar]
- 6.Moran A, Hardin D, Rodman D, Allen HF, Beall RJ, Borowitz D, Brunzell C, Campbell PW, 3rd, Chesrown SE, Duchow C, Fink RJ, Fitzsimmons SC, Hamilton N, Hirsch I, Howenstine MS, Klein DJ, Madhun Z, Pencharz PB, Quittner AL, Robbins MK, Schindler T, Schissel K, Schwarzenberg SJ, Stallings VA, Zipf WB. Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract 1999; 45: 61– 73 [DOI] [PubMed] [Google Scholar]
- 7.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183– 1197 [DOI] [PubMed] [Google Scholar]
- 8.Bennett PH, Burch TA, Miller M. Diabetes mellitus in American (Pima) Indians. Lancet 1971; 2: 125– 128 [DOI] [PubMed] [Google Scholar]
- 9.van den Berg JM, Morton AM, Kok SW, Pijl H, Conway SP, Heijerman HG. Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J Cyst Fibros 2008; 7: 515– 519 [DOI] [PubMed] [Google Scholar]
- 10.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, Stokes DC, Wohl ME, Wagener JS, Regelmann WE, Johnson CAthe Scientific Advisory Group and the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr 2007; 151: 134– 139 [DOI] [PubMed] [Google Scholar]
- 11.Lai HJ, Shoff SM, Farrell PMthe Wisconsin Cystic Fibrosis Neonatal Screening Group. Recovery of birth weight z score within 2 years of diagnosis is positively associated with pulmonary status at 6 years of age in children with cystic fibrosis. Pediatrics 2009; 123: 714– 722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld M. Overview of published evidence on outcomes with early diagnosis from large US observational studies. J Pediatr 2005; 147: S11– S14 [DOI] [PubMed] [Google Scholar]
- 13.Courtney JM, Bradley J, Mccaughan J, O'Connor TM, Shortt C, Bredin CP, Bradbury I, Elborn JS. Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol 2007; 42: 525– 532 [DOI] [PubMed] [Google Scholar]
- 14.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487– 1495 [DOI] [PubMed] [Google Scholar]
- 15.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 1983; 127: 725– 734 [DOI] [PubMed] [Google Scholar]
- 16.Swets JA. Measuring the accuracy of diagnostic systems. Science 1988; 240: 1285– 1293 [DOI] [PubMed] [Google Scholar]
- 17.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr 1997; 131: 809– 814 [DOI] [PubMed] [Google Scholar]
- 18.O'Riordan SM, Robinson PD, Donaghue KC, Moran Athe ISPAD Clinical Practice Consensus. Management of cystic fibrosis- related diabetes. Pediatr Diabetes 2008; 9: 338– 344 [DOI] [PubMed] [Google Scholar]
- 19.Hardin DS, Leblanc A, Marshall G, Seilheimer DK. Mechanisms of insulin resistance in cystic fibrosis. Am J Physiol Endocrinol Metab 2001; 281: E1022– E1028 [DOI] [PubMed] [Google Scholar]
- 20.Mohan K, Miller H, Dyce P, Grainger R, Hughes R, Vora J, Ledson M, Walshaw M. Mechanisms of glucose intolerance in cystic fibrosis. Diabet Med 2009; 26: 582– 588 [DOI] [PubMed] [Google Scholar]
- 21.Lanng S, Hansen A, Thorsteinsson B, Nerup J, Koch C. Glucose tolerance in patients with cystic fibrosis: five year prospective study. BMJ 1995; 311: 655– 659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerem E, Conway S, Elborn S, Heijerman H. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros 2005; 4: 7– 26 [DOI] [PubMed] [Google Scholar]
- 23.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis related diabetes: current trends in prevalence, incidence and mortality. Diabetes Care 2009; 32: 1626– 1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobson L, Sheldon CD, Hattersley AT. Conventional measures underestimate glycaemia in cystic fibrosis patients. Diabet Med 2004; 21: 691– 696 [DOI] [PubMed] [Google Scholar]
- 25.Palerm C. Physiologic insulin delivery with insulin feedback: a control systems perspective. In Proceedings of the 7th IFAC Symposium on Modelling and Control in Biomedical Systems, Aalborg, Denmark, 2009. Laxenburg, Austria, International Federation of Autonomic Control, p. 31 [Google Scholar]
- 26.Lippe BM, Sperling MA, Dooley RR. Pancreatic α and β cell functions in cystic fibrosis. J Pediatr 1977; 90: 751– 755 [DOI] [PubMed] [Google Scholar]
- 27.Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, Tullis E, Liou TG, Allen Hthe Cystic Fibrosis Related Diabetes Therapy Study Group. Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: results of the Cystic Fibrosis Related Diabetes Therapy Trial. Diabetes Care 2009; 32: 1783– 1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jefferies C, Solomon M, Perlman K, Sweezey N, Daneman D. Continuous glucose monitoring in adolescents with cystic fibrosis. J Pediatr 2005; 147: 396– 398 [DOI] [PubMed] [Google Scholar]